Paper Menu >>
Journal Menu >>
 Engineering, 2012, 5, 118-120 doi:10.4236/eng.2012.410B030 Published Online October 2012 (http://www.SciRP.org/journal/eng) Copyright © 2012 SciRes. ENG The Poly(vinyl alcohol)-Immobilized Photobacteria for Toxicology Monitoring Kristina A. Alenina, Leyla E.Aleskerova, Polina B.Kascheyeva, Anvar D. Ismailov Dept. of Microbiology, Biological Faculty, Moscow State University, Moscow, Russia Email: anvaris@list.ru Received 2012 ABSTRACT The present work is undertaken to studies the important factors affecting the stability of a light emission at immobilized photobacte- ria, and application PVAGs luminous biosensors for biomonitoring of toxicants. The intensity and stability of a light emission is competently controlled by: 1) intensity and persistens of a luminescent cycle using bacterial strain; 2) type of the carrier and the composition of the gel-formation medium; 3) freeze-thawing procedures; 4) physical and chemical conditions of storage and applica- tion. Keywords: Component; Bioluminescen ce; Photobacter ia; Immobilization; Po ly (Vinyl Alcohol) 1. Introduction The luminous bacteria are widely used as a biosensor for bio- monitoring of toxicants [1-3]. The application of photobacteria is defined by high sensitivity of bioluminescence to a wide spectrum of toxic substances, and a fast responsibility of light reaction. Bioluminescent activity reflects of a cell metabolism to chemical compounds with toxic action, including the heavy metals, aliphatic and aromatic hydrocarbons, phenols and a number of other xenobiotics. The emission value serves as the quantitative indicator of the general and specific toxicity of the sa mp l e . Light-emitting bacteria can be used for toxicity monitoring both using the free and immobilized forms. The immobilization of photobacteria allows to raise stability of light emission by biosensors, what is could form the basis of a cost-effective, real-time system for detecting environmental pollutions [4,5]. Many diver se gel mat rices has be en pro posed as po ssible carri- ers for immobilisation of luminous bacteria, In this case, either natural: agar, agarose, alginate, carrageenan, gelatin, collagen, or synthetic (polyacrilates, polyurethanes, polyethers) bio- po- limers has b een used as the gel-forming agents [6,7]. Taking into account that a luminescence activity extremely sensitive to temperature influence, in quality gel-forming agent is chosen poly (vinyl alcohol) (PVA). The crioPVA gel forma- tion proceeds at negative temperatures. This carrier on struc- tural, physical and chemical properties possess a number of advantages in comparison with other carriers. PVA gels have very high micro-and macro-porosities which provide favored conditions for the non hindered mass transfer of substrates and toxicants. Thermo-stability of cryoPVA gels exceeds that of other commonly employed thermo-reversible gel carriers, such as agar, agarose, ets. PVAGs are highly resistant to biological degradation and practically insensitive to media composition, — solutes, buffers, pH. PVA itself is a biologically compatible, nontoxic, readily available low-cost polymer. Rheological pa- rameters of cryoPVAGs are quite stable and allow for this car- rier to by used under variety biomonitoring conditions [6,7]. The aim of this study was the analyses of the factors affect- ing the stability of a light emission at PVA-immobilized pho- tobacteria and application PVAGs luminous biosensors for biom onitoring of tox ic a nt s . 2. Materials and Methods Bacterial strains, media and culture grow conditions. The strains used in this study were Photobacterium phosphoreum strain №331 MC MSU Immobilization procedures. The immobilization was car- ried out according to a technique [6, 9]. Liquid biomass were mixed with 13% solution of poly(vinyl alcohol), (Mm – 48000 Da), Gel solution were prepared at 24h in 1) the GM media; 2) Na-phosphat buffer + 2% NaCl, pH 7.6, and 3) 3% NaCl. The 0.2 ml gel-cell solution pour out on 96-hole planshet, and transferred to the freezing chamber (20°C) for gel formation with simultaneous inclusion of cells in a polymeric matrix. The final cell co n centration in granules about 1–5×107 cells/granule. Prio r to each measure ment s sampl es were d e frosted at 4 °C over 24 h and placed on incubation media, and stored at 4°С. The procedures of cryogenic gel-formation and cell reactivation in detail s ar e described in [9]. Measurement of light intensity and соncentration of cells. A bioluminescence registered on luminometer 1250 LKB- Wallac and expressed in relative units, or absolute values of photons output according the standard [10]. The cell contain carried out under maintenance АТР using a bioluminescent method with firefly luciferase [11] 3. Results CryoPVAGs-cell immobilization. The intensity and duration of light output by the isolated cells is defined not only by natural emission properties of strains and composition of incubation mixture. Except energet- 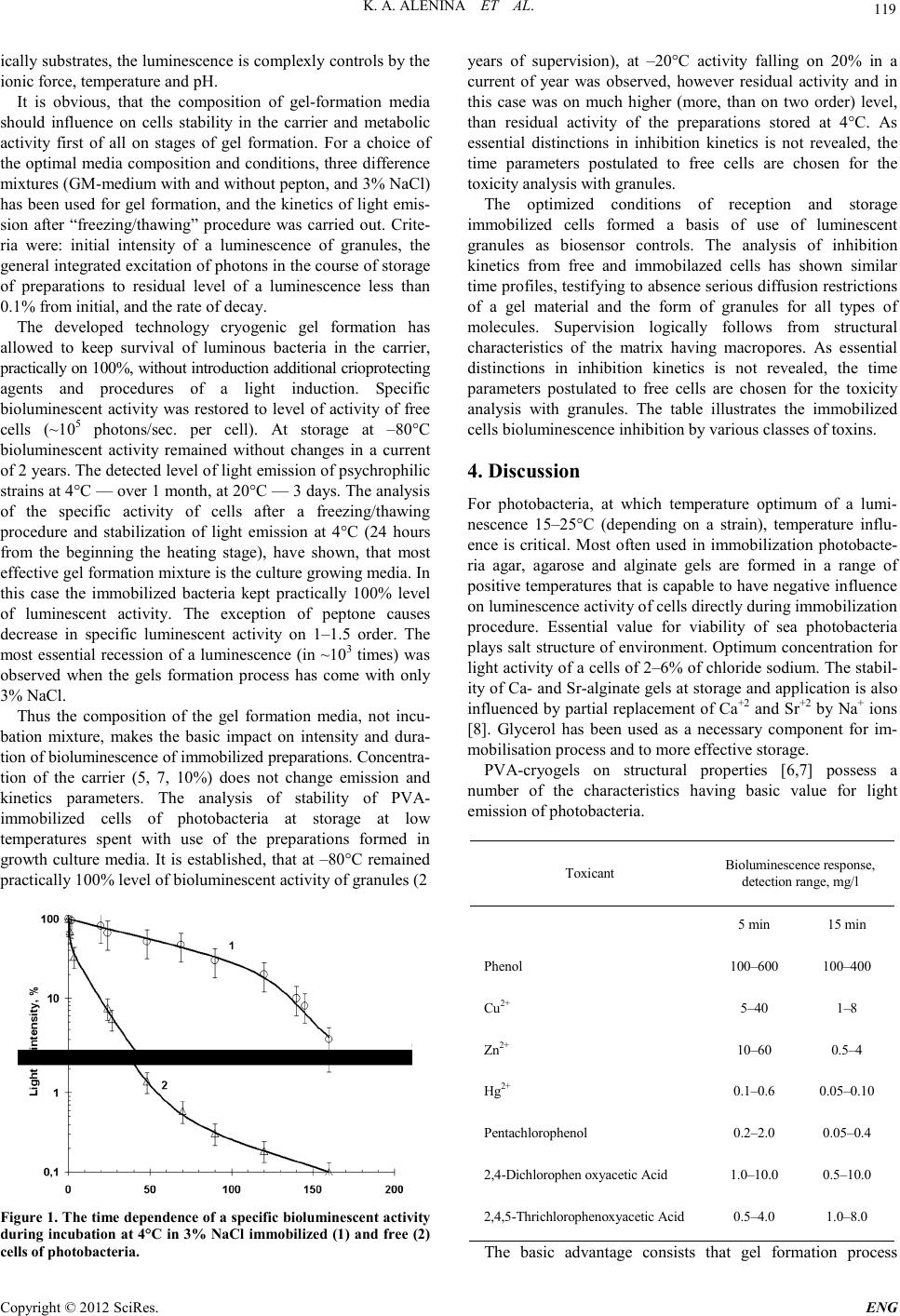 K. A. ALENINA ET AL. Copyright © 2012 SciRes. E NG 119 ically substrates, the luminescence is complexly controls by the ion ic force, temperat ure and рН. It is obvious, that the composition of gel-formation media should influence on cells stability in the carrier and metabolic activity first of all on stages of gel formation. For a choice of the optimal media composition and conditions, three difference mi xtu r e s (G M -medium with and without pepton, and 3% NaCl) has been used for gel formation, and the kinetics of light emis- sion after “freezing/thawing” procedure was carried out. Crite- ria were: initial intensity of a luminescence of granules, the general integrated excitation of photons in the course of storage of preparations to residual level of a luminescence less than 0.1% from initial, and the rate of decay. The developed technology cryogenic gel formation has allowed to keep survival of luminous bacteria in the carrier, practically on 100%, without introduction additional crioprotecting agents and procedures of a light induction. Specific bioluminescent activity was restored to level of activity of free cells (~105 photons/sec. per cell). At storage at –80°C bioluminescent activity remained without changes in a current of 2 years. The d etect ed l evel o f light emission of psychrophilic strains at 4°С — over 1 month, at 20°С — 3 d ays . Th e analysis of the specific activity of cells after a freezing/thawing procedure and stabilization of light emission at 4°С (24 hours from the beginning the heating stage), have shown, that most effective gel for matio n mixt ur e is th e cu lture gr owin g media. In this case the immobilized bacteria kept practically 100% level of luminescent activity. The exception of peptone causes decrease in specific luminescent activity on 1–1.5 order. The most essential recession of a luminescence (in ~103 times) was observed when the gels formation process has come with only 3% NaCl. Thus the composition of the gel formation media, not incu- bation mixture, makes the basic impact on intensity and dura- tion of bioluminescence of immobilized preparations. Concentra- tion of the carrier (5, 7, 10%) does not change emission and kinetics parameters. The analysis of stability of PVA- immobilized cells of photobacteria at storage at low temperatures spent with use of the preparations formed in growth culture media. It is established, that at –80°C remained practically 10 0% level of b ioluminescent activity of granules (2 Figure 1. The time depende nce of a specific bioluminescent activity during incubation at 4°С in 3% NaCl immobili zed (1) and fr ee (2) cells of photobacteria. years of supervision), at –20°C activity falling on 20% in a current of year was observed, however residual activity and in this case was on much higher (more, than on two order) level, than residual activity of the preparations stored at 4°С. As essential distinctions in inhibition kinetics is not revealed, the time parameters postulated to free cells are chosen for the toxicity analysis with granules. The optimized conditions of reception and storage immobilized cells formed a basis of use of luminescent granules as biosensor controls. The analysis of inhibition kinetics from free and immobilazed cells has shown similar time profiles, testifying to absence serious diffusion restrictions of a gel material and the form of granules for all types of molecules. Supervision logically follows from structural characteristics of the matrix having macropores. As essential distinctions in inhibition kinetics is not revealed, the time parameters postulated to free cells are chosen for the toxicity analysis with granules. The table illustrates the immobilized cells bioluminescence inhibition by various classes of toxins. 4. Discussion For photobacteria, at which temperature optimum of a lumi- nescence 15–25°С (depending on a strain), temperature influ- ence is critical. Most often used in immobilization photobacte- ria agar, agarose and alginate gels are formed in a range of posi tive temperatures t hat is capab le to have negati ve influen ce on luminescence activity of cells directly during immobilization procedure. Essential value for viability of sea photobacteria plays salt structure of environment. Optimum concentration for light activity of a cells of 2–6% of chloride sodium. The stabil- ity of Ca- and Sr-alginate gels at storage an d app licatio n is al so influen ced by partial rep lacement of Ca+2 and Sr+2 by Na+ ions [8]. Glycerol has been used as a necessary component for im- mobi li s a ti on proces s and to m or e e f fe ctiv e stor a ge . PVA-cryogels on structural properties [6,7] possess a number of the characteristics having basic value for light emission of photobacteria. Toxicant Bioluminescence response, detection range, mg/l 5 min 15 min Phenol 100–600 100–400 Сu2+ 5–40 1–8 Zn2+ 10–60 0.5–4 Hg2+ 0.1–0.6 0.05–0.10 Pentachlorophenol 0.2–2.0 0.05–0.4 2,4-Dichlorophen oxyacetic Acid 1.0–10.0 0.5–10.0 2,4,5-Thrichlorophenoxyacetic Acid 0.5–4.0 1.0–8.0 The basic advantage consists that gel formation process  K. A. ALENINA ET AL. Copyright © 2012 SciRes. ENG 120 proceeds at negative temperatures. The PVA-gels possess high thermo stability in a wide range of positive temperatures up to 80°С, that creates possibility of use of a bioreactor in different temperature modes. Physical and chemical para meters of carrier slightly depend on structure of the environment of formation of gel, in particular salt, which has great value for bioluminescen t activity of bacteria. The PVA-gels has note been damage by microorganisms. Essentially, that PVA it is nontoxic in relation to the in clud ed pho tob acteri a. Conseq uen tly, th ese gel material s can be considered as very promising carrier for use in photo- bacteria entrap ment technologies The results presented testify the long-term stability and high intensity of a luminescence with PVA-Gs immobilized psyc- hrophilic strains of photobacteria. It is established, that the major importance fo r s tability of a lumin es cence has a choice of photobacteria strain and of the gel formation mixture. A com- position of incubation medium, рН, and especially temperature, have complex an effect as on intensity and duration of a lumi- nescence. Results suggests that the most stability of light output by immobilized preparations can be achieved with incu- bation at relative lo w (no more then 20°C) temper ature in al ka- line (pH 8.5) mixtures. High survival of cells, with preservation of specific activity of light emission, at level of the free cells, presented to the given work, reflect advantages cryogenic immobilization photobacteria in PVA carrier and application PVAGs-lum biosensors for biodetection of toxicants REFERENCES [1] Bulich AA (1979) Use of luminescent bacteria for determining toxicity in aquatic environments. In: Markings LL, Kimerle RA (eds.) Aquatic Toxicology. American Society for Testing and Materials , Phi ladelphia [2] Lee J H, Mi tch ell RJ, Kim BC , Cullen DC, Gu MB (2005) A c el l array bi osensor for en viron mental t oxicit y analysis. Biosen s Bio- electron 21(3):500–507 [3] Scheller F, Schubert F (1992) Biosensors. Techniques and in- strumentation in analytical chemistry. Elsevier, Amsterdam 11:359 [4] Park KS, Baumstark-Khan Ch, Rettberg P, Horneck G, Rabbow E, Gu MB (2005) Immobilisation as a technical possibility for long-term storage of bacterial biosensors. Radiat Environ Bio- phys 44:69–71 [5] Lee B, Lee J, Shin D, Kim E (2006) Statistical optimization of bioluminescence Photobacterium phosphoreum KCTC 2852. Envi ron Int 32(2):265–268 [6] Lozinsky VI, Zubov AL, Titova EF (1996) Swelling behaviour of poly(vinyl alcohol) cryogels employed as matrices for cell immobilization. Enzyme Microb Technol 18(6):561-569 [7] Lozinsky VI, Plieva FM (1998) PoIy(vinyl alcohol) cryogels employed as matrices for cell immobilization. 3. Overview of recent research and developments. Enzyme Microb Technol 23:227–242 [8] Chun UH, Simonov N, Chen Y, Britzb ML (1996) Continuous pollution monitoring using Photobacterium phosphoreum. Resour Conserv Recycl 18:25–40 [9] Efremenko EN, Senko OV, Kuts VV, Alenina KA, Kholstov AV, Ismailov AD (2010) The luminescent biocatalyst for detecting toxicants. Patent RU 2394910 [10] Hastings JW, Weber G (1963) Total quantum flux of isotropic sourc es. Opt Soc Am 53(12):14 10–1415 [11] Efremenko EN, Tatarinova NYu (2007) The effect of long-term preservat ion of bacteria l cells immobilized in poly(vin yl alcoh ol) cryogel on their viability and biosynthesis of target metabolites. Microbiol 76( 3):383–389 |

