 Open Journal of Applied Sciences, 2013, 3, 381-392 http://dx.doi.org/10.4236/ojapps.2013.36048 Published Online October 2013 (http://www.scirp.org/journal/ojapps) The Effect of Hygroscopic Growth on Continental Aerosols Bello Idrith Tijjani1, Aliyu Aliyu2, Fatima Shuaibu3 1Department of Physics, Bayero University, Kano, Nigeria 2Department of Science Laboratory Technology, School of Technology, Kano State Polytechnic, Kano, Nigeria 3Girl’s Science and Technical College, Kano, Nigeria Email: idrith@yahoo.com, idrithtijjani@gmail.com Received July 11, 2013; revised August 30, 2013; accepted September 12, 2013 Copyright © 2013 Bello Idrith Tijjani et al. This is an open access article distributed under the Creative Commons Attribution Li- cense, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT In this paper, the authors investigated some microphysical and optical properties of continental clean aerosols from OPAC to determine the effect of hygroscopic growth at the spectral range of 0.25 μm to 2.5 μm and eight relative hu- midities (RHs) (0%, 50%, 70%, 80%, 90%, 95%, 98% and 99%). The microphysical properties extracted were radii, volume mix ratio, number mix ratio and mass mix ratio as a function of RH while the optical properties are scattering and absorption coefficients and asymmetric parameters. Using the microphysical properties, growth factors of the mix- tures were determined while using optical properties the enhancement parameters were determined and then parameter- ized using some models. We observed that the data fitted the models very well. The angstrom coefficients show that the mixture has bimodal type of distribution with the dominance of fine mode particles. Keywords: Microphysical Properties; Optical Properties; Hygroscopic Growth; Parametrization; Enhancement Parameters; Angstrom Coefficients 1. Introduction Aerosol in the atmosphere is comprised of numerous and diverse components originating from both natural and an- thropogenic activities. An important factor affecting the role aerosols play in climate change is their hygroscopicity and is currently modeled in global climate models (GCMs), mostly to better predict the scattering properties and size distribu- tion under varying humidity conditions [1]. The swelling of aerosols due to water vapor uptake will enhance their ability to scatter radiation. Numerous studies have inves- tigated the relationship between aerosol scattering and relative humidity RH in terms of the hygroscopic growth factor gf(RH) using humidified nephelometers. These have been used for airborne or ground-based determina- tion of the growth factor considering a “dry” RH over the range from 20% - 40% and a ‘‘wet’’ RH up to 90% [2-5]. The characterization of particle hygroscopicity has primary importance for climate monitoring and predic- tion. Model studies have demonstrated that relative hu- midity (RH) has a critical influence on aerosol climate forcing. Hygroscopic properties of aerosols influence particle size distribution and refractive index and hence their radiative effects. Aerosol particles tend to grow at large relative humidity values as a result of their hygro- scopicity. Some aerosol particles, such as ammonium sulphate (NH4)2SO4, sea salt and ammonium nitrate NH4NO3 are hygroscopic. Changes in relative humidity modify their size distribution and refractive index and hence the opti- cal properties of the aerosol, including the scattering co- efficient [6-9]. Jeong et al. [10] demonstrated an expo- nential dependence of the aerosol optical thickness on relative humidity. A strong correlation of spectral aerosol optical thickness with precipitable water, especially for continental air masses, was shown by Rapti [11]. Water-soluble organic carbon (WSOC) species are emitted as primary particles, especially during biomass combustion, and produced as a result of reactions in the gas and aqueous phases [12-18]. Moreover, WSOC has been suggested as a marker for secondary organic aerosol (SOA) in the absence of biomass burning (e.g., Docherty et al. [19]). In a study of aged continental aerosols, Swietlicki et al. [7] observed 2 modes, a less hygroscopic mode with a gf(RH) of 1.12 and a more hygroscopic mode with a gf(RH) between 1.44 and 1.65. They postulated that the hygroscopic growth could be attributed entirely to the inorganic content of the aerosol: sulfate, nitrate and am- monium ions. Particle hygroscopicity may vary as a fun- C opyright © 2013 SciRes. OJAppS  B. I. TIJJANI ET AL. 382 ction of time, place and particle size [20-22]. The size and the solubility of a particle determine the response of an ambient particle to changes in RH. The water vapor pressure above a water droplet containing dissolved ma- terial is lowered by the Raoult effect. The equilibrium size of a droplet was first described by Kohler [23], who considered the Kelvin (curvature) and Raoult (solute) effect. Using optical properties, several previous studies (e.g. Sheridan et al. [24]) have measured and modeled enhancement factors for continental aerosols. The aim of this study is to determine the aerosols’ hy- groscopic growth and enhancement factors for continent- tal clean aerosols from the data extracted from OPAC. One variable and two variables parameterizations models will be performed to determine the relationship of the particles’ hygroscopic growth and enhancement parame- ters with the RH. Angstrom coefficients are used to de- termine the particles’ type and the type mode size distri- butions. 2. Methodology The models extracted from OPAC are given in Table 1. The main parameter used to characterize the hygro- scopicity of the aerosol particles is the aerosol hygro- scopic growth factor gf(RH), which indicates the relative increase in mobility diameter of particles due to water absorption at a certain RH and is defined as the ratio of the particle diameter at any RH to the particle diameter at RH = 0 and RH is taken for seven values 50%, 70%, 80%, 90%, 95%, 98% and 99% [22,26]: RH RH RH 0 D gf D (1) The gf(RH) can be subdivided into different classes with respect hygroscopicity. One classification is based on diameter growth factor by Liu et al. [27] and Swiet- licki et al., [22] as barely hygroscopic (gf(RH) = 1.0 – 1.11), less Hygroscopic (gf(RH) = 1.11 − 1.33), more Hygroscopic (gf(RH) = 1.33 − 1.85) and most hygro- scopic growth (gf(RH) > 1.85). Atmospheric particles of a defined dry size typically exhibit different growth factors. This is due to either ex- ternal mixing of particles in an air sample or variable relative fractions of different compounds in individual Table 1. Compositions of aerosol type [25]. Aerosol model types Components Concentration Ni (cm−3) Continental clean WASO INSO Total 2600.0 0.15 26000.15 Note: Ni is the mass concentration of the component, water soluble compo- nents (WASO, consists of scattering aerosols, that are hygroscopic in nature, such as sulfates and nitrates present in anthropogenic pollution) and water insoluble (INSO). particles (the latter here in after referred to as quasi-in- ternally mixed). A mono-modal growth distribution with- out spread can only be expected in very clean and ho- mogeneous air parcels. For further details on mixing states see e.g. Buzorius et al. [28]. Most atmospheric aerosols are externally mixed with respect to hygroscopicity, and consist of more and less hygroscopic sub-fractions [22]. The ratio between these fractions as well as their content of soluble material de- termine the hygroscopic growth of the overall aerosol. Prediction of hygroscopic growth factors with Kohler theory requires detailed knowledge of particle composi- tion as well as a thermodynamic model, which describes the concentration dependence of the water activity for such a mixture. The hygroscopic growth factor of a mix- ture, gfmix(RH), can be estimated from the growth factors of the individual components of the aerosol and their respective volume fractions, Vk, using the Zdanovskii- Stokes-Robinson relation and other researchers [29-32]: 13 3 RH mixk k k gfV gf (2) where the summation is performed over all compounds present in the particles. Solute-solute interactions are neglected in this model and volume additivity is also assumed. The model assumes spherical particles, ideal mixing (i.e. no volume change upon mixing) and inde- pendent water uptake of the organic and inorganic com- ponents. It can also be computed using the corresponding num- ber fractions nk as [33,34]; 13 3 RH mixk k k gfn gf (3) where nk is the number fraction of particles having the growth factor gfk . We now proposed the gfmix(RH) to be a function of mass mix ratio as 13 3 RH mixk k k gfm gf (4) where mk represents the mass mix ratio of particles hav- ing the growth factor gfk. The RH dependence of gfmix(RH) were parameterized in a good approximation by a one-parameter equation, proposed e.g. by Petters and Kreidenweis [35]: 1 3 11 w mix w w a gf aa (5) Here, aw is the water activity, which can be replaced by the relative humidity RH, if the Kelvin effect is negli- gible, as for particles with sizes more relevant for light scattering and absorption. At equilibrium, it can be shown that, over a flat surface, the water activity equals the ambient relative humidity in the sub-saturated humid Copyright © 2013 SciRes. OJAppS  B. I. TIJJANI ET AL. 383 environment [36,37]. The coefficient κ is a simple meas- ure of the particle’s hygroscopicity and captures all sol- ute properties (Raoult effect). Humidograms of the ambient aerosols obtained in various atmospheric conditions showed that gfmix(RH) could as well be fitted well with a γ-law [38-42] as RH RH1 100 mix gf 3 1ln mix w Bgfa (6) Particle hygroscopicity is a measure that scales the volume of water associated with a unit volume of dry particle [35] and depends on the molar volume and the activity coefficients of the dissolved compounds [43]. The bulk hygroscopicity factor B under subsaturation RH conditions was determined using the relation: (7) where aw is the water activity, which can be replaced by the RH as explained earlier. The impact of hygroscopic growth on the aerosol op- tical properties is usually described by the enhancement factor fχ(RH,λ): RH, RH, RH 0, f (8) where χ(RH,λ) can be denoting the aerosol scattering and absorption coefficients, and asymmetry parameters. RH corresponds to any condition, and can cover the entire RH spectrum. In this paper we will only use scattering, absorption and asymmetric parameter. The reason for using asymmetric parameter is to determine the effect of hygroscopic growth on forward scattering. This method was initially introduced by Covert et al. [2]. In general the relationship between RH,f and RH is nonlinear (e.g. Jeong et al. [10]). In this paper we determine the empirical relations between the enhance- ment parameter and RH [44] as: 100 RH RH, RH, ref fRH 0,100 RHhigh (9) where in our study RHref is 0%. The known as the hu- midification factor represents the dependence of aerosol optical properties on RH, which results from changes in the particle size and refractive index upon humidification. The parameter in our case was obtained by combining the eight RH, parameters at 0%, 50%, 70%, 80%, 90%, 95%, 98% and 99% RH. The use of has the ad- vantage of describing the hygroscopic behavior of aero- sols in a nonlinear manner over a broad range of RH values; it also implies that particles are deliquesced [45], a reasonable assumption for this data set due to the high ambient relative humidity during the field study. The parameter is dimensionless, and it increases with in- creasing particle water uptake. From previous studies, typical values of γ for ambient aerosol ranged between 0.1 and 1.5 [45-47]. Two parameters empirical relation is also used [10,48] as: RH % 1100 b fa RH, (10) The model assumes equilibrium (metastable) growth of the aerosol scattering with RH such that the humidi- graph profile does not display a deliquescent growth pro- file. For aerosol in a humid environment, this behavior will hold true. Most aerosols are a mixture of metastable and deliquescent particles and will exhibit some deli- quescent behavior. To verify the non-linearity of the re- lation between RH,f and RH, the Equations (9) and (10) were modeled at = 0.25, 1.25 and 2.50 µm. The Angstrom exponent being an indicator of the aerosol spectral behaviour of aerosols [49], the spectral behavior of the aerosol optical parameter (X, say), with the wavelength of light (λ) is expressed as inverse power law [50]: X (11) where X(λ) can represent scattering and absorption coef- ficients. The variable X(λ) can be characterized by the Angstron parameter, which is a coefficient of the fol- lowing regression, lnXln ln (12) however the Angstrom exponent itself varies with wave- length, and a more precise empirical relationship be- tween aerosol extinction and wavelength is obtained with a 2nd-order polynomial [51-61], as: 2 21 lnlnln lnX (13) and then we proposed the cubic 23 12 3 lnln lnlnlnX (14) where X can be any of the optical parameter, β, α, α1, α2, α3 are constants that are determined using regres- sion analysis with SPSS16.0. We also determine the effect of hygroscopic growth on the effective refractive indices of the two mixed aerosols using the following formula [62]: 2 00 1 00 22 eff i i i eff i f (15) where fi and εi are the volume fraction and dielectric con- stant of the ith component and ε0 is the dielectric constant of the host material. For the case of Lorentz-Lorentz [63,64], the host material is taken to be vacuum, ε0 = 1. Copyright © 2013 SciRes. OJAppS 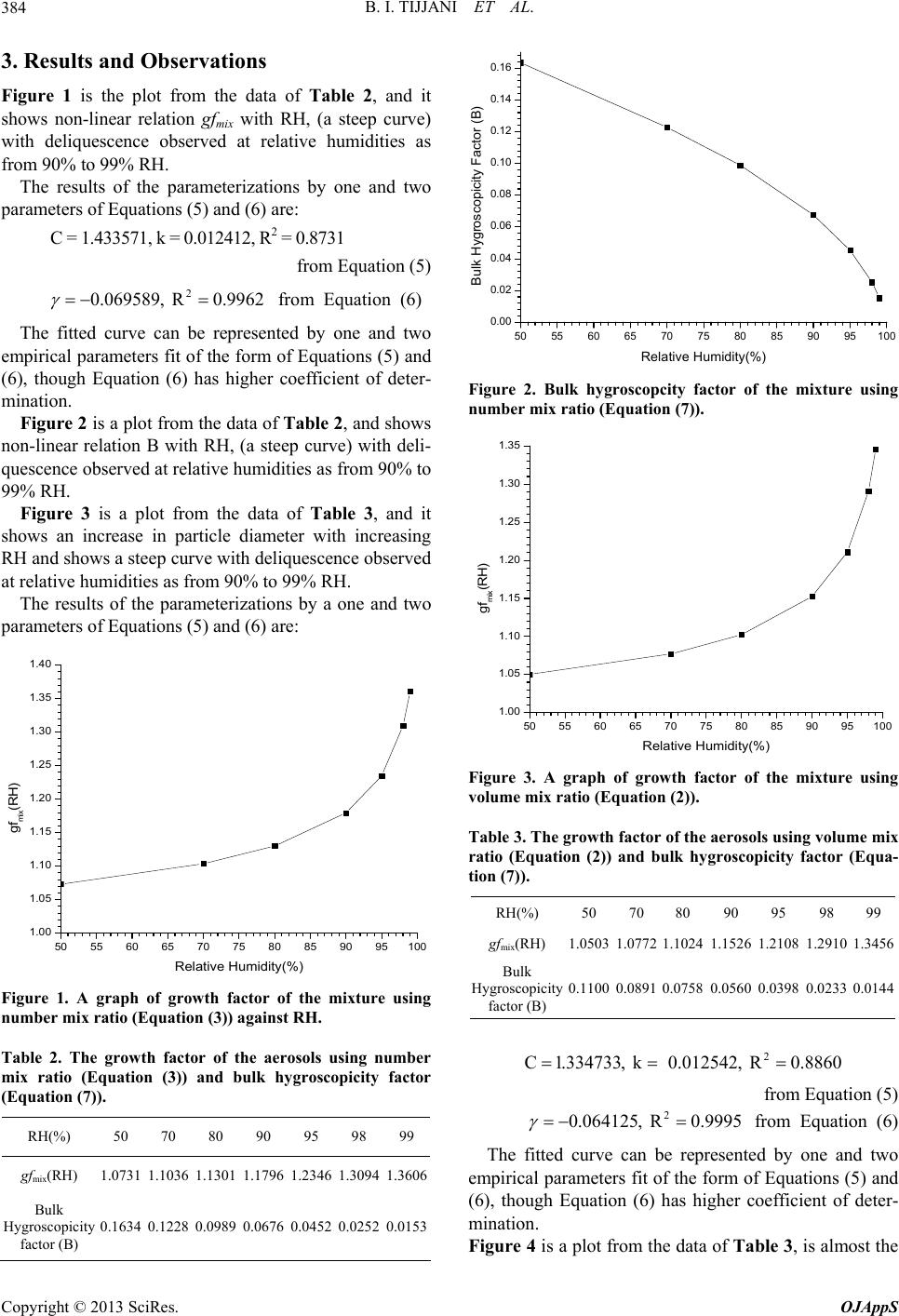 B. I. TIJJANI ET AL. 384 3. Results and Observations Figure 1 is the plot from the data of Table 2, and it shows non-linear relation gfmix with RH, (a steep curve) with deliquescence observed at relative humidities as from 90% to 99% RH. The results of the parameterizations by one and two parameters of Equations (5) and (6) are: C = 1.433571, k = 0.012412, R2 = 0.8731 from Equation (5) 2 0.069589, R0.9962 from Equation (6) The fitted curve can be represented by one and two empirical parameters fit of the form of Equations (5) and (6), though Equation (6) has higher coefficient of deter- mination. Figure 2 is a plot from the data of Table 2, and shows non-linear relation B with RH, (a steep curve) with deli- quescence observed at relative humidities as from 90% to 99% RH. Figure 3 is a plot from the data of Table 3, and it shows an increase in particle diameter with increasing RH and shows a steep curve with deliquescence observed at relative humidities as from 90% to 99% RH. The results of the parameterizations by a one and two parameters of Equations (5) and (6) are: 50 55 60 65 70 75 80 85 90 95100 1.00 1.05 1.10 1.15 1.20 1.25 1.30 1.35 1.40 gf mix (RH) Relative Humidity(%) Figure 1. A graph of growth factor of the mixture using number mix ratio (Equation (3)) against RH. Table 2. The growth factor of the aerosols using number mix ratio (Equation (3)) and bulk hygroscopicity factor (Equation (7)). RH(%) 50 70 80 90 95 98 99 gfmix(RH) 1.0731 1.1036 1.13011.1796 1.2346 1.30941.3606 Bulk Hygroscopicity factor (B) 0.1634 0.1228 0.0989 0.0676 0.0452 0.0252 0.0153 50 55 60 65 70 75 80 85 90 95100 0.00 0.02 0.04 0.06 0.08 0.10 0.12 0.14 0.16 Bulk Hygroscopicity Factor (B) Relative Humidity(%) Figure 2. Bulk hygroscopcity factor of the mixture using number mix ratio (Equation (7)). 50 55606570 758085 90 95100 1.00 1.05 1.10 1.15 1.20 1.25 1.30 1.35 gf mix (RH) Relative Humidity(%) Figure 3. A graph of growth factor of the mixture using volume mix ratio (Equation (2)). Table 3. The growth factor of the aerosols using volume mix ratio (Equation (2)) and bulk hygroscopicity factor (Equa- tion (7)). RH(%) 50 70 80 90 95 98 99 gfmix(RH) 1.05031.07721.1024 1.1526 1.2108 1.29101.3456 Bulk Hygroscopicity factor (B) 0.11000.08910.0758 0.0560 0.0398 0.02330.0144 2 C1.334733, k 0.012542, R0.8860 from Equation (5) 2 0.064125, R0.9995 from Equation (6) The fitted curve can be represented by one and two empirical parameters fit of the form of Equations (5) and (6), though Equation (6) has higher coefficient of deter- mination. Figure 4 is a plot from the data of Table 3, is almost the Copyright © 2013 SciRes. OJAppS 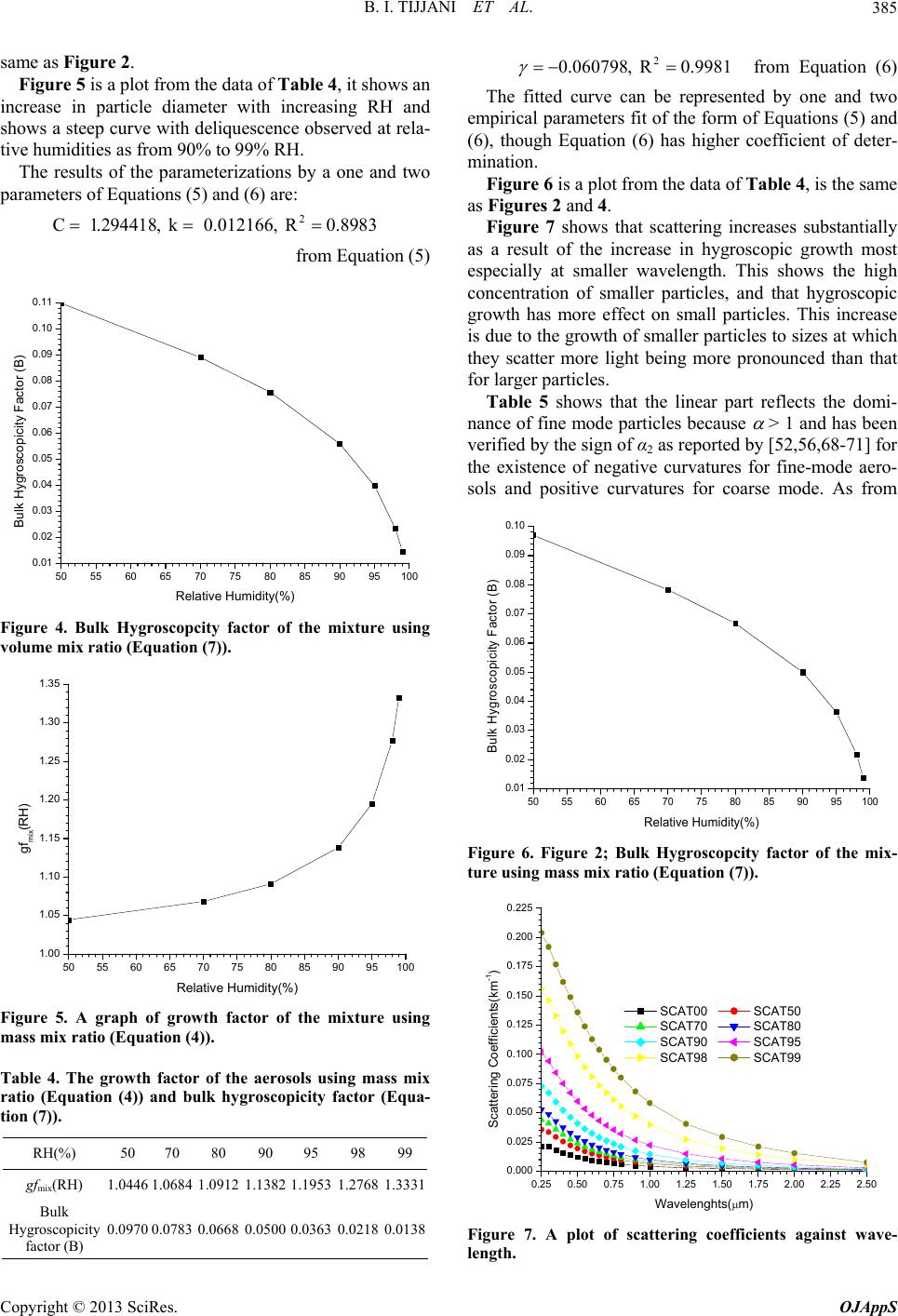 B. I. TIJJANI ET AL. 385 same as Figure 2. Figure 5 is a plot from the data of Table 4, it shows an increase in particle diameter with increasing RH and shows a steep curve with deliquescence observed at rela- tive humidities as from 90% to 99% RH. The results of the parameterizations by a one and two parameters of Equations (5) and (6) are: 2 C 1.294418, k 0.012166, R0.8983 from Equation (5) 50 55 60 65 70 75 80 85 90 95100 0.01 0.02 0.03 0.04 0.05 0.06 0.07 0.08 0.09 0.10 0.11 Bulk Hygroscopicity Factor (B) Relative Humidity(%) Figure 4. Bulk Hygroscopcity factor of the mixture using volume mix ratio (Equation (7)). 50 55 60 65 70 75 8085 90 95100 1.00 1.05 1.10 1.15 1.20 1.25 1.30 1.35 gf mix (RH) Relative Humidity(%) Figure 5. A graph of growth factor of the mixture using mass mix ratio (Equation (4)). Table 4. The growth factor of the aerosols using mass mix ratio (Equation (4)) and bulk hygroscopicity factor (Equa- tion (7)). RH(%) 50 70 80 90 95 98 99 gfmix(RH) 1.0446 1.0684 1.0912 1.1382 1.1953 1.2768 1.3331 Bulk Hygroscopicity factor (B) 0.0970 0.0783 0.0668 0.0500 0.0363 0.0218 0.0138 2 0.060798, R0.9981 from Equation (6) The fitted curve can be represented by one and two empirical parameters fit of the form of Equations (5) and (6), though Equation (6) has higher coefficient of deter- mination. Figure 6 is a plot from the data of Table 4, is the same as Figures 2 and 4. Figure 7 shows that scattering increases substantially as a result of the increase in hygroscopic growth most especially at smaller wavelength. This shows the high concentration of smaller particles, and that hygroscopic growth has more effect on small particles. This increase is due to the growth of smaller particles to sizes at which they scatter more light being more pronounced than that for larger particles. Table 5 shows that the linear part reflects the domi- nance of fine mode particles because > 1 and has been verified by the sign of α2 as reported by [52,56,68-71] for the existence of negative curvatures for fine-mode aero- sols and positive curvatures for coarse mode. As from 50 55 60 65 70 75 80 8590 95100 0.01 0.02 0.03 0.04 0.05 0.06 0.07 0.08 0.09 0.10 Bulk Hygroscopicity Factor (B) Relative Humidity(%) Figure 6. Figure 2; Bulk Hygroscopcity factor of the mix- ture using mass mix ratio (Equation (7)). 0.25 0.50 0.75 1.00 1.25 1.50 1.75 2.00 2.25 2.50 0.000 0.025 0.050 0.075 0.100 0.125 0.150 0.175 0.200 0.225 Scattering Co ef fic i en ts (km - ) Wavelenghts( m) SCAT00 SCAT50 SCAT70 SCAT80 SCAT90 SCAT95 SCAT98 SCAT99 Figure 7. A plot of scattering coefficients against wave- length. Copyright © 2013 SciRes. OJAppS  B. I. TIJJANI ET AL. Copyright © 2013 SciRes. OJAppS 386 Table 5. The results of the Angstrom coefficients of scattering coefficients using Equations (12)-(14) for continental clean model at the respective relative humidities using regression analysis with SPSS16.0. RH Linear Quadratic Cubic (%) R2 Α R 2 α1 α2 R 2 α1 α2 α3 0 0.9887 1.3858 0.9956 −1.4647 −0.1718 0.9991 −1.6016 −0.0156 0.2048 50 0.9839 1.5029 0.9985 −1.6276 −0.2715 0.9997 −1.7142 −0.1727 0.1295 70 0.9802 1.5327 0.9992 −1.6781 −0.3164 0.9998 −1.7403 −0.2454 0.0931 80 0.9763 1.5483 0.9995 −1.7108 −0.3537 0.9998 −1.7522 −0.3065 0.0619 90 0.9682 1.5522 0.9998 −1.7429 −0.4151 0.9998 −1.7469 −0.4106 0.0060 95 0.9586 1.5227 0.9995 −1.7371 −0.4666 0.9997 −1.7067 −0.5013 −0.0455 98 0.9451 1.4423 0.9988 −1.6764 −0.5096 0.9995 −1.6141 −0.5807 −0.0933 99 0.9356 1.3710 0.9983 −1.6127 −0.5260 0.9993 −1.5366 −0.6129 −0.1138 0% to 90% the hygroscopic growth has caused increase in α and increase in the curvature from the quadratic part. As from 95%, α started decreasing and continued to de- crease with the increase in RH despite the fact that α2 continued to increase. This shows that as the deliques- cence point increase the values of α continued to de- crease. It also shows that hygroscopic growth enhances mode size growth. The increase in 2 signifies the in- crease in the domination of fine particles. The cubic part shows that the mixture has bimodal type of distribution with the dominance of fine mode particles because the magnitude of α1 > 1. 0.25 0.50 0.75 1.00 1.25 1.50 1.75 2.00 2.25 2.50 1.25 1.30 1.35 1.40 1.45 1.50 1.55 Effective Real Refractive Indicies Wavelength( m) RFR00 RFR50 RFR70 RFR80 RFR90 RFR95 RFR98 RFR99 Figure 8 shows that refractive indicies decrease with the increase in RH. It also shows that the non-sphericity increases with the increase in RH. This shows that in- crease in hygroscopic growth causes the particles to be more non-spherical with wavelengths. Figure 8. A plot of effective real refractive indices against wavelength. Figure 9 shows that enhancement increases with the increase in hygroscopic growth and is also a function of wavelengths. Enhancement factor as a function of RH shows a nonlinear relation. 0.25 0.50 0.75 1.00 1.25 1.50 1.75 2.00 2.25 2.50 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Scattering Enhancement Wavelenghts( m) SCATEN50 SCATEN70 SCATEN80 SCATEN90 SCATEN95 SCATEN98 SCATEN99 The results of the fitted curves of Equations (9) and (10) are presented as follows: For one parameter (Equation (9)) At λ = 0.25μ, γ = 0.512704, R2 = 0.9961 At λ = 1.25μ, γ = 0.552129, R2 = 0.9984 At λ = 2.50μ, γ = 0.367149, R2 = 0.9722 For two parameters (Equation (10)) At λ = 0.25μ, a = 1.204648, b = −0.454391, R2 = 0.9992 At λ = 1.25μ, a = 0.925221, b = −0.576471, R2 = 0.9957 At λ = 2.50μ, a = 0.737846, b = −0.462366, R2 = 0.9753 Because of the very good correlations, they verify the non-linearity relation between the enhancements pa- rameters and RH. Figure 9. A plot of scattering enhancement parameters against wavelengths.  B. I. TIJJANI ET AL. 387 Figure 10 shows that absorption is barely dependent of hygroscopic growth at smaller wavelengths but in- creases as the wavelengths increases. This shows that larger particles absorbs more than smaller as the hygro- scopic growth increases. The plots can be approximated by power law. From Table 6, the values of α shows the dominance of coarse particles because it is less than 1. As the RH in- creases it also continued to decrease and the values of α2 continued to increase and positive throughout. This shows that increase in hygroscopic growth increases the particle size and shows the dominance of the coarse par- ticles. This also verifies bi-modal type of particle size distribution. From Figure 11, the behavior of the effective imagi- nary refractive indicies with wavelengths shows the dominance of non-spherical particles. It also shows de- crease in refractive indicies as a result of the increase in hygroscopic growth. Comparing Figures 10 and 11 shows that particle has more dominance in absorption than the imaginary effective refractive indicies. This is because as a result of the decrease in the effective imag- inery refractive indicies, we expect decrease in absorp- tion instead of the increase as shown in Figure 10. Figure 12 shows that the enhancement parameter in- creases with the increase in wavelengths and this implies increase with the increases of the particle size. This shows that it increases with the increase in particle size as observed in Figure 10. Enhancement factor as a function of RH shows a nonlinear relation. The results of the fitted curves of Equations (9) and (10) are presented as follows: For one parameter (Equation (9)) At λ = 0.25μ, γ = 0.033611, R2 = 0.9799 At λ = 1.25μ, γ = 0.032435, R2 = 0.9931 0.25 0.50 0.75 1.00 1.25 1.50 1.75 2.00 2.25 2.50 0.000 0.001 0.002 0.003 0.004 0.005 0.006 Absorption Coefficients (km -1 ) Wavelengths( m) ABS00 ABS50 ABS70 ABS80 ABS90 ABS95 ABS98 ABS99 Figure 10. A plot of absorption coefficients against wave- length. 0.25 0.50 0.75 1.00 1.25 1.50 1.75 2.00 2.25 2.50 0.0000 0.0125 0.0250 0.0375 E ffe ct iv e Imag in a ry R e fra c tiv e Ind ic ie s Wavelength( m) RFI00 RFI50 RFI70 RFI80 RFI90 RFI95 RFI98 RFI99 Figure 11. A plot of effective imaginary refractive indices against wavelength. Table 6. The results of the Angstrom coefficients of absorption coefficients using Equations (12)-(14) for continental clean model at the respective relative humidities using regression analysis with SPSS16.0. RH Linear Quadratic Cubic (%) R2 α R2 α1 α2 R 2 α1 α2 α3 0 0.7321 0.8145 0.7574 −0.7114 0.2243 0.9472 −0.0227 −0.5615 −1.0300 50 0.6988 0.7801 0.7326 −0.6633 0.2542 0.9397 0.0419 −0.5504 −1.0547 70 0.6866 0.7655 0.7252 −0.6419 0.2690 0.9368 0.0638 −0.5362 −1.0555 80 0.6757 0.7518 0.7195 −0.6214 0.2838 0.9343 0.0824 −0.5193 −1.0527 90 0.6537 0.7228 0.7108 −0.5773 0.3168 0.9293 0.1166 −0.4749 −1.0378 95 0.6226 0.6824 0.7026 −0.5158 0.3626 0.9219 0.1567 −0.4047 −1.0058 98 0.5596 0.6126 0.6940 −0.4082 0.4450 0.9083 0.2214 −0.2733 −0.9416 99 0.4973 0.5532 0.6912 −0.3180 0.5120 0.8954 0.2707 −0.1597 −0.8805 Copyright © 2013 SciRes. OJAppS  B. I. TIJJANI ET AL. 388 0.25 0.50 0.75 1.00 1.25 1.50 1.75 2.00 2.25 2.50 0.9 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 2.0 2.1 2.2 2.3 Enhancement Parameter Wavelengths( m) ABSEN50 ABSEN70 ABSEN80 ABSEN90 ABSEN95 ABSEN98 ABSEN99 Figure 12. A plot of Absorption enhancement parameters against wavelengths. At λ = 2.50μ, γ = 0.153640, R2 = 0.9740 For two parameters (Equation (10)) At λ = 0.25μ, a = 1.027460, b = −0.025126, R2 = 0.9845 At λ = 1.25μ, a = 1.016064, b = −0.027444, R2 = 0.9995 At λ = 2.50μ, a = 0.894238, b = −0.188649, R2 = 0.9669 Because of the very good correlations, they verify the non-linearity relation between the enhancements pa- rameters and RH. Figure 13 shows that hygroscopic growth causes smaller particles to scatter more in the forward and for- ward scattering decreases with the increase in particle size. Figure 14 shows that hygroscopic growth causes en- hancement in the forward direction to decrease with the increase in particle size. The results of the fitted curves of Equations (9) and (10) are presented as follows: For one parameter (Equation (9)) At λ = 0.25μ, γ = 0.029089, R2 = 0.9291 At λ = 1.25μ, γ = 0.027159, R2 = 0.9826 At λ = 2.55μ, γ = −0.060856, R2 = 0.9717 For two parameters (Equation (10)) At λ = 0.25μ, a = 1.014671, b = −0.014565, R2 = 0.9348 At λ = 1.25μ, a = 0.980473, b = −0.033335, R2 = 0.9920 At λ = 2.50μ, a = 0.954266, b = 0.046195, R2 = 0.9136 Because of the very good correlations, they verify the non-linearity relation between the enhancements pa- rameters and RH. 4. Conclusions From the gfmix(RH) determined, it can be observed that 0.25 0.50 0.751.001.251.501.75 2.00 2.25 2.50 0.60 0.62 0.64 0.66 0.68 0.70 0.72 0.74 0.76 0.78 0.80 0.82 Assymmetric Parameter Wavelengths( m) ASP00 ASP50 ASP70 ASP80 ASP90 ASP95 ASP98 ASP99 Figure 13. A plot of Asymmetric parameter against wave- length. 0.25 0.500.75 1.001.25 1.501.75 2.002.25 2.50 0.75 0.80 0.85 0.90 0.95 1.00 1.05 1.10 1.15 1.20 1.25 Enhancement Parameter Wavelengths(m) ASYEN50 ASYEN70 ASYEN80 ASYEN90 ASYEN95 ASYEN98 ASYEN99 Figure 14. A plot of Asymmetric parameter enhancement parameters against wavelengths. despite the higher fractions of more strongly absorbing particles, very low values of gfmix(RH) were observed, and this is in line with what Sheridan et al. [24] deter- mined. It shows that increase in RH increases forward scat- tering because particle growth enhances forward. These hygroscopic growth behaviors also reveal an immense potential of light scattering enhancement in the forward scattering [65] for smaller particles while in lar- ger particles it causes increase in the backward scattering at high humidities and the potential for being highly ef- fective cloud condensation nuclei. It also shows that the mixture is internally mixed for smaller particles because of the increase in forward scat- tering as a result of the hygroscopic growth [66]. Field measurements have noted a k value of 0.01 for fresh soot rich biomass [67]. The overall, modeled k ranges from 0.012 to 0.163 depending on the RH and the Copyright © 2013 SciRes. OJAppS  B. I. TIJJANI ET AL. 389 type of the mixing ratio used. Finally, it can be observed that the absorption and scattering coefficients together with their enhancement parameters have exponential dependence with RH. The modeling shows that hygroscopic growth at higher relative humidity increases the effective radii, scattering coefficients, scattering enhancement parameters, absorp- tion coeffeicnts, absorption enhancement parameters, but decreases effective real refractive indices, effective imaginary refractive indices. However, the asymmetric and enhancement asymmetric parameters increase with the increase in RH but decreases with the increase in wavelength. Jeong et al. [10] demonstrated an exponential de- pendence of the aerosol optical thickness on relative hu- midity. Finally, the data fit our models very well and can be used to extrapolate the hygroscopic growth at any RH and enhancement parameters at any RH and wavelengths. The importance of determining gfmix(RH) as a function of RH and volume fractions, mass fractions and number fractions, and enhancement parameters as a function of RH and wavelengths can be potentially important be- cause it can be used for efficiently representing aero- sols-water interactions in global models. REFERENCES [1] D. A. Randall, R. A. Wood, S. Bony, R. Colman, T. Fi- chefet, J. Fyfe, V. Kattsov, A. Pitman, J. Shukla, J. Srini- vasan, R. J. Stouf-fer, A. Sumi and K. E. Taylor, “Con- tribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change—Climate Models and their Evaluation,” Cam- bridge University Press, Cambridge, New York, 2007, pp. 589-662. [2] D. S. Covert, R. J. Charlson and N. C. Ahlquist, “A Study of the Relationship of Chemical Composition and Hu- midity to Light Scattering by Aerosols,” Journal of Ap- plied Meteorology, Vol. 11, No. 6, 1972, pp. 968-976. http://dx.doi.org/10.1175/1520-0450(1972)011<0968:AS OTRO>2.0.CO;2 [3] L. McInnes, M. Bergin, J. Ogren and S. Schwartz, “Ap- portionment of Light Scattering and Hygroscopic Growth to Aerosol Composition,” Geophysical Research Letters, Vol. 25, No. 4, 1998, pp. 513-516. http://dx.doi.org/10.1029/98GL00127 [4] R. A. Kotchenruther, P. V. Hobbs and D. A. Hegg, “Hu- midification Factors for Atmospheric Aerosols off the Mid-Atlantic Coast of the United States,” Journal of Geophysical Research, Vol. 104, No. D2, 1999, pp. 2239- 2251. http://dx.doi.org/10.1029/98JD01751 [5] W. C. Malm, D. E. Day, S. M. Kreidenweis, J. L. Collett and T. Lee, “Humidity-Dependent Optical Properties of Fine Particles during the Big Bend Regional Aerosol and Visibility Observational Study,” Journal of Geophysical Research, Vol. 108, No. D9, 2003, p. 4279. http://dx.doi.org/10.1029/2002JD002998 [6] I. N. Tang, “Chemical and Size Effects of Hygroscopic Aerosols on Light Scattering Coefficient,” Journal of Geophysical Research, Vol. 101, No. D14, 1996, pp. 19245-19250. http://dx.doi.org/10.1029/96JD03003 [7] E. Swietlicki, J. Zhou, O. H. Berg, B. G. Martinsson, G. Frank, S.-I. Cederfelt, U. Dusek, A. Berner, W. Birmilli, A. Wiedensohler, B. Yuskiewicz and K. N. Bower, “A Closure Study of Sub-Micrometer Aerosol Particle Hy- groscopic Behaviour,” Atmospheric Research, Vol. 50 No. 3-4, 1999, pp. 205-240. http://dx.doi.org/10.1016/S0169-8095(98)00105-7 [8] S. A. Terpugowa, M. V. Panchenko, V. S. Kozlov, V. V. Polkin and E. P. Yausheva, “The Study of the Growth Factor of the Aerosol Scattering Coefficient in the Near- Ground Layer of the Atmosphere in West Siberia,” Euro- pean Aerosol Conference, Budapest, 2004 [9] J. Kuśmierczyk-Michulec, “Angstrom Coefficient as an Indicator of the Atmospheric Aerosol Type for a Well- Mixed Atmospheric Boundary Layer: Part 1: Modelde- velopment,” Oceanologia, Vol. 51 No. 1, 2009, pp. 5-38. http://dx.doi.org/10.5697/oc.51-1.005 [10] M. J Jeong, Z. Li, E. Andrews and S. C. Tsay, “Effect of Aerosol Humidification on the Column Aerosol Optical Thickness over the Atmospheric Radiation Measurement Southern Great Plains Site,” Journal of Geophysical Re- search, Vol. 112, No. D10, 2007, p. 202. http://dx.doi.org/10.1029/2006JD007176 [11] A. S. Rapti, “Spectral Optical Atmospheric Thickness Dependence on the Specific Humidity in the Presence of Continental and Marine Air Masses,” Atmospheric Re- search, Vol. 78, No. 1-2, 2005, pp. 13-32. http://dx.doi.org/10.1016/j.atmosres.2005.02.004 [12] Y. Miyazaki, Y. Kondo, N. Takegawa, Y. Komazaki, M. Fukuda, K. Kawamura, M. Mochida, K. Okuzawa and R. J. Weber, “Time-Resolved Measurements of Water-Solu- ble Organic Carbon in Tokyo,” Journal of Geophysical Research, Vol. 111, No. D23, 2006, p. 206. http://dx.doi.org/10.1029/2006JD007125 [13] A. P. Sullivan, R. E. Peltier, C. A. Brock, J. A. de Gouw, J. S. Holloway, C. Warneke, A. G. Wollny and R. J. We- ber, “Airborne Measurements of Carbonaceous Aerosol Soluble in Water over Northeastern United States: Method Development and an Investigation into Wa- ter-Soluble Organic Carbon Sources,” Journal of Geo- physical Research, Vol. 111, No. D23, 2006, p. S46. http://dx.doi.org/10.1029/2006JD007072 [14] Y. Kondo, Y. Miyazaki, N. Takegawa, T. Miyakawa, R. J. Weber, J. L. Jimenez, Q. Zhang and D. R. Worsnop, “Oxygenated and Water-Soluble Organic Aerosols in Tokyo,” Journal of Geophysical Research, Vol. 122, No. D01, 2007, p. 203. http://dx.doi.org/10.1029/2006JD007056 [15] R. J. Weber, A. P. Sullivan, R. E. Peltier, A. Russell, B. Yan, M. Zheng, J. de Gouw, C. Warneke, C. Brock, J. S. Holloway, E. L. Atlas and E. Edgerton, “A Study of Sec- ondary Organic Aerosol Formation in the Anthropogenic Influenced Southeastern United States,” Journal of Geo- physical Research, Vol. 112, No. D13, 2007, p. 302. Copyright © 2013 SciRes. OJAppS  B. I. TIJJANI ET AL. 390 http://dx.doi.org/10.1029/2007JD008408 [16] B. Ervens and R. Volkamer, “Glyoxal Processing by Aerosol Multiphase Chemistry: Towards a Kinetic Mod- eling Framework of Secondary Organic Aerosol Forma- tion in Aqueous Particles,” Atmospheric Chemistry and Physics, Vol. 10, 2010, pp. 8219-8244. http://dx.doi.org/10.5194/acp-10-8219-2010 [17] A. Sorooshian, S. M. Murphy, S. Hersey, R. Bahreini, H. Jonsson, R. C. Flagan and J. H. Seinfeld, “Constraining the Contribution of Organic Acids and AMS m/z44 to the Organic Aerosol Budget: On the Importance of Meteor- ology, Aerosol Hygroscopicity and Region,” Geophysical Research Letters, Vol. 37, No. 21, 2010. http://dx.doi.org/10.1029/2010GL044951 [18] H. Timonen, M. Aurela, S. Carbone, K. Saarnio, S. Saarikoski, T. Makela, M. Kulmala, V. M. Kerminen, D. R. Worsnop and R. Hillamo, “High Time-Resolution Chemical Characterization of the Water-Soluble Fraction of Ambient Aerosols with PILS-TOC-IC and AMS,” At- mospheric Measurement Techniques, Vol. 3, 2010, pp. 1063-1074. http://dx.doi.org/10.5194/amt-3-1063-2010 [19] K. S. Docherty, E. A. Stone, I. M. Ulbrich, P. F. DeCarlo, D. C. Snyder, J. J. Schauer, R. E. Peltier, R. J. Weber, S. M. Murphy, J. H. Seinfeld, D. J. Eatough, B. D. Grover and J. L. Jimenez, “Apportionment of Primary and Sec- ondary Organic Aerosols in Southern California during the 2005 Study of Organic Aerosols in Riverside (SOAR),” Environmental Science & Technology, Vol. 42, No. 20, 2008, pp. 7655-7662. [20] P. McMurry and M. Stolzenburg, “On the Sensitivity of Particle Size to Relative Humidity for Los Angeles Aero- sols,” Atmospheric Environment, Vol. 23, No. 2, 1989, pp. 497-507. http://dx.doi.org/10.1016/0004-6981(89)90593-3 [21] D. Cocker, N. Whitlock, R. Flagan and J. H. Seinfeld, “Hygroscopic Properties of Pasadena, California Aero- sol,” Aerosol Science and Technology, Vol. 35, No. 2, 2001, pp. 637-647. http://dx.doi.org/10.1080/02786820120653 [22] E. Swietlicki, et al., “Hygroscopic Properties of Submi- crometer Atmospheric Aerosol Particles Measured with H TDMA Instruments in Various Environments—A Re- view,” Tellus B, Vol. 60, No. 3, 2008, pp. 432-469. http://dx.doi.org/10.3402/tellusb.v60i3.16936 [23] H. Kohler, “The Nucleus and Growth of Hygroscopic Droplets,” Transactions of the Faraday Society, Vol. 32, 1936, pp. 1152-1161. http://dx.doi.org/10.1039/tf9363201152 [24] P. J. Sheridan, D. J. Delene and J. A. Ogren, “Four Years of Continuous Surface Aerosol Measurements from the Department of Energy’s Atmospheric Radiation Meas- urement Program Southern Great Plains Cloud and Ra- diation Testbedsite,” Journal of Geophysical Research, Vol. 106, No. 20, 2001, pp. 735-747. http://dx.doi.org/10.1029/2001JD000785 [25] M. Hess, P. Koepke and I. Schult, “Optical Properties of Aerosols and Clouds: The Software Package OPAC,” Bulletin of the American Meteorological Society, Vol. 79, No. 5, 1998, pp. 831-844. [26] C. A. Randles, L. M. Russell and V. Ramaswamy, “Hy- groscopic and Optical Properties of Organic Sea Salt Aerosol and Consequences for Climate Forcing,” Geo- physical Research Letters, Vol. 31, No. 16, 2004, Article ID: L16108. http://dx.doi.org/10.1029/2004GL020628 [27] P. F. Liu, C. S. Zhao, T. Gobel, E. Hallbauer, A. Nowak, L. Ran, W. Y. Xu, Z. Z. Deng, N. Ma, K. Mildenberger, S. Henning, F. Stratmann and A. Wiedensohler, “Hygro- scopic Proper Ties of Aerosol Particles at High Relative Humidity and Their Diurnal Variations in the North China Plain,” Atmospheric Chemistry and Physics, Vol. 11, 2011, pp. 2991-3040. http://dx.doi.org/10.5194/acpd-11-2991-2011 [28] G. Buzorius, A. Zelenyuk, F. Brechtel and D. Imre, “Si- multaneous Determination of Individual Ambient Particle Size, Hygroscopicity and Composition,” Geophysical Re- search Letters, Vol. 29, 1974, 2002. http://dx.doi.org/10.1029/2001GL014221 [29] S. Sjogren, M. Gysel, E. Weingartner, U. Baltensperger, M. J. Cubison, H. Coe, A. A. Zardini, C. Marcolli, U. K. Krieger and T. Peter, “Hygroscopic Growth and Water Uptake Kinetics of Two-Phase Aerosol Particles Consist- ing of Ammonium Sulfate, Adipic and Humic Acid Mix- tures,” Journal of Aerosol Science, Vol. 38, No. 2, 2007, 157-171. http://dx.doi.org/10.1016/j.jaerosci.2006.11.005 [30] R. H. Stokes and R. A. Robinson, “Interactions in Aque- ous Nonelec-trolyte Solutions. I. Solute-Solvent Equilib- ria,” The Journal of Physical Chemistry, Vol. 70, No. 7, 1966, pp. 2126-2130. http://dx.doi.org/10.1021/j100879a010 [31] N. K. Meyer, J. Duplissy, M. Gysel, A. Metzger, J. Dom- men, E. Weingartner, M. R. Alfarra, A. S. H. Prevot, C. Fletcher, N. Good, G. McFiggans, A. M. Jonsson, M. Hallquist, U. Baltensperger and Z. D. Ristovski, “Analy- sis of the Hy-groscopic and Volatile Properties of Am- monium Sulphate Seeded and Unseeded SOA Particles,” Atmospheric Chemistry and Physics, Vol. 9, 2009, pp. 721-732. http://dx.doi.org/10.5194/acp-9-721-2009 [32] M. Stock, Y. F. Cheng, W. Birmili, A. Massling, B. Weh- ner, T. Muller, S. Leinert, N. Kalivitis, N. Mihalopoulos and A. Wiedensohler, “Hygroscopic Properties of At- mospheric Aerosol Particles over the Eastern Mediterra- nean: Implications for Regional Direct Radiative Forcing under Clean and Polluted Conditions,” Atmospheric Chemistry and Physics, Vol. 11, 2011, pp. 4251-4271. www.atmos-chem-phys.net/11/4251/2011/ http://dx.doi.org/10.5194/acp-11-4251-2011 [33] J. Duplissy, P. F. DeCarlo, J. Dommen, M. R. Alfarra, A. Metzger, I. Barmpadimos, A. S. H. Prevot, E. Weingart- ner, T. Tritscher, M. Gysel, A. C. Aiken, J. L. Jimenez, M. R. Canagaratna, D. R. Worsnop, D. R. Collins, J. Tom- linson and U. Baltensperger, “Relating Hygroscopicity and Composition of Organic Aerosol Particulate Matter,” Atmospheric Chemistry and Physics, Vol. 11, 2011, pp. 1155-1165. www.atmos-chem-phys.net/11/1155/2011/ http://dx.doi.org/10.5194/acp-11-1155-2011 [34] J. Meier, B. Wehner, A. Massling, W. Birmili, A. Nowak, T. Gnauk, E. Bruggemann, H. Herrmann, H. Min and A. Wiedensohler, “Hygroscopic Growth of Urban Aerosol Copyright © 2013 SciRes. OJAppS  B. I. TIJJANI ET AL. 391 Particles in Beijing (China) during Wintertime: A Com- parison of Three Experimental Methods,” Atmospheric Chemistry and Physics, Vol. 9, 2009, pp. 6865-6880. www.atmos-chem-phys.net/9/6865/2009/ http://dx.doi.org/10.5194/acp-9-6865-2009 [35] M. D. Petters and S. M. Kreidenweis, “A Single Parame- ter Representation of Hygroscopic Growth and Cloud Condensation Nucleus Activity,” Atmospheric Chemistry and Physics, Vol. 7, No. 8, 2007, pp. 1961-1971. http://dx.doi.org/10.5194/acp-7-1961-2007 [36] J. H. Seinfeld and S. N. Pandis, “Atmospheric Chemistry and Physics,” Wiley-Interscience Publication, Hoboken, 1998. [37] J. Seinfeld and S. Pandis, “Atmospheric Chemistry and Physics,” 2nd Edition, Wiley-Interscience, New York, 2006. [38] E. Swietlicki, J. Zhou, D. S. Covert, K. Hameri, B. Busch, M. Vakeva, U. Dusek, O. H. Berg, A. Wiedensohler, P. Aalto, J. Makela, B. G. Martinsson, G. Papaspiropoulos, B. Mentes, G. Frank and F. Stratmann, “Hygroscopic Pro- perties of Aerosol Particles in the Northeastern Atlantic during ACE-2,” Tellus, Vol. 52, No. 2, 2000, pp. 201- 227. [39] W. Birmili, A. Nowak, K. Schwirn, K. Lehmann, et al., “A New Method to Accurately Relate Dry and Humidi- fied Number Size Distributions of Atmospheric Aero- sols,” Journal of Aerosol Science, Vol. 1, No. 1, 2004, pp. 15-16. [40] F. Kasten, “Visibility Forecast in the Phase of Pre-Con- densation,” Tellus, Vol. 21, No. 5, 1969, pp. 631-635. [41] M. Gysel, G. B. McFiggans and H. Coe, “Inversion of Tandem Differential Mobility Analyser (TDMA) Meas- urements,” Journal of Aerosol Science, Vol. 40, No. 2, 2009, pp. 134-151. http://dx.doi.org/10.1016/j.jaerosci.2008.07.013 [42] J.-P. Putaud, “Interactive Comment on ‘Aerosol Hygro- scopicity at Ispra EMEP-GAW Station’ by M. Adam et al.,” Atmospheric Chemistry and Physics Discussions, Vol. 12, No. 1, 2012, pp. C200-C202. [43] S. I. Christensen and M. D. Petters, “The Role of Tem- perature in Cloud Droplet Activation,” The Journal of Physical Chemistry A, Vol. 116, No. 39, 2012, pp. 9706- 9717. http://dx.doi.org/10.1021/jp3064454 [44] S. J. Doherty, et al., “A Comparison and Summary of Ae- rosol Optical Properties as Observed in Situ from Air- craft, Ship, and Land during ACE-Asia,” Journal of Geo- physical Research: Atmospheres, Vol. 110, No. D4, 2005, Article ID: D04201. http://dx.doi.org/10.1029/2004JD004964 [45] P. K. Quinn, et al., “Impact of Particulate Organic Matter on the Relative Humidity Dependence of Light Scattering: A Simplified Parameterization,” Geophysical Research Letters, Vol. 32, No. 22, 2005, Article ID: L22809. http://dx.doi.org/10.1029/2005GL024322 [46] S. Gasso, et al., “Influence of Humidity on the Aerosol Scattering Coefficient and Its Effect on the Upwelling Ra- diance during ACE-2,” Tellus B, Vol. 52, No. 2, 2000, pp. 546-567. [47] A. Clarke, et al., “Biomass Burning and Pollution Aero- sol over North America: Organic Components and Their Influence on Spectral Optical Properties and Humidifica- tion Response,” Journal of Geophysical Research: At- mospheres, Vol. 112, No. D12, 2007, Article ID: D12S18. http://dx.doi.org/10.1029/2006JD007777 [48] G. Hanel, “The Properties of Atmospheric Aerosol Parti- cles as Functions of Relative Humidity at Thermody- namic Equilibrium with Surrounding Moist Air,” In: H. E. Landsberg and J. Van Mieghem, Eds., Advances in Geo- physics, Academic Press, New York, 1976, pp. 73-188. [49] M. K. Latha and K. V. S. Badarinath, “Factors Influen- cing Aerosol Characteristics over Urban Environment,” Environmental Monitoring and Assessment, Vol. 104, No. 1-3, 2005, pp. 269-280. http://dx.doi.org/10.1007/s10661-005-1615-7 [50] A. Angstrom, “Techniques of Determining the Turbidity of the Atmosphere,” Tellus, Vol. 13, No. 2, 1961, pp. 214- 223. http://dx.doi.org/10.1111/j.2153-3490.1961.tb00078.x [51] M. D. King and D. M. Byrne, “A Method for Inferring Total Ozone Content from Spectral Variation of Total Optical Depth Obtained with a Solar Radiometer,” Jour- nal of the Atmospheric Sciences, Vol. 33, No. 11, 1976, pp. 2242-2251. http://dx.doi.org/10.1175/1520-0469(1976)033<2242:AM FITO>2.0.CO;2 [52] T. F. Eck, B. N. Holben, J. S. Reid, O. Dubovic, A. Smirnov, N. T. O’Neil, I. Slutsker and S. Kinne, “Wave- length Dependence of the Optical Depth of Biomass Bur- ning, Urban, and Desert Dust Aerosols,” Journal of Geo- physical Research: Atmospheres, Vol. 104, No. D24, 1999, pp. 31333-31349. [53] T. F. Eck, B. N. Holben, O. Dubovic, A. I. Smirnov, J. Slutsker, M. Lobert and V. Ramanathan, “Column-Inte- grated Aerosol Optical Properties over the Maldives dur- ing the Northeast Mon-Soon for 1998-2000,” Journal of Geophysical Research: Atmospheres, Vol. 106, 2001, pp. 28555-28566. [54] T. F. Eck, B. N. Holben, D. E. Ward, O. Dubovic, J. S. Reid, A. Smirnov, M. M. Mukelabai, N. C. Hsu, N. T. O’ Neil and I. Slutsker, “Characterization of the Optical Pro- perties of Biomass Burning Aerosols in Zambia during the 1997 ZIBBEE Field Campaign,” Journal of Geophy- sical Research: Atmospheres, Vol. 106, No. D4, 2001, pp. 3425-3448. http://dx.doi.org/10.1029/2000JD900555 [55] Y. J. Kaufman, “Aerosol Optical Thickness and Atmos- pheric Path Radiance,” Journal of Geophysical Research: Atmospheres, Vol. 98, No. D2, 1993, pp. 2677-2992. http://dx.doi.org/10.1029/92JD02427 [56] N. T. O’Neill, O. Dubovic and T. F. Eck, “Modified Ån- gström Exponent for the Characterization of Submicro- meter Aerosols,” Applied Optics, Vol. 40, No. 15, 2001, pp. 2368-2375. http://dx.doi.org/10.1364/AO.40.002368 [57] N. T. O’Neill, T. F. Eck, A. Smirnov, B. N. Holben and S. Thulasiraman, “Spectral Discrimination of Coarse and Fine Mode Optical Depth,” Journal of Geophysical Research: Atmospheres, Vol. 108, No. D17, 2003. Copyright © 2013 SciRes. OJAppS  B. I. TIJJANI ET AL. Copyright © 2013 SciRes. OJAppS 392 [58] R. Pedros, J. A. Martinez-Lozano, M. P. Utrillas, J. L. Gomez-Amo and F. Tena, “Column-Integrated Aerosol, Optical Properties from Ground-Based Spectroradiometer Measurements at Barrax (Spain) during the Digital Air- borne Imaging Spectrometer Experiment (DAISEX) Cam- paigns,” Journal of Geophysical Research: Atmospheres, Vol. 108, No. D18, 2003, pp. 1-12. http://dx.doi.org/10.1029/2002JD003331 [59] D. G. Kaskaoutis and H. D. Kambezidis, “Investigation on the Wavelength Dependence of the Aerosol Optical Depth in the Athens Area,” Quarterly Journal of the Royal Meteorological Society, Vol. 132, No. 620, 2006, pp. 2217-2234. http://dx.doi.org/10.1256/qj.05.183 [60] B. Schmid, D. A. Hegg, J. Wang, D. Bates, J. Redemann, P. B. Russell, J. M. Livingston, H. H. Jonsson, E. J. Wel- ton, J. H. Seinfeld, R. C. Flagan, D. S. Covert, O. Dubo- vik and A. Jefferson, “Column Closure Studies of Lower Tropospheric Aerosol and Water Vapor during ACE-Asia Using Airborne Sun Photometer and Airborne in Situ and Ship-Based Lidar Measurements,” Journal of Geophysi- cal Research: Atmospheres, Vol. 108, No. D23, 2003, pp. 1-19. http://dx.doi.org/10.1029/2002JD003361 [61] J. A. Martinez-Lozano, M. P. Utrillas, F. Tena, R. Pedros, J. Canada, J. V. Bosca and J. Lorente, “Aerosol Optical Characteristics from Summer Campaign in an Urban Coas- tal Mediterranean Area,” IEEE Transactions on Geosci- ence and Remote Sensing, Vol. 39, No. 7, 2001, pp. 1573- 1585. http://dx.doi.org/10.1109/36.934089 [62] D. E. Aspens, “Local-Field Effect and Effective Medium Theory: A Microscopic Perspective,” American Journal of Physics, Vol. 50, No. 8, 1982, pp. 704-709. http://dx.doi.org/10.1119/1.12734 [63] H. A. Lorentz, “Ueber Die Beziehungzwischen der Fortp- flanzungsgeschwindigkeit des Lichtes und der Körperdi- chte,” Annalen der Physik, Vol. 245, No. 4, 1880, pp. 641-665. http://dx.doi.org/10.1002/andp.18802450406 [64] L. Lorenz, “Ueber Die Refractionconstante,” Annalen der Physik, Vol. 11, No. 1, 1880, pp. 70-103. [65] K. N. Liou, “An Introduction to Atmospheric Radiation,” Elsevier, New York, 2002. [66] J. Wang and S. T. Martin, “Satellite Characterization of Urban Aerosols: Importance of Including Hygroscopicity and Mixing State in the Retrieval Algorithms,” Journal of Geophysical Research: Atmospheres, Vol. 112, No. D17203, 2007, pp. 1-18. http://dx.doi.org/10.1029/2006JD008078 [67] M. O. Andreae and D. Rosenfeld, “Aerosol-Cloud-Pre- cipitation Interactions. Part 1. The Nature and Sources of Cloud-Active Aerosols,” Earth-Science Reviews, Vol. 89, No. 1-2, 2008, pp. 13-41. http://dx.doi.org/10.1016/j.earscirev.2008.03.001 [68] T. F. Eck, B. N. Holben, D. E. Ward, M. M. Mukelabai, O. Dubovik, A. Smirnov, J. S. Schafer, N. C. Hsu, S. J. Piketh, A. Queface, J. Le Roux, R. J. Swap and I. Slutsker, “Variability of Biomass Burning Aerosol Optical Char- acteristics in Southern Africa during the SAFARI 2000 Dry Season Campaign and a Comparison of Single Scat- tering Albedo Estimates from Radiometric Measure- ments,” Journal of Geophysical Research: Atmosphere, Vol. 108, No. D13, 2003, pp. [69] G. L. Schuster, O. Dubovik and B. N. Holben, “Angstrom Exponent and Bimodal Aerosol Size Distributions,” Journal of Geophysical Research: Atmosphere, Vol. 111, No. D17, 2006, Article ID: D07207. http://dx.doi.org/10.1029/2005JD006328 [70] D. G. Kaskaoutis, H. D. Kambezidis, N. Hatzianastassiou, P. G. Kosmopoulos and K. V. S. Badarinath, “Aerosol Climatology: Dependence of the Angstrom Exponent on Wavelength over Four AERONET Sites,” Atmospheric Chemistry and Physical Discussion, Vol. 7, No. 3, 2007, pp. 7347-7397. http://dx.doi.org/10.5194/acpd-7-7347-2007 [71] D. G. Kaskaoutis, H. D. Kambezidis, N. Hatzianastassiou, P. G. Kosmopoulos and K. V. S. Badarinath, “Aerosol Climatology: On the Discrimination of Aerosol Types over four AERONET Sites,” Atmospheric Chemistry and Physical Discussion, Vol. 7, No. 3, 2007, pp. 6357-6411. http://dx.doi.org/10.5194/acpd-7-6357-2007
|