H. Y. CHEN ET AL.
Copyright © 2012 SciRes. ENG
which is harmful for the nucleophilic reaction of amino groups.
So the nucleophilic reaction falls into hydroxyl groups of CS,
emerging as selectively alkylation of hydroxyl groups without
amin o groups protecting.
3.3. XRD Characterization of OACS
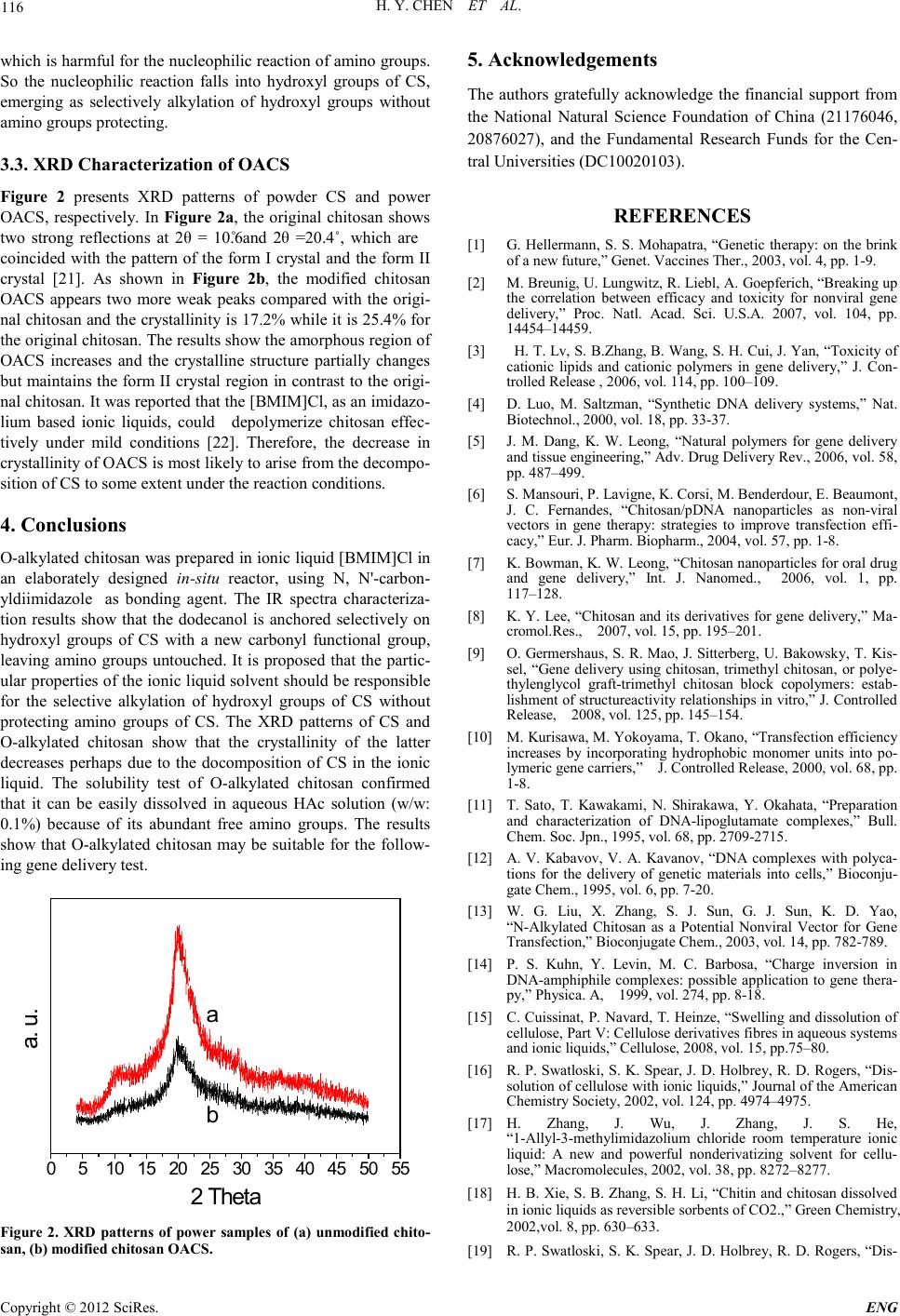
Figure 2 presents XRD patterns of powder CS and power
OACS, respectively. In Figure 2a, the original chitosan shows
two strong reflections at 2θ = 10.6˚ and 2θ =20.4˚, which are
coincided with the pattern of the form I crystal and the form II
crystal [21]. As shown in Figure 2b, the modified chitosan
OACS appears two more weak peaks compared with the origi-
nal chitosan and the crystallinity is 17.2% while it is 25.4% for
the original chitosan. The results show the amorphous region of
OACS increases and the crystalline structure partially changes
but maintains the form II crystal region in contrast to the origi-
nal chitosan. It was reported that the [BMIM]Cl, as an imidazo-
lium based ionic liquids, could depolymerize chitosan effec-
tively under mild conditions [22]. Therefore, the decrease in
crystallinity of OACS is most likely to arise from the decompo-
sition of CS to some extent under the reaction conditions.
4. Conclusions
O-alkylated chitosan was p repared in ionic liquid [BMIM]Cl in
an elaborately designed in-situ reactor, using N, N'-carbon-
yldiimidazole as bonding agent. The IR spectra characteriza-
tion results show that the dodecanol is anchored selectively on
hydroxyl groups of CS with a new carbonyl functional group,
leaving amino groups untouched. It is proposed that the partic-
ular properties of the ionic liquid solvent should be responsible
for the selective alkylation of hydroxyl groups of CS without
protecting amino groups of CS. The XRD patterns of CS and
O-alkylated chitosan show that the crystallinity of the latter
decreases perhaps due to the docomposition of CS in the ionic
liquid. The solubility test of O-alkylated chitosan confirmed
that it can be easily dissolved in aqueous HAc solution (w/w:
0.1%) because of its abundant free amino groups. The results
show that O-alkylated chitosan may be suitable for the follow-
ing gene delivery test.
05 10 15 20 25 30 35 40 45 50 55
a
b
2 Theta
a. u.
Fig ure 2. XRD patterns of power samples of (a) unmodified chito-
san, (b) modifie d chi tosan OACS.
5. Acknowledgements
The authors gratefully acknowledge the financial support from
the National Natural Science Foundation of China (21176046,
20876027), and the Fundamental Research Funds for the Cen-
tral Universities (DC10020103).
REFERENCES
[1] G. Hellermann, S. S. Mohapatra, “Genetic therapy: on the brink
of a new future,” Genet. Vaccines Ther., 2003, vol. 4, pp. 1-9.
[2] M . Breun ig, U . Lungwit z, R . Liebl, A. Goep feri ch , “B reakin g up
the correlation between efficacy and toxicity for nonviral gene
delivery,” Proc. Natl. Acad. Sci. U.S.A. 2007, vol. 104, pp.
14454–14459.
[3] H. T. Lv, S. B.Zhang, B. Wang, S. H. Cui, J. Yan, “Toxicity of
cationic lipids and cationic polymers in gene delivery,” J. Con-
trolled Release , 2006, vol. 114, pp. 100–109.
[4] D. Luo, M. Saltzman, “Synthetic DNA delivery systems,” Nat.
Biotechnol., 2000, vol. 18, pp. 33-37.
[5] J. M. Dang, K. W. Leong, “Natural polymers for gene delivery
and ti ssu e engin eerin g, ” Adv. Dru g Deliv ery Re v., 2 006, vol. 5 8,
pp. 487–499.
[6] S. Mansouri, P. Lavigne, K. Corsi, M. Benderdour, E. Beaumont,
J. C. Fernandes, “Chitosan/pDNA nanoparticles as non-viral
vectors in gene therapy: strategies to improve transfection effi-
cacy,” Eur. J. Pharm. Biopharm., 2004, vol. 57, pp. 1-8.
[7] K. Bowman, K. W. Leong, “Chitosan nanoparticles for oral drug
and gene delivery,” Int. J. Nanomed., 2006, vol. 1, pp.
117–128.
[8] K. Y. Lee, “Ch itosan an d its deri vatives for g ene deli very,” M a-
cromol.Res., 2007, vol. 15, pp. 195–201.
[9] O. Germershaus, S. R. Mao, J. Sitterberg, U. Bakowsky, T. Kis-
sel, “Gene delivery using chitosan, trimethyl chitosan, or polye-
thylenglycol graft-trimethyl chitosan block copolymers: estab-
lishment of structureactivity relationships in vitro,” J. Controlled
Release, 2008, vol. 125, pp. 145–154.
[10] M. Kurisawa, M. Yokoyama, T. Okano, “Transfection efficiency
increases by incorporating hydrophobic monomer units into po-
lymeric gene carriers,” J. Controlled Release, 2000, vol. 68, pp.
1-8.
[11] T. Sato, T. Kawakami, N. Shirakawa, Y. Okahata, “Preparation
and characterization of DNA-lipoglutamate complexes,” Bull.
Chem. Soc. Jpn., 1995, vol. 68, pp. 2709-2715.
[12] A. V. Kabavov, V. A. Kavanov, “DNA complexes with polyca-
tions for the delivery of genetic materials into cells,” Bioconju-
gate Chem., 1995, vol. 6, pp. 7-20.
[13] W. G. Liu, X. Zhang, S. J. Sun, G. J. Sun, K. D. Yao,
“N-Alkylated Chitosan as a Potential Nonviral Vector for Gene
Transfection,” Bioconjugate Chem., 2003, vol. 14, pp. 782-789.
[14] P. S. Kuhn, Y. Levin, M. C. Barbosa, “Charge inversion in
DNA-amphiphile complexes: possible application to gene thera-
py,” Physica. A, 1999, vol. 274, pp. 8-18.
[15] C . Cuissin at, P. Nava rd, T. Heinze, “Swellin g and dis solution of
cellu los e, P ar t V : C el lu los e d eri v at i ves fi b res i n aqu eou s s yst ems
and ionic liquids,” Cellulose, 2008, vol. 15, pp.75–80.
[16] R. P. Swatloski, S. K. Spear, J. D. Holbrey, R. D. Rogers, “Dis-
solut ion of c ellulose with ionic liquids, ” Jou rnal of th e America n
Chemistry Societ y, 2002 , vol. 124, pp. 4974–4975.
[17] H. Zhang, J. Wu, J. Zhang, J. S. He,
“1-Ally l-3 -methylimidazolium chloride room temperature ionic
liquid: A new and powerful nonderivatizing solvent for cellu-
lose,” M acromolec ules, 2 002, vol. 38, pp. 8272–8277.
[18] H. B. Xie, S. B. Zhang, S. H. Li, “Chitin and chitosan dissolved
in i oni c li qui d s a s revers i b le sor b en ts of C O2 ., ” Green C h emis t ry,
2002,vol. 8, pp. 630–633.
[19] R. P. Swatloski, S. K. Spear, J. D. Holbrey, R. D. Rogers, “Dis-