 International Journal of Medical Physics, Clinical Engineering and Radiation Oncology, 2013, 2, 1-6 doi:10.4236/ijmpcero.2013.23B001 Published Online August 2013 (http://www.scirp.org/journal/ijmpcero) Candidate Molecules and ki-67/MIB1 as Novel Diagnostic Biomarker for Human Uterine Mesenchymal Tumors Takuma Hayashi1,2, Akiko Horiuchi3, Nobuo Yaegashi4, Susumu Tonegawa5, Ikuo Konishi2 1Department of Immunology and Infectious Disease, Shinshu University School of Medicine, Matsumoto, Nagano, Japan 2Department of Obstetrics and Gynecology, Kyoto University Graduate School of Medicine, Kyoto-city, Kyoto, Japan 2Promoting Business using Advanced Technology, Japan Science and Technology Agency (JST), Chiyoda-ku, Tokyo, Japan 3Horiuchi Ladies Clinic, Matsumoto, Nagano 390-0821, Japan 4Department of Obstetrics and Gynecology, Tohoku University Graduate School of Medicine, Miyagi Japan 5Picower Institution and Department of Biology, Massachusetts Institute of Technology, Cambridge, MA 02139-4307 USA Email: yoyoyo224@hotmail.com Received April, 2013 ABSTRACT Human uterine leiomyosarcoma (LMS) develops more often in the muscle tissue layer of the uterine body than in the uterine cervix. The development of gynecologic tumors is often correlated with female hormone secretion; however, the development of uterine LMS is not substantially correlated with hormonal conditions, and the risk factors are not yet known. Importantly, a diagnostic-biomarker, which distinguishes malignant uterine LMS from benign tumor leio- myoma (LMA), is yet to be established. Accordingly, it is necessary to analyze risk factors associated with uterine LMS, to establish a clinical treatment method. Protea some β-ring subunit LMP2/β1i-deficient mice spontaneously develop uterine LMS, with a disease prevalence of ~40% by 14 months of age. We found LMP2/β1i expression to be absent in human uterine LMS, but present in human LMA. Therefore, defective-LMP2/β1i expression may be one of the risk factors for human uterine LMS. LMP2/β1i is a potential diagnostic-biomarker under the combination of candidate mo- lecules, for instance cyclin B1, cyclin E and calponin h1 and ki-67/MIB1 counts for uterine mesenchymal tumors, espe- cially human uterine LMS, and may be a targeted-molecule for a new therapeutic approach. Keywords: LMP2/β1i; Uterine Leiomyosarcoma; Uterine Leiomyoma; Biomarker 1. Introduction The uterus, the organ in which the embryo grows, is composed of three layers, the uterine endometrium which serves as a bed for the embryo; the myometrium of the wall which protects the embryo; and a serous membrane enveloping the uterus. In general, the term uterine tumor refers to an epithelial malignant tumor of the uterus, which is roughly classified as a tumor of the uterine cer- vix or the uterine body. Because of the prevalence of screening, uterine cervix cancer is decreasing in inci- dence, and usually detected at a very early stage. In con- trast, cancer of the uterine body is increasing in incidence, and rarely detected at the initial stages. While most tu- mors of the uterine body are adenocarcinomas (derived from the subintimal gland), tumors of the uterine cervix are classified into squamous cancer and adenocarcinoma. Smooth muscle tumors (SMTs) which develop in the myometrium have been traditionally divided into benign uterine leiomyoma (LMA) and malignant uterine leio- myosarcoma (LMS) based on cytological atypia, mitotic activity and other criteria. Uterine LMS is relatively rare, having an estimated annual incidence of 0.64 per 100,000 women [1]. Uterine LMS accounts for 2% to 5% of tumors of the uterine body and develops more often in the muscle layer of the uterine body than in the uterine cervix. As uterine LMS is resistant to chemotherapy and radiotherapy, surgical intervention is virtually the only means of clinical treatment [2-4]. The prognosis for uterine LMS is not good, and the five-year survival rate is approximately 35% [5]. However, developing an effi- cient adjuvant therapy is expected to improve survival rate. Uterine LMA may occur in as many as 70% ~ 80% of women by the age of 50 years [6]. Distinguishing uterine LMA from uterine LMS is very difficult, and a diagnosis generally requires surgery and cytoscopy [7]. Copyright © 2013 SciRes. IJMPCERO  T. HAYASHI ET AL. 2 Diagnostic categories for uterine SMTs and morphologi- cal criteria are used to assign cases [8,9] (Attention 1). The non-standard subtypes of uterine SMTs such as the epithelioid and myxoid types are classified in a different way using these features, so the establishment of a dia- gnostic method for the identification of non-standard smooth muscle differentiation is important [8,9]. High estrogen levels are considered to significantly in- fluence the development of tumors in the uterine body [10-12]. The mechanisms by which uterine LMA and uterine LMS develop are not yet known, though tumors that have developed in the myometrium for some reason gradually become larger due to the influence of the fe- male hormone and generate tumors. However, no corre- lation between the development of uterine LMS and hormonal conditions, and no obvious risk factors has been found. Although cases accompanied by hypocal- caemia or eosinophilia have been reported, neither cli- nical abnormality is an initial risk factor for uterine LMS. The identification of a risk factor associated with the development of uterine LMS would significantly contri- bute to the development of preventive and therapeutic treatments. 2. Spontaneous Development of Uterine Leiomyosarcoma in LMP2/β1i-Deficient Mice Cytoplasmic proteins are mostly degraded by a protease complex, which has many substrates consisting of twen- ty-eight 20 to 30-kDa subunits, referred to as the 20S proteasome [13,14]. The proteasomal degradation is essential for many cellular processes, including the cell cycle, the gene expression and immunological function [15]. Interferon (IFN)- induces the expression of large numbers of responsive genes, proteasome subunits, i.e., low-molecular mass polypeptide (LMP)2/β1i, LMP7/β5i, and LMP10/β2i [16] (Figure 1). The individual expression of LMP2/β1i, LMP7/β5i and LMP10/β2i subunits in various cell types or tissues is believed to contribute to the initiation and development of disorders. A recent study revealed a unique role for LMP7/β5i in controlling pathogenic immune responses and provided a therapeutic rationale for targeting LMP7/β5i in autoimmune disorders, especially rheuma- toid arthritis [17]. Homozygous mice deficient in LMP2/β1i show tissue- and substrate-dependent abnormalities in the biological functions of the proteasome [18]. Here we identify LMP2/β1i, as obligatory for tumor surveillance and demonstrate a tissue-specific role for LMP2/β1i in pro- tection from spontaneous uterus neoplasms. In short, uterine LMS reportedly occurred in female LMP2/β1i- deficient mice at age 6 months or older, and the inci- dence at 14 months of age was about 40% [19,20] (Fig- Figure 1. The immuno-proteasomal degradation pathway is essential for antigen presentation by MHC class I. ure 2). The curve indicating the incidence in mice is similar to that indicating the incidence of human uterine LMS, which occurs after menopause. Histological stud- ies of LMP2/β1i-lacking uterine tumors have revealed characteristic abnormalities of uterine LMS, and the tu- mors lacked lymphoid infiltrates, a sign of immune rec- ognition, and consisted of uniform elongated myo- me- trium cells arranged into bundles [19] (Figure 2). The nuclei of the tumor cells varied in size and shape, furthermore, mitosis was frequent. In contrast, the myo- metrium cells of its parental mice, C57BL/6 mice were normal in appearance. Whereas relatively few ki-67/ MIB1-positive cells, the proliferating cells of solid tu- mors, were observed in the basal cell layer of the normal myometrium, most of the basal cells vividly expressed ki-67/MIB1 in LMP2/β1i-deficient mice [19] (Figure 2). Immunohistochemistry (IHC) study indicates abnormal proliferation of the LMP2/β1i-lacking cells in the basal layer. LMP2/β1i-deficient mice that have developed ute- rine LMS undergo considerable weight loss, and then die by 14 months of age [19,20]. The LMP2/β1i-deficient mice also exhibit skeletal muscle metastasis from uterine LMS. Therefore it is likely that LMP2/β1i-deficient mice with uterine LMS die as a result of the tumor mass and metastasis [19,20]. In general, it is not easy to distinguish uterine LMA from uterine LMS, however, in mice, be- cause of such characteristic pathological findings, sig- nificant weight loss, and skeletal muscle metastasis, a tumor that develops in the uterus of an LMP2/β1i-defi- cient mouse can be considered malignant, i.e., uterine LMS [19,20]. 3. Defective LMP2/β1i Expression in Human Leiomyosarcoma IHC studies were performed to demonstrate the validity and reliability of LMP2/β1i as a diagnostic biomarker Copyright © 2013 SciRes. IJMPCERO 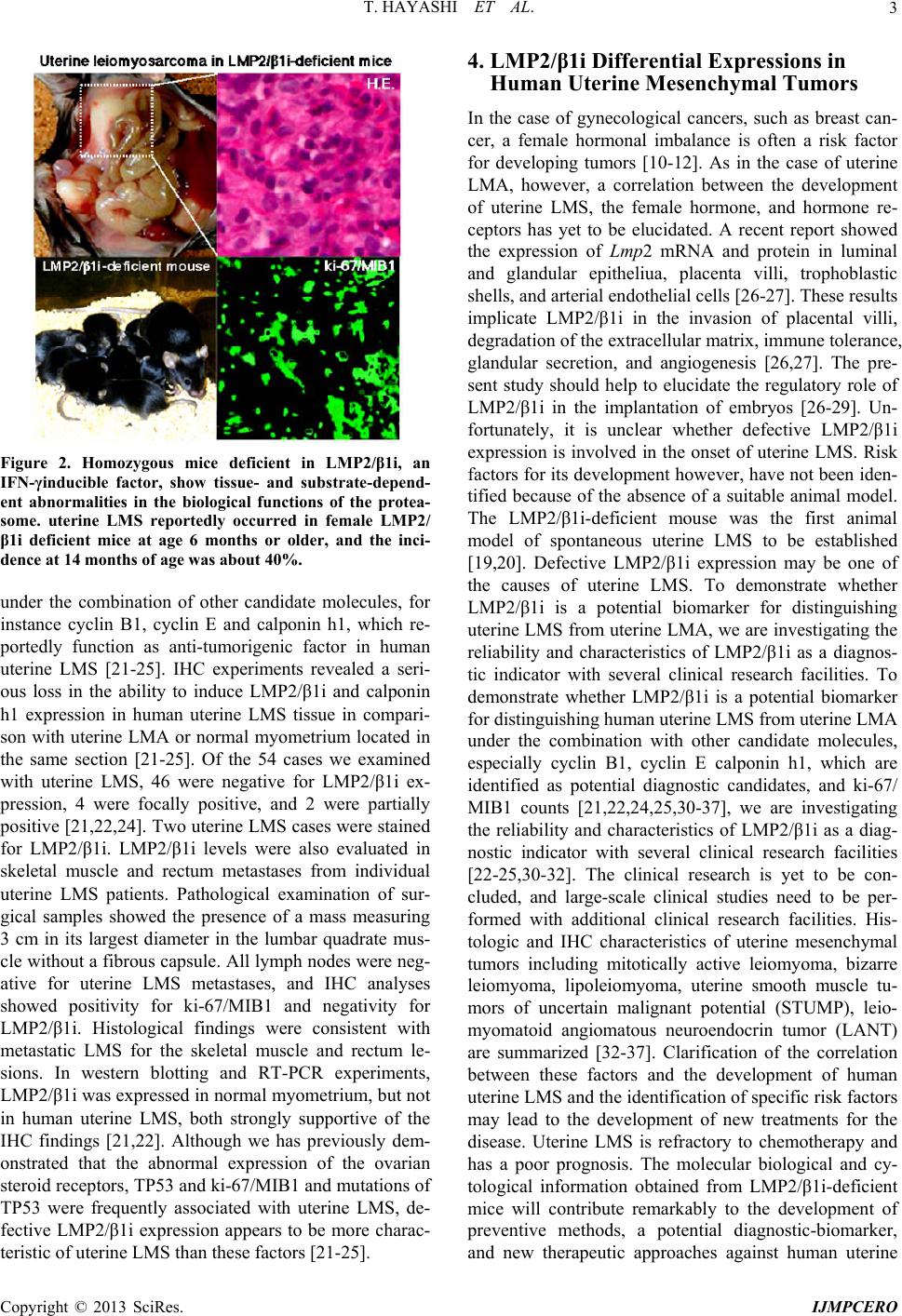 T. HAYASHI ET AL. 3 Figure 2. Homozygous mice deficient in LMP2/β1ian nder the combination of other candidate molecules, for Human Uterine Mesenchymal Tumors an- cector , IFN-γinducible factor, show tissue- and substrate-depend- ent abnormalities in the biological functions of the protea- some. uterine LMS reportedly occurred in female LMP2/ β1i deficient mice at age 6 months or older, and the inci- dence at 14 months of age was about 40%. u instance cyclin B1, cyclin E and calponin h1, which re- portedly function as anti-tumorigenic factor in human uterine LMS [21-25]. IHC experiments revealed a seri- ous loss in the ability to induce LMP2/β1i and calponin h1 expression in human uterine LMS tissue in compari- son with uterine LMA or normal myometrium located in the same section [21-25]. Of the 54 cases we examined with uterine LMS, 46 were negative for LMP2/β1i ex- pression, 4 were focally positive, and 2 were partially positive [21,22,24]. Two uterine LMS cases were stained for LMP2/β1i. LMP2/β1i levels were also evaluated in skeletal muscle and rectum metastases from individual uterine LMS patients. Pathological examination of sur- gical samples showed the presence of a mass measuring 3 cm in its largest diameter in the lumbar quadrate mus- cle without a fibrous capsule. All lymph nodes were neg- ative for uterine LMS metastases, and IHC analyses showed positivity for ki-67/MIB1 and negativity for LMP2/β1i. Histological findings were consistent with metastatic LMS for the skeletal muscle and rectum le- sions. In western blotting and RT-PCR experiments, LMP2/β1i was expressed in normal myometrium, but not in human uterine LMS, both strongly supportive of the IHC findings [21,22]. Although we has previously dem- onstrated that the abnormal expression of the ovarian steroid receptors, TP53 and ki-67/MIB1 and mutations of TP53 were frequently associated with uterine LMS, de- fective LMP2/β1i expression appears to be more charac- teristic of uterine LMS than these factors [21-25]. 4. LMP2/β1i Differential Expressions in In the case of gynecological cancers, such as breast c r, a female hormonal imbalance is often a risk fa for developing tumors [10-12]. As in the case of uterine LMA, however, a correlation between the development of uterine LMS, the female hormone, and hormone re- ceptors has yet to be elucidated. A recent report showed the expression of Lmp2 mRNA and protein in luminal and glandular epitheliua, placenta villi, trophoblastic shells, and arterial endothelial cells [26-27]. These results implicate LMP2/β1i in the invasion of placental villi, degradation of the extracellular matrix, immune tolerance, glandular secretion, and angiogenesis [26,27]. The pre- sent study should help to elucidate the regulatory role of LMP2/β1i in the implantation of embryos [26-29]. Un- fortunately, it is unclear whether defective LMP2/β1i expression is involved in the onset of uterine LMS. Risk factors for its development however, have not been iden- tified because of the absence of a suitable animal model. The LMP2/β1i-deficient mouse was the first animal model of spontaneous uterine LMS to be established [19,20]. Defective LMP2/β1i expression may be one of the causes of uterine LMS. To demonstrate whether LMP2/β1i is a potential biomarker for distinguishing uterine LMS from uterine LMA, we are investigating the reliability and characteristics of LMP2/β1i as a diagnos- tic indicator with several clinical research facilities. To demonstrate whether LMP2/β1i is a potential biomarker for distinguishing human uterine LMS from uterine LMA under the combination with other candidate molecules, especially cyclin B1, cyclin E calponin h1, which are identified as potential diagnostic candidates, and ki-67/ MIB1 counts [21,22,24,25,30-37], we are investigating the reliability and characteristics of LMP2/β1i as a diag- nostic indicator with several clinical research facilities [22-25,30-32]. The clinical research is yet to be con- cluded, and large-scale clinical studies need to be per- formed with additional clinical research facilities. His- tologic and IHC characteristics of uterine mesenchymal tumors including mitotically active leiomyoma, bizarre leiomyoma, lipoleiomyoma, uterine smooth muscle tu- mors of uncertain malignant potential (STUMP), leio- myomatoid angiomatous neuroendocrin tumor (LANT) are summarized [32-37]. Clarification of the correlation between these factors and the development of human uterine LMS and the identification of specific risk factors may lead to the development of new treatments for the disease. Uterine LMS is refractory to chemotherapy and has a poor prognosis. The molecular biological and cy- tological information obtained from LMP2/β1i-deficient mice will contribute remarkably to the development of preventive methods, a potential diagnostic-biomarker, and new therapeutic approaches against human uterine Copyright © 2013 SciRes. IJMPCERO  T. HAYASHI ET AL. 4 LMS. 5. Final Considerations tory to chemotherapy and e LMP2/β1i expression is nowledgements r Luc Van Kaer (Vanderbilt This study was suppor REFERENCES [1] C. Zaloudek n, “Mesenchymal tumors of the Blaus tors and Im- Human uterine LMS is refrac has a poor prognosis. Defectiv likely to be one of the risk factors in the development of human uterine LMS as it is in the LMP2/β1i-deficient mouse. LMP2/β1i might function as an anti-tumorgenic factor in human uterine LMS. The molecular biological and cytological information obtained from LMP2/β1i- deficient mice will contribute remarkably to the devel- opment of preventive methods, a potential diagnostic biomarker, and new therapeutic approaches against hu- man mesenchymal tumors, especially human uterine LMS. 6. Ack We sincerely thank Professo University Medical Center). ted in part by grants from the Ministry of Education, Culture, Science and Technology, and The Foundation of Osaka Cancer Research, The Ichiro Kanehara Foundation for the Promotion of Medical Science and Medical Care, The foundation for the Promotion of Cancer Research, The Kanzawa Medical Research Foundation, The Shinshu Medical Foundation, and The Takeda Foundation for Medical Science. and M. R. Hendrickso uterus, in R. J. Kurman (ed):tein’s Pathology of the Female Genital Tract (ed 5),” New York, Springer-Verlag, Vol. 5, 2002, pp. 561-578. [2] T. I. Wu, T. C. Chang, S. Hsueh, K. H. Hsu, H. H. Chou, H. J. Huang and C. H. Lai, “Prognostic Fac pact of Adjuvant Chemotherapy for Uterine Leiomyosar- coma,” Gynecologic Oncology, Vol. 100, No. 1, 2006, pp.166-172.doi: /10.1016/j.ygyno.2005.08.010 [3] M. M. Leitao, R. A. Soslow, D. Nonaka, A. B. Olshen, C. Aghajanian, P. Sabbatini, J. Dupont, M. Hensley, Y. So- noda, R. R. Barakat and S. Anderson, “Tissue Microarray Immunohisto Chemical Expression of Estrogen, Proges- terone, and Androgen Receptors in Uterine Leiomyomata and Leiomyosarcoma,” Cancer ,Vol. 101, No. 6, 2004, pp. 1455-1462. doi:10.1002/cncr.20521 [4] E. A. Perez, L Patient Care through Mo . Pusztai and M. Van de Vijver, “Improving lecular Diagnostics,” Seminars in Oncology, Vol. 31, No. 10, 2004, pp. 14-20. doi:10.1053/j.seminoncol.2004.07.017 [5] http://www.cancer.gov/cancertopics/pdq/treatment/uterine sarcoma/HealthProfessional/page1#Section_87 inn, of the Uterus Other than Or- [6] http://cancer.gov/cancertopics/pdq/treatment/uterine sarcoma/HealthProfessional [7] H. L. Evans, S. P. Chawla, C. Simpson and K. P. F “Smooth Muscle Neoplasms dinary Leiomyoma,” A Study of 46 Cases, with Emphasis on Diagnostic Criteria and Prognostic Factors, Cancer, Vol. 62, No. 10, 1988, pp. 2239-2247. doi:10.1002/1097-0142(19881115)62:10<2239::AID-CN CR2820621028>3.0.CO;2-Y [8] R. J. Kurma, “Pathology of the Female Genital Tract,” 4th edition, Springer-Verlag; New York, Vol. 4, 2001, p. lines: World Health Organization Classification of Vol. 10, No. 6, 2008, pp. 499. [9] Diagnostic Criteria for LMS, Adapted from 2003 WHO Guide Tumours: Pathology and Genetics, Pathology and Genet- ics of Tumours of the Breast and Female Genital Organs, IARC Press, France, 2003. [10] J. F. Lin and B. M. Slomovitz, “Uterine Sarcoma,” Current Oncology Reports, 512-518. doi:10.1007/s11912-008-0077-9 [11] F. Amant, A. Coosemans, M. Debiec-Rychter, D. Timmerman and I. Vergote, “Clinical Management of Uterine Sarcomas,” The Lancet Oncology, Vol. 10, No. 12, 2009, pp. 1188-1198. doi:10.1016 /S1470- 2045( 09)70 226-8 [12] M. Miettinen an Potential of Smooth Muscle Tumours,” Histopathology, d J. F. Fetsch, “Evaluation of Biological Vol. 48, No. 1, 2006, pp. 97-105. doi:10.1111 /j.1365- 2559.200 5.02292 .x [13] J. M. Peters, W. W. Franke and J. tinct 19 S and 20 S Subcomplexes of the 26 A. Kleinschmidt, Dis- S Proteasome cott, S. L. Zipursky and J. Darnell, 2004 ellular Regulation,” In- and Their Distribution in the Nucleus and the Cyto- plasm,” Journal of Biological Chemistry, Vol. 269, 1994, pp.7709-7718. [14] H. Lodish, A. Berk, P. Matsudaira, C. A. Kaiser, M. Krieger, M. P. S "3", Mol Cell Biol (5th ed.), New York: W. H. Freeman and CO. Vol. 5, 2004, pp. 66-72. [15] I. M. Konstantinova, A. S. Tsimokha and A. G. Mitten- berg, “Role of Proteasomes in C ternational Review Cell and Molecular Biology, Vol. 267, 2008, pp. 59-124. doi:10.1016 /S1937- 6448( 08)00 602-3 [16] J. Wang and M. A. me System and Its Role in Infla Maldonado, “TheUbiquitin-Proteaso mmatory and , E. R. Ring, J. Shields, J. Ji- Autoimmune Diseases,” Cell and Molecular Immunology, Vol. 3, 2006, pp. 255–261. [17] T. Muchamuel, M. Basler, M. A. Aujay, E. Suzuki, K. W. Kalim, C. Lauer, C. Sylvain ang, P. Shwonek, F. Parlati, S. D. Demo, M. K. Bennett, C. J. Kirk and M. Groettrup, “A Selective Inhibitor of the Immunoproteasome Subunit LMP7 Blocks Cytokine Production and Attenuates Progression of Experimental Arthritis,” Nature Medicine, Vol. 15, 2009, pp.781-788. doi:10.1038/nm.1978 [18] L. Van Kaer, P. G. Ashton-Rickardt, M. Eichelberger, M. Gaczynska, K. Nagashima, K. L. Rock, A. L. Goldberg, P. C. Doherty and S. Tonegawa, “Altered Peptidase and Viral-specifi c T Cell Response in LMP2 Mutant Mice,” Immunity, Vol. 1, 1994, pp. 533-541. Copyright © 2013 SciRes. IJMPCERO  T. HAYASHI ET AL. Copyright © 2013 SciRes. IJMPCERO 5 lecular Mass Poly- oriuchi, K. Sano, N. Hiraoka, Y. Kanai, olecular [19] T. Hayashi and D. L. Faustman, “Development of Spon- taneous Uterine Tumors in Low Mo peptide-2 Knockout Mice,” Cancer Research, Vol. 62, 2002, pp. 24-27. http://cancerres.aacrjournals.org/content/62/1/24 [20] T. Hayashi, A. H T. Shiozawa, S. Tonegawa and I. Konishi, “M Approach on Uterine Leiomyosarcoma: LMP2-deficient Mice as an Animal Model of Spontaneous Uterine Leio- myosarcoma,” Sarcoma 2011: 476498, Epub 2011 Mar 8, http://www.hindawi.com/journals/srcm/2011/476498/ [21] T. Hayashi, Y. Kobayashi, S. Kohsaka and K. Sano, “Mutation in the ATP-binding Region of JAK1, Identi- fied in Human Uterine Leiomyosarcomas, Results in De- fective Interferon-gamma Inducibility of TAP1 and LMP2,” Oncogene, Vol. 25, 2006, pp. 4016-4026. doi:10.1038 /sj.o nc.120943 4 [22] T. Hayashi, A. Horiuchi, K. Sano, N. Hiraoka, M. K T. Ichimura, S. Nagase, O. Ishik asai, o, Y. Kanai, N. Yaegashi, H. Aburatani, T. Shiozawa, S. Tonegawa and I. Konishi, “Potential Role of LMP2 as Tumor-suppressor Defines New Targets for Uterine Leiomyosarcoma Therapy,” Scientific Re p or ts, Vol. 1, 2011, p. 180. doi:10.1038 /srep001 80 [23] Y. L. Zhai, Y. Kobayashi, A. Mori, A. O Konishi and S. Fujii, “Ex rii, T. Nikaido, I pression of Steroid Receptors, . Ki-67, and p53 in Uterine Leiomyosarcomas,” Interna- tional Journal Gynecological Pathology, Vol. 18, No. 1,2004, pp. 20-28. doi:10.1097 /000043 47-199 901000 -00 004 [24] T. Hayashi, A. Horiu T. Ichimura, S. Nagase, O. Ishiko, Y. Kanai, N. chi, K. Sano, N. Hiraoka, M. Kasai Yaegashi, , H. Aburatani, T. Shiozawa, S. Tonegawa and I. Konishi, “Potential role of LMP2 as an Anti-oncogenic Factor in Human Uterine Leiomyosarcoma: Morphological Sig- nificance of Calponin h1,” FEBS Letter, Vol. 586, 2012, pp. 1824-1831.doi:10.1016/j.febslet.2012.05 .029 [25] T. Hayashi, A. Horiuchi, K. Sano, N. Hiraoka, T. Ichi- mura, T. Sudo, O. Ishiko, N. Yaegashi, H. Aburatani and ssion of Proteasome Subunits Low Molecular I. Konishi, “Potential Diagnostic Biomarkers for Human Uterine Mesenchymal Tumors: Especially LMP2/β1i and Cyclin B1-differential Expression,” Oncology Letters, 2013. [26] H. X. Wang, H. M. Wang, Q. L. Li and P. L. Judoson, “Expre Mass Polypeptide (LMP) 2 and LMP7 in the Endo- metrium and Placenta of Rhesus Monkey (Macaca mu- latta) during early Pregnancy,” Biology of Reproduction, Vol. 71, No. 4, 2004, pp. 1317-1324. doi:10.1095 /bio lrep rod.104 .03021 3 [27] H. X. Wang, H. M. Wang, H. Y. Lin, B. K.Tsang and C. Zhu, “Proteasome S Q. Yang, H. Zhang ubunit LMP2 is , Required for Matrix Metalloproteinase-2 and -9 expres- sion and Activities in Human Invasive Extravillous Tro- phoblast Cell Line,” Journal of Cellular Physiology, Vol. 206, No. 3, pp. 616-623. doi:10.1002/jcp .2 0508 [28] J. J. Fu, P. Lin, X. Y. Lv, X. J. Yan, H. X. Wang, C. Zhu, B. K. Tsang, X. G. Yu and H. Wang, “Low Molecular Mass Polypeptide-2 in Human Trophoblast: Over-expression in Hydatidiform Moles and Possible Role in Trophoblast Cell Invasion,” Placenta, Vol. 30, No. 4, 2009, pp. 305-312. doi:10.1016/j.placenta.2009.01.005 [29] T. Hayashi, A. Horiuchi, H. Aburatani, N. Y Tonegawa and I. Konishi, “A Potential aegashi, S. Diagnostic Bio- ed Expression of or in Hu- marker: Proteasome LMP2/β1i-differential Expression in Human Uterus Neoplasm,” Nature Proceedings 2012, March 2: hdl:10101/npre.2012.7082.1. [30] A. Horiuchi, T. Nikaido, K. Ito, Y. Zhai, A. Orii, S. Ta- niguchi, T. Toki and S. Fujii, “Reduc Calponin h1 in Leiomyosarcoma of the Uterus,” Lab In- vestment, Vol. 78, 1998, pp. 839-846. http://www.ncbi.nlm.nih.gov/pubmed/9690561 [31] A. Horiuchi, T. Nikaido, S. Taniguchi and S. Fujii, “Pos- sible Role of Calponin h1 as a Tumor Suppress man Uterine Leiomyosarcoma,” Journal of the National Cancer Institute, Vol. 91, No. 9, 1999, pp.790-796. doi:10.1093/jnci/91.9.790 [32] Y. L. Zhai, T. Nikaido, T. Shiozawa, A. Orii and S. Fujii, “Expression of Cyclins and Cyclin-dependent Kinases in l Smooth Muscle Tumors of the Uterus,” International Journal of Cancer, Vol. 84, 1999, pp. 224-250. [33] P. P. Ip, A. N. Cheung and P. B. Clement, “Uterine Smooth Muscle Tumors of Uncertain Malignant Potentia (STUMP): A Clinicopathologic Analysis of 16 Cases,” The American Journal of Surgical Pathology, Vol. 33, 2009, pp. 992-1005. doi:10.1097 /PAS .0b013 e3181a02d 1c [34] iomyomas and P. P. Ip, K. Y. Tse and K. F. Tam, “Uterine Smooth Mus- cle Tumors Other than the Ordinary Le LeioMyosarcomas: A Review of Selected Variants with Emphasis on Recent Advances and Unusual Morphology that may Cause Concern for Malignancy,” Advances in Anatomic Pathology, Vol. 17, 2010, pp. 91-112. doi:10.1097 /PAP .0b013 e3181cfb9 01 [35] hrist and R. W. Neuroendocrine I. Vajtai, R. Sahli, A. Kappeler, E. R. C Seiler, “Leiomyomatoid Angiomatous Tumor (LANT) of the Pituitary: A Distinctive Biphasic Neoplasm with Primitive Secretory Phenotype and Smooth Muscle-rich Stroma,” Acta Neuropathologica, Vol. 111, No. 4, 2006, pp. 397-399. doi:10.1007 /s00401 -00 6-0065 -9 [36] N. Sakashita, M. Yamada, T. Nakag and M. Takeya, “A Leiomyomatoid awa, H. Yamasaki Angiomatous Neu- roendocrine tumor of the Myometrium: Case Study with Ultrastructural Analysis,” Human Pathology, Vol. 39, No. 5, 2008, pp. 788-792. doi:10.1016 /j.humpath .2007 .09 .017 [37] R. Avritscher, R. B. Iyer1, J. Ro and G. leioMyoma of the Uterus,” American Jo Whitman, “Lipo- urnal of Radiol- ogy, Vol. 177, 2001, p. 856.  T. HAYASHI ET AL. 6 Attention 1 The typical gross appearance is a large (>10 cm), poorly circumscribed mass with a soft, fleshy consistency and a variegated cut surface that is grey-yellow to pink, with foci of hemorrhage and necrosis [8,9]. The histologic classification of uterine sarcomas is based upon homol- ogy to normal cell types and include uterine LMS (analo- gous to myometrium), stromal sarcoma (analogous to endometrial stroma), and other heterologous cell types (i.e., osteosarcoma, liposarcoma). Microscopically, most human uterine LMS are overtly malignant, with hyper- cellularity, coagulative tumor cell necrosis, abundant mitoses [>10 to 20 mitotic figures (mf) per 10 high power fields (hpf)], atypical mitoses, cytologic atypia, and infiltrative borders. Mitotic rate is the most important determinant of malignancy, but is modified by the pres- ence of necrosis and cytologic atypia. The diagnosis of human uterine LMS may be made in the presence of tu- mor necrosis and any mitoses. In the absence of tumor necrosis, the diagnosis can be made with moderate to severe cytologic atypia and a mitotic index greater than 10mf/10hpf. Without tumor necrosis and significant atypia, a high mitotic index is compatible with a benign clinical course; however, data is limited [8,9]. Copyright © 2013 SciRes. IJMPCERO
|