Paper Menu >>
Journal Menu >>
 Engineering, 2012, 5, 94-98 doi:10.4236/eng.2012.410B024 Published Online October 2012 (http://www.SciRP.org/journal/eng) Copyright © 2012 SciRes. ENG Electronic Detection of Escherichia coli O157︰H7 Using Single-Walled Carbon Nanotubes Field-Effect Tr ansistor Biosensor Xiaoxian Zhang1, Dongwei Wang1, Danna Yang1, Sai Li1*, Zhiqiang Shen2 1School of Chemical Engineeri ng, Sichuan University, Chengdu, China 2Academy of Military Medical sciences, Chinese PLA Center for Disease Control & Prevention, Tianjin, China Email: *lisai@scu.edu.cn Received 2012 ABSTRACT Field effect tr ansisto rs (FET) based on Single-Walled Carbo n Nanotubes ( SWNTs) become the ho t topic in fields of nano-electronic, clinical diagnostics, environmental testing etc. in recent years. In this paper, we reported a simple, scalable way to enrich semicon- ducting SWNTs by using HNO3/H2SO4. Then carbon nanotube field-effect transistors (CNTFET) biosensor was fabricated with the enrich ment SWNTs for E scherich ia coli O15 7︰H7 detect ion. The resp onse of each CN TFET was moni tored in real time before an d after introduction of the Escherichia coli O157︰H7 at various con centration s. The results show that CNT-FET biosensors we fabri- cated are sensitive t o chan ge of concen tration of so lution and response time is really short. Keywords: Sin gle-walled Carbon Nanotubes (SWNTs); Field Effect Transistors; Biosensor; Escherichia Coli O157 ︰H7 1. Introduction Biosensor plays an important role in many areas of environ- mental monitoring, food analysis and life sciences. But tradi- tional biological sensing techniques require multiple reagents, signal amplification, and complex data analysis [1]. Because SWNTs have outstanding properties such as possible biocom- patibility, size compatibility and sensitivity towards minute electrical perturbations [2], it has a great potential for biosens- ing applications. Besides, CNTFET fabricated with semicon- ducting SWNTs can operate at room temperature, reagentless and l abel-free. CNTFET has been used as biosensors to detect target bio- molecules such as virus [3], bacterium [4,5], DNA [6] and en- zymes [ 7]. Star et al. [8] h ave fabricated CNTFE T devices sen- sitive to streptavidin by using individual biotin-functional- ized CNTFET. Li et al. [9] repo rted the comple mentar y detectio n of prostate-specific antigen (PSA) by using an anti-PSA monoc- lonal antibody CNTFET. The mechanism of detection of bio- molecules is still controversial. There are two major explains: charge-transfer mechanism (electrostatic gating) [10] and Schottky–Barrier modulation effect [11]. But there’s a hurdle to the widespread application of CNT- FET. SWMTs synthesized are the mixture of semiconducting SWNTs (sem-SWNTs) and metallic SWNTs (met-SWNTs). The undesired met-SWNTs will hinder effective electronic switching. Therefore, it’s essential to enrich or separate sem- SWNTs from met-SWNTs. Many proposed methods were put forward, such as d ielectr ophoresis [12], aromatic mol ecules [ 13] and DNA wrapped [14]. But there’s not a scalable, nondestruc- tive, iteratively repeatable and affordable sorting strategy emerged [15]. In this paper, we separate metallic from semiconducting na- notubes by using HNO3/H2SO4. Then we fabricate CNTFET biosensor with treated SWNTs by applying AC dielectrophore- sis [16] method. The CNTFET biosensor was functionalized with linker molecule and the corresponding antibody. The binding of the target E. coli O157︰H7 onto the receptor de- tected by monitoring the electronic properties between source and drain electrodes. We hope our work could contribute to fast detection and comprehend of mechanism. 2. Materials and Methods 2.1. Materials E. coli O157︰H7 antibody and E. coli O157︰H7 were sup- plied by Academy of Military Medical sciences, Chinese PLA Center for Disease Control & Prevention. 1-pyrenebutanoic acid succinimidyl ester (PASE) was purchased from Sigma- Aldrich. The reagents Dimethyl Formamide was purchased from Kelong Chemical Company. All chemicals were used without purification. CVD SWCNTs were produced by Chengdu Organ ic Chemistry Research C enter. 2.2. Preparation of CNTFET Biosensor The procedure of separate metallic from semiconducting nano- tub es and fabricate C NTFET can be seen in our precious works [17,18 ]. Immerse CNT channels in a 6 mM solution of PASE in di- methyl formamide solution in room temperature overnight. Then washed it with deionized water and blown dry with nitro- gen. We can get a PASE-CNTFET. Secondly, the channels of *Corresponding author. 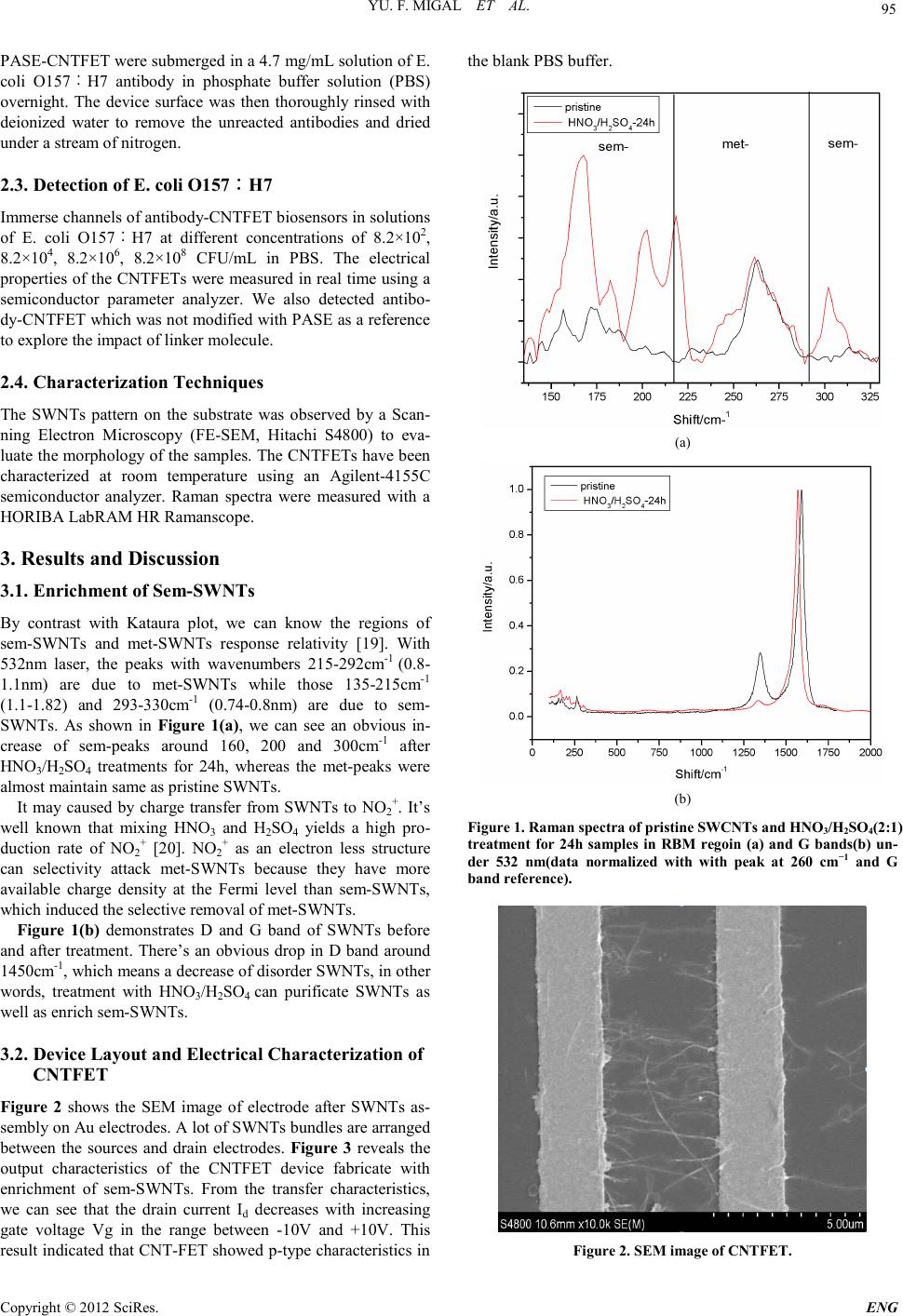 YU. F. MIGAL ET AL. Copyright © 2012 SciRes. ENG 95 P AS E -CNTFET were submerged in a 4.7 mg/mL solution of E. coli O157︰H7 antibody in phosphate buffer solution (PBS) overnight. The device surface was then thoroughly rinsed with deionized water to remove the unreacted antibodies and dried under a stream of nitrogen. 2.3. Detection of E. coli O157︰H7 Immerse ch ann els of ant ibody-CNTFET biosensors in solutions of E. coli O157︰H7 at different concentrations of 8.2×102, 8.2×104, 8.2×106, 8.2×108 CFU/mL in PBS. The electrical propert ies of the CNTFETs were measured in real time using a semiconductor parameter analyzer. We also detected antibo- dy-CNTFET which was not modified with PASE as a reference to explore the impact of linker molecul e. 2.4. Characterization Techniques The SWNTs pattern on the substrate was observed by a Scan- ning Electron Microscopy (FE-SEM, Hitachi S4800) to eva- luate the morphology of the samples. The CNTFETs have been characterized at room temperature using an Agilent-4155C semiconductor analyzer. Raman spectra were measured with a HORIBA LabRAM HR Ramansc ope. 3. Results and Discussion 3.1. Enrichment of Sem-SWNTs By contrast with Kataura plot, we can know the regions of sem-SWNTs and met-SWNTs response relativity [19]. With 532nm laser, the peaks with wavenumbers 215-292cm-1 (0.8- 1.1nm) are due to met-SWNTs while those 135-215cm-1 (1.1-1.82) and 293-330cm-1 (0.74-0.8nm) are due to sem- SWNTs. As shown in F igure 1( a), we can see an obvious in- crease of sem-peaks around 160, 200 and 300cm-1 after HNO3/H2SO4 treatments for 24h, whereas the met-peaks were al mo s t ma i nt ai n same as pristin e S WNTs. It may caused by charge transfer from SWNTs to NO2+. It’s well known that mixing HNO3 and H2SO4 yields a high pro- duction rate of NO2+ [20]. NO2+ as an electron less structure can selectivity attack met-SWNTs because they have more available charge density at the Fermi level than sem-SWNTs, which induced t he selecti ve r emoval of met-S WN Ts . Figure 1(b) demonstrates D and G band of SWNTs before and after treatment. There’s an obvious drop in D band around 1450cm-1, which means a decreas e o f diso rder S WNTs, in other words, treatment with HNO3/H2SO4 can purificate SWNTs as well as enrich sem-S WN Ts. 3.2. Device Layout and Electrical Characterization of CNTFET Figure 2 shows the SEM image of electrode after SWNTs as- sembly on Au electrodes. A lot of SWNTs bundles are arranged between the sources and drain electrodes. F igure 3 reveals the output characteristics of the CNTFET device fabricate with enrichment of sem-SWNTs. From the transfer characteristics, we can see that the drain current Id decreases with increasing gate voltage Vg in the range between -10V and +10V. This result indicated that CNT-FET showed p-type char acteristics in the blank PB S buffer . (a) (b) Figure 1. Raman spectra of pristine SWCNTs and HNO3/H2SO4(2:1) treatment for 24h samples in RBM regoin (a) and G bands(b) un- der 532 nm(data normalized with with peak at 260 cm−1 and G band reference). Figure 2. SEM image of CNTFET.  YU. F. MIGAL ET AL. Copyright © 2012 SciRes. ENG 96 3.3. Detection of the E. coli O157︰H7 on CNTFET Modified by Linker and Antibody It’s a common way to functionalize SWNTs by using corres- ponding receptor molecules as recognition layer to realize spe- cific detecti on of bio molecul es. We detected E. col i O157︰H7 using CNTFET biosensors, in which CNTFET channels were modified with E. coli O157︰H7 antibody. Linker bifunctional molecule PASE can attach with antibody and SWNTs respec- tively, playing a role as the bridge medium. The illustration for fabrication of CNTFET immune biosensor was showed in Fig- ure 4. Resistance-volt a ge curves of CNTFET were demonstrated in Figure 5 and Figure 6. Figure 5 shows that after modified with PASE, the antibody-CNTFET biosensor causes a signifi- cant decrease in the source-drain resistance contrast with the pristine CNTFET. The significant change means E. coli O157 ︰H7 could be captured by the antibody, then resistance change follows. Figure 6 shows Resistance-voltage curve of CNTFET after introduction of O157︰H7 into antibody-CNTFET with- out PASE modified has slight change, which means CNTFET without modified by linker could not attach effective antibody to cap ture E. coli O157︰H7 . Fig ure 3. Id -Vds curves of a CNTFET measured at room temper- ature. (Vg =-10 to 10 V and steps are 5V). Figure 4. Schematic illustration for fabrication of CNTFET immu ne biosensor. Figure 5. Resistance of Voltages of antibody-CNTFET after intro- duction of O157︰H7 into antibody- CNTFET with PSE. a: PASE-CNTFET, b: pristine CNTFET. Figure 6. Resistance of Voltages of antibody-CNTFET after intro- duction of O157︰H7 into antibody-CNTFET without PASE.a:CNTFET witho u t PASE, b:pristine CNTFET . After measuring of electrical properties of the CNTFE T bio- sensors, we washed the device surface with deionized water several times and dried under a stream of nitrogen. Then we observed using SEM to confirm whether E. coli O157︰H7 were attached on channel of CNTFET or not. Figure 7 shows SEM resu lts. CNTFET after P ASE modified could attach E. coli O157︰H7 (Figure 7(a) ) whereas those CNTFET without PASE could not catch the E. coli O157︰H7 (Figure 7(b)). Combine R-V Curves and SEM results, we can get the con- clusio n that the CENFET biosen sors cou ld realize t he effective detection of E. coli O157︰H7. Only the CNTFET modified by linker PASE and antibody have adsorption and response effect on E. coli O157︰H7. The linker PASE plays an important role to immobilize nanotubes and antibody. It’s due to the special structure of PASE. It has two functional groups pyrene and succinimidyl ester. Pyrene moiety could functionalize with the SWNTs by π–π stacking and succinimidyl ester could react with -NH2 in antibody to be a covalent bond.  YU. F. MIGAL ET AL. Copyright © 2012 SciRes. ENG 97 (a) (b) Figure 7. SEM images of CNTFET after introduction of O157︰H7 (a) PASE-CNT FET (b ) CNTFET without PASE. Time dependence of resistance of CNTFET after introduc- tion of O157︰H7 at different concentration into antibody- CNTFET was showed in Figure 8. Solution of concentrations 8.2×102, 8.2 ×104, 8.2×106, 8.2×108 CFU/mL of E. coli O157︰ H7 in PBS was dropped in channels of antibody-CNTFE T bio- sensors. The electrical properties of the CNTFETs were meas- ured in real time using a semiconductor parameter analyzer. Resistan ce increases sharpl y to the maximum after dropped the solution. Then it decreases rapidly in around 50s to a relative stable level. Same trend occurred in different concentrations, and maximum of resistance increased from 84kΩ to 110kΩ along with the increase of concentration of E. coli O157︰H7 solution. The main reason is more E. coli O157︰H7 could be captured by antibodies on CNTFET along with the increase of concentration, then resistance between source and drain elec- trode rises consequently. The changes of resistance indicates the possibility of CNTFET biosensor to detect E. coli O157︰H7, and concentra- Figure 8. Time dependence of resistance of CNTFET after intro- duction of O157︰H7 at different concentration into antibo- dy-modifie d CNTFET. tions as low as 8.2×102 CFU/mL can be readily detected with response times of about 50s. 4. Conclusion In this paper, we successfully fabricated CNTFET biosensor with enriched semiconducting SWNTs by using a simple, scal- able way of HNO3/H2SO4. Fr om SEM images and R-V cu rves, we confirmed the effect of linker PASE. It can functionalize with the SWNTs as well as the antibody. Then solutions of concentrations 8.2×102, 8.2×104, 8.2×106, 8.2×108 CFU/mL of E. coli O157︰H7 were detected with antibody-CNTFET bio- sensor. There’s an obvious increase of resistance between sour ce and drain with increas e of concen tration . We can draw a conclusion that the CNTFET biosensors we fabricated are sen- sitive to change of concentration of solution. The strategy can be applied to general antibody-based detection schemes for detecti ng concentratio n of target bi omolecules . 5. Acknowledgements This research was supported by NSFC (20805033; 30901199), SRF for ROCS, SEM (2008890-19-9). REFERENCES [1] Gruner G, “Carbon nanotube transistors for biosensing applica- tions,” Anal. Bioanal. Chem., vol.384, pp. 322-335, 2006. [2] Allen B L, Kichambare P D, Star A, “Carbon nanotube field-effect-transistor-based bi osen s ors , ”. Ad v M at er. , vol. 19 , pp. 1439-1451, 2007. [3] Oh J, Yoo S, Chan g Y W et al. “Carbon nanotube-based bi osen- sor for detection hepatitis B,” Current Applied Physics, vol. 9, pp. E229-E231, 2009. [4] Raquel A. Villamizar1, Alicia Maroto and F. Xavier Rius, “Im- proved detection of Candida albicans with carbon nanotubefield-effect transistors,” Sensors and Actuators B. vol. 136, pp. 451–457, N ovember 2009 . [5] Villamizar R A., Maroto A, Riusa F.X, Inza I and Figueras M J., “Fast detection of Salmonella Infantis with carbon nanotube field effect transistors,” Biosens. Bioelectron.. vol. 24, pp. 279-283,  YU. F. MIGAL ET AL. Copyright © 2012 SciRes. ENG 98 April 2008. [6] M artinez M T, Ts eng Y C, Ormat egui N et al. “Lab el-free DNA biosensors based on functionalized carbon nanotube field effect transistors,” Nano Letters, vol. 9, pp.530-536,2009. [7] B est em an K, Lee J O, Wi ert z F G M, Heeri n g H A and Dekker C, “E n zym e -coated carbon nanotubes as single-molecule biosen- sors,” N ano Letters, vol. 3, pp. 727-730, 2003. [8] Alexander Star,Jean-Christophe P. Gabriel, Keith Bradley, and George Gru1ner, “Electronic detection of specific protein bind- ing using nanotube FET devices,”Nano Letts. vol. 3, pp. 459-463, February 2003. [9] Li C, Curreli M, Lin H, Lei B, Ishikawa F.N., Datar R, et al, “Complementary detection of prostate-specific antigen using ln2O3 nanowires and carbon nanotubes,” J. Am. Oil Chem. Soc., vol. 127, pp. 12484-12485, 2005. [10] Bradley K, Briman M, Star A and Gruner G, “Charge transfer from adsorbed proteins,” Nano Letters, vol. 4, pp. 253-256,2004. [11] Chen C, Zhang W, Zhao B and Zhang Y, “Investigation of Schottky-Barrier carbon nanotube field-effect t ran sist or by an ef- ficient semi-classical numerical modeling,” Phys. Lett. A, vol. 374, pp. 309-312, 2009. [12] Ralph Krupke, Frank Hennrich, Hilbert v. Lohneysen and Man- fred M.Kappes, “Separation of metallic from semi-conducting single walled carbon nanotubes,” Science, vol. 3 01 , pp . 34 4 -347, July 2003 [13] Douglas R. Kauffman, Oleksandr Kuzmych and Alexander Star, “Interactions between single-walled carbon nanotubes and tetra- phenyl metalloporphyrins:correlation between spectroscopic and FET mea su r emen t s ,” J . Phys. C h em. C, vol. 111, pp . 3539–3543, 2007. [14] Zheng M, Jagota A, Semke E D., Bruce A. Diner, Robert S. Mclean, Steve R. Lustig, et al., “DNA-assisted dispersion and sepa rat ion of ca rb on nano tub es,” N at. Ma ter, vol. 2, p p. 338–342, April 2003 [15] M ark C. Hersa M, “P rogr ess towards monodisperse single-walled carbon nanotubes,” Nature Nanotechnology, vol. 3, pp.387-394, July 2008. [16] Stokes P, Silbar E, Zayas Y M., and Khondaker S I.,“Solution processed large area field effect transistors from dielectrophore- ticly aligned arrays of carbon nanotubes,” Appl. Phys. Lett., vol 94, pp. 113104-1-3, March 20 0 9. [17] Zhang X, Wang D, Yang D, Li S and He Y, “Field-effect Tran- sistor Biosensor Fabricated With Selective Enrichment of Semi- conducting Single-Walled Carbon Nanotubes,” ICoBE, pp. 27-28, February 2012. [18] Yang D, Wang L, Zhao Q and Li S, “Fabrication Of Sin- gle-Walled Carbon Nanotubes (SWNTs) Field-Effect Transistor (FET) Bi os ensor,” BMEI 3rd,vol. 4, pp.1482-1485, 2010. [19] H.Kataura, Y. Kumazawa, Y. Maniwa, I. Umezu, S. Suzuki, Y. Ohtsuka, et al,, “Optical properities of single-walled carbon na- notubes,” Synth.Met. vol. 103, pp . 2555-2558, 1999. [20] Francis A. C and Richard J. S, Advantaged Organic Chemistry; McGraw-Hill: New York, 1996. |

