Synthesis, Growth, Crystal Structure and Characterization of the o-Toluidinium Picrate
Copyright © 2013 SciRes. JCPT
128
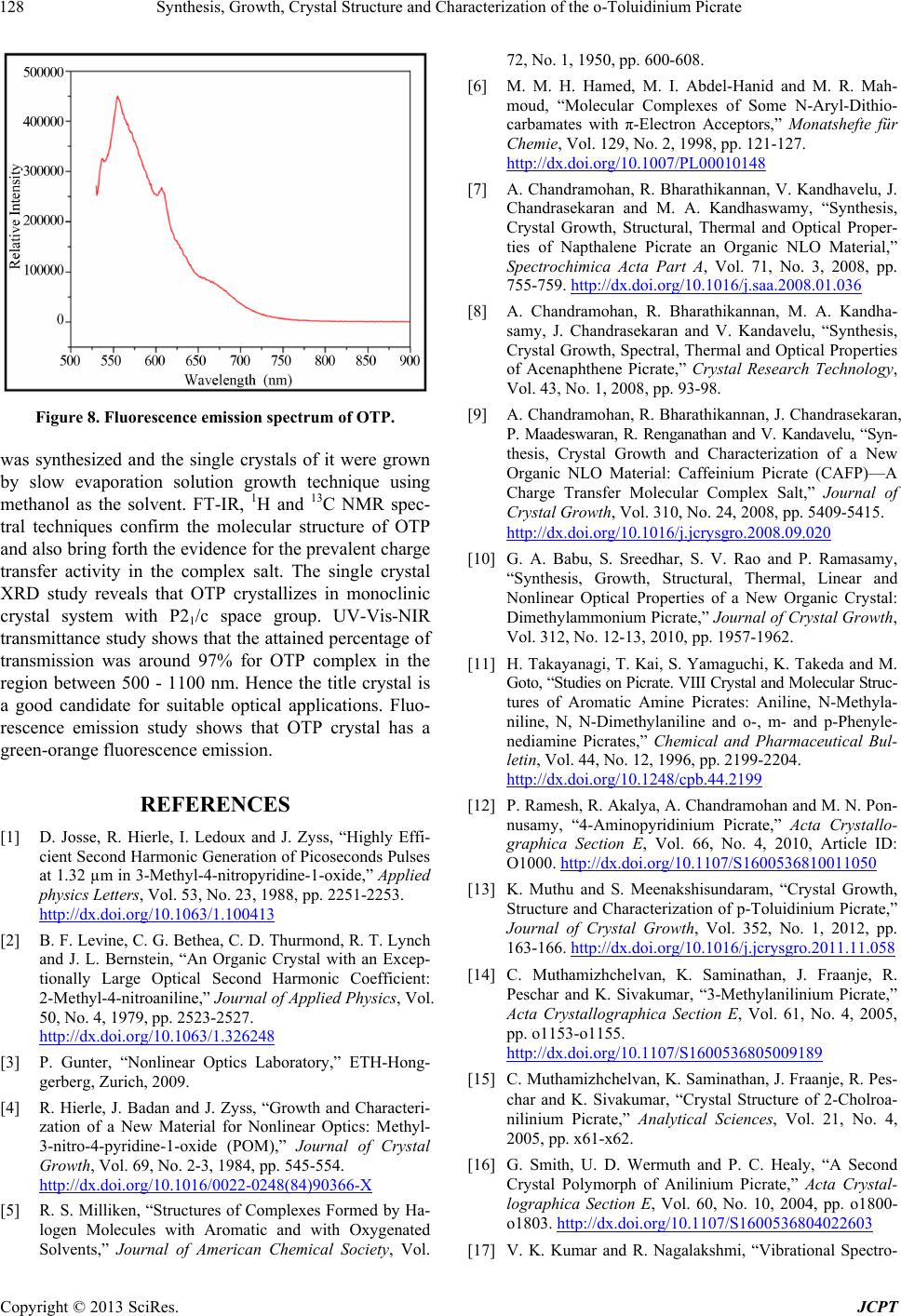
Figure 8. Fluorescence emission spectrum of OTP.
was synthesized and the single crystals of it were grown
by slow evaporation solution growth technique using
methanol as the solvent. FT-IR, 1H and 13C NMR spec-
tral techniques confirm the molecular structure of OTP
and also bring forth the evidence for the prevalent charge
transfer activity in the complex salt. The single crystal
XRD study reveals that OTP crystallizes in monoclinic
crystal system with P21/c space group. UV-Vis-NIR
transmittance study shows that the attained percentage of
transmission was around 97% for OTP complex in the
region between 500 - 1100 nm. Hence the title crystal is
a good candidate for suitable optical applications. Fluo-
rescence emission study shows that OTP crystal has a
green-orange fluorescence emission.
REFERENCES
[1] D. Josse, R. Hierle, I. Ledoux and J. Zyss, “Highly Effi-
cient Second Harmonic Generation of Picoseconds Pulses
at 1.32 µm in 3-Methyl-4-nitropyridine-1-oxide,” Applied
physics Letters, Vol. 53, No. 23, 1988, pp. 2251-2253.
http://dx.doi.org/10.1063/1.100413
[2] B. F. Levine, C. G. Bethea, C. D. Thurmond, R. T. Lynch
and J. L. Bernstein, “An Organic Crystal with an Excep-
tionally Large Optical Second Harmonic Coefficient:
2-Methyl-4-nitroaniline,” Journal of Applied Physics, Vol.
50, No. 4, 1979, pp. 2523-2527.
http://dx.doi.org/10.1063/1.326248
[3] P. Gunter, “Nonlinear Optics Laboratory,” ETH-Hong-
gerberg, Zurich, 2009.
[4] R. Hierle, J. Badan and J. Zyss, “Growth and Characteri-
zation of a New Material for Nonlinear Optics: Methyl-
3-nitro-4-pyridine-1-oxide (POM),” Journal of Crystal
Growth, Vol. 69, No. 2-3, 1984, pp. 545-554.
http://dx.doi.org/10.1016/0022-0248(84)90366-X
[5] R. S. Milliken, “Structures of Complexes Formed by Ha-
logen Molecules with Aromatic and with Oxygenated
Solvents,” Journal of American Chemical Society, Vol.
72, No. 1, 1950, pp. 600-608.
[6] M. M. H. Hamed, M. I. Abdel-Hanid and M. R. Mah-
moud, “Molecular Complexes of Some N-Aryl-Dithio-
carbamates with π-Electron Acceptors,” Monatshefte für
Chemie, Vol. 129, No. 2, 1998, pp. 121-127.
http://dx.doi.org/10.1007/PL00010148
[7] A. Chandramohan, R. Bharathikannan, V. Kandhavelu, J.
Chandrasekaran and M. A. Kandhaswamy, “Synthesis,
Crystal Growth, Structural, Thermal and Optical Proper-
ties of Napthalene Picrate an Organic NLO Material,”
Spectrochimica Acta Part A, Vol. 71, No. 3, 2008, pp.
755-759. http://dx.doi.org/10.1016/j.saa.2008.01.036
[8] A. Chandramohan, R. Bharathikannan, M. A. Kandha-
samy, J. Chandrasekaran and V. Kandavelu, “Synthesis,
Crystal Growth, Spectral, Thermal and Optical Properties
of Acenaphthene Picrate,” Crystal Research Technology,
Vol. 43, No. 1, 2008, pp. 93-98.
[9] A. Chandramohan, R. Bharathikannan, J. Chandrasekaran,
P. Maadeswaran, R. Renganathan and V. Kandavelu, “Syn-
thesis, Crystal Growth and Characterization of a New
Organic NLO Material: Caffeinium Picrate (CAFP)—A
Charge Transfer Molecular Complex Salt,” Journal of
Crystal Growth, Vol. 310, No. 24, 2008, pp. 5409-5415.
http://dx.doi.org/10.1016/j.jcrysgro.2008.09.020
[10] G. A. Babu, S. Sreedhar, S. V. Rao and P. Ramasamy,
“Synthesis, Growth, Structural, Thermal, Linear and
Nonlinear Optical Properties of a New Organic Crystal:
Dimethylammonium Picrate,” Journal of Crystal Growth,
Vol. 312, No. 12-13, 2010, pp. 1957-1962.
[11] H. Takayanagi, T. Kai, S. Yamaguchi, K. Takeda and M.
Goto, “Studies on Picrate. VIII Crystal and Molecular Struc-
tures of Aromatic Amine Picrates: Aniline, N-Methyla-
niline, N, N-Dimethylaniline and o-, m- and p-Phenyle-
nediamine Picrates,” Chemical and Pharmaceutical Bul-
letin, Vol. 44, No. 12, 1996, pp. 2199-2204.
http://dx.doi.org/10.1248/cpb.44.2199
[12] P. Ramesh, R. Akalya, A. Chandramohan and M. N. Pon-
nusamy, “4-Aminopyridinium Picrate,” Acta Crystallo-
graphica Section E, Vol. 66, No. 4, 2010, Article ID:
O1000. http://dx.doi.org/10.1107/S1600536810011050
[13] K. Muthu and S. Meenakshisundaram, “Crystal Growth,
Structure and Characterization of p-Toluidinium Picrate,”
Journal of Crystal Growth, Vol. 352, No. 1, 2012, pp.
163-166. http://dx.doi.org/10.1016/j.jcrysgro.2011.11.058
[14] C. Muthamizhchelvan, K. Saminathan, J. Fraanje, R.
Peschar and K. Sivakumar, “3-Methylanilinium Picrate,”
Acta Crystallographica Section E, Vol. 61, No. 4, 2005,
pp. o1153-o1155.
http://dx.doi.org/10.1107/S1600536805009189
[15] C. Muthamizhchelvan, K. Saminathan, J. Fraanje, R. Pes-
char and K. Sivakumar, “Crystal Structure of 2-Cholroa-
nilinium Picrate,” Analytical Sciences, Vol. 21, No. 4,
2005, pp. x61-x62.
[16] G. Smith, U. D. Wermuth and P. C. Healy, “A Second
Crystal Polymorph of Anilinium Picrate,” Acta Crystal-
lographica Section E, Vol. 60, No. 10, 2004, pp. o1800-
o1803. http://dx.doi.org/10.1107/S1600536804022603
[17] V. K. Kumar and R. Nagalakshmi, “Vibrational Spectro-