 Open Journal of Ophthalmology, 2013, 3, 103-117 Published Online November 2013 (http://www.scirp.org/journal/ojoph) http://dx.doi.org/10.4236/ojoph.2013.34024 Open Access OJOph 1 Cryotherapy in Ophthalmology* Shandiz Tehrani, Frederick W. Fraunfelder# Casey Eye Institute, Oregon Health & Science University, Portland, USA. Email: #fraunfer@ohsu.edu Received January 7th, 2013; revised February 8th, 2013; accepted March 5th, 2013 Copyright © 2013 Shandiz Tehrani, Frederick W. Fraunfelder. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Cryogens have been used to freeze living tissue for the purpose of treating benign and malignant lesions. Within the last century, ophthalmologists have found cryotherapy to be useful in treating a variety of ocular pathologies. Here, we re- view the history of cryotherapy, its introduction to the field of ophthalmology, its proposed mechanism of action, and its current applications in treating surface and intraocular eye disease. Keywords: Cryotherapy; Cryosurgery; Cryogenic Surgery; Cryoprobe; Cryospray; Liquid Nitrogen; Surface Eye Disease 1. Introduction Cryotherapy (also known as cryocautery, cryogenic sur- gery, and cryosurgery) has been used to treat a variety of ophthalmic conditions, including surface and intraocular pathology. Cryotherapy may be preferable in treating certain surface and intraocular diseases (including benign and malignant lesions), as there are few post-operative adverse events and limited long-term complications when compared to radiation and chemotherapy. Here, we re- view the historical and current role of cryotherapy in ophthalmology. 2. Historical Review The history of medical cryotherapy is a captivating one, and has been well documented by Lubritz [1], Cooper and Dawber [2], and Freiman and Bouganim [3], among others. The known benefits of cryotherapy date back to 2500 B.C., when ancient Egyptians used cold to soothe injuries [1,4]. Hippocrates used cold to relieve swelling, bleeding, and pain [5]. The first published report on freezing biologic tissue came from Arnott (1797-1883), who used freezing of tissue locally in the setting of tu- mors [6]. Using a salt and crushed ice mixture, Arnott used cryotherapy for palliation of pain in breast cancer, uterine cancers, and treatment of some skin cancers. He also used his cold treatment for acne, neuralgia, and headaches, and was able to achieve temperatures of −24˚C. With the advent of liquefied gases, it was realized that a rapid freeze with a colder cryogen could effectively treat tumors. In 1899, White became the first to use commercially available refrigerants (liquefied gases) for medical care. He published reports on using liquid air for the treatment of lupus, herpes zoster, nevi, warts, chan- croid, varicose leg ulcers, carbuncles, and epitheliomas [7,8]. Irvine and Turnacliffe expanded the uses of liquid air treatment to seborrheic keratosis, senile keratosis, lichen simplex, and poison ivy dermatitis [9]. Allington was the first to use liquid nitrogen in the treatment of skin lesions [10]. This became the preferred gas because liquid oxygen is explosive and the two gases have similar boiling points (oxygen = −182.9˚C, nitrogen = −195.6˚C). Allington used a cotton swab dipped in liquid nitrogen to treat skin tumors. Unfortunately, there was loss of the heat sink potential with the transfer be- tween the cotton swab and the skin, rendering this method insufficient for effectively treating skin tumors and malignancies. Cooper designed a liquid nitrogen probe that achieved a temperature of −195.6˚C [11]. He treated Parkinson’s disease and inoperable brain tumors by freezing the thalamus and the lesion, respectively. Torre and Zacarian further advanced the field by developing hand held de- *This report was supported by an unrestricted grant from Research to Prevent Blindness to Casey Eye Institute. The authors have no relevant financial disclosures or interests. #Corresponding author.  Cryotherapy in Ophthalmology 104 vices, which facilitated one-handed cryospray and cryo- probe procedures [12]. Liquid nitrogen cryotherapy spread to multiple spe- cialties from that point forward [2]. Dermatologists ad- vanced cryotherapy treatments of benign and malignant tumors of the skin. Neurosurgeons were able to perform liquid nitrogen-assisted transsphenoidal hypophysectomy. Liquid nitrogen treatment of oral and cervical cancers, as well as cryosurgery to the uterus, was described. Today, liquid nitrogen is the most popular medical cryogen. Carbon dioxide (melting point = −79˚C) is still used worldwide because of relatively easy storage. How- ever, because of its higher boiling point, carbon dioxide is generally suitable for treating benign conditions only. Similarly, nitrous oxide (boiling point = −89˚C) is fa- vored by some gynecologists and oral surgeons, but only for benign lesions. Freon (boiling point = −29.8˚C to −40.8˚C) has also been used to treat benign tumors. Cur- rently, the acceptable minimal temperature to achieve cell death is −50˚C to −55˚C [13,14]. The standard of care in dermatology, oral surgery, otolaryngology, neu- rosurgery, and gynecology is liquid nitrogen cryotherapy for malignancies [2,15,16]. Interestingly, this has yet to translate fully to ophthalmology, where multiple cryo- gens are used in lieu of liquid nitrogen for tumors in and around the eye. 3. Early Advancements of Cryotherapy in Ophthalmology Cryotherapy of the eye was first reported by Bietti in 1933 [17]. He reported on the use of cryogenics to pro- duce a thermal chorioretinitis to seal a retinal hole. The technique used a metal probe, pre-cooled in a mixture of carbon dioxide and acetone, with application of the probe to the outer wall of the eye overlying the retinal hole. Two years later, Deutschmann used cryosurgery in the form of solid carbon dioxide probes applied in the same fashion to treat retinal detachments [18]. It was not until 1961 that cryotherapy reemerged within the subspecialty of ophthalmology. Krwawicz developed a metal probe, which he immersed in a mixture of alcohol and solid carbon dioxide for the use of intracapsular cataract ex- traction [19]. Amoils improved cryoextraction techniques by developing a liquid nitrogen probe [20]. Over the next few years, cryoextraction of cataracts was adopted by many ophthalmologists around the world [21]. Advances in treatment of retinal detachments and retinal tears were also made with cryotherapy. Cryore- tinopexy was done with various types of cryoapplica- tors. Bellows and Kelman created retinal cryopexy in- struments for the treatment of retinal tears and for cryo- extraction [22,23]. Schepens, Lincoff, and others were key figures in the advancement of cryoretinopexy surgi- cal techniques and in using cryoretinopexy with scleral buckling for retinal detachments [24-26]. Application of a cryoprobe to the sclera for 5 seconds was shown to create a white area in the underlying retina and seal reti- nal tears and holes [23,25]. In most instances, solid car- bon dioxide was utilized to achieve a temperature of −50˚C to −60˚C. After popularization of cryoretinopexy and cryoex- traction, cryotherapy with different cryogens was used to treat a variety of benign and malignant eye diseases. In the 1950s and 1960s, advances in keratomileusis and lamellar corneal grafting techniques using a cryolathe and carbon dioxide snow, were put forth by Barraquer and Kaufman [27,28]. In 1972, Zacarian published the first series of cases using liquid nitrogen cryosurgery around the eye and orbit for tumors [29]. Fraunfelder and colleagues described cryosurgical treatment of ocular and periocular squamous cell carcinomas in cattle [30-32] and in humans [33,34]. 4. Cryogens Ophthalmologists who do use cryotherapy primarily use freon (boiling point = −29.8˚C to −40.8˚C), nitrous oxide (boiling point = −88.5˚C), or solid carbon dioxide (melt- ing point = −79˚C) rather than liquid nitrogen (boiling point = −195.6˚C) [16]. The boiling point of liquid ni- trogen is by far the lowest of the available cryogens used in medicine, making it the most effective in cell destruc- tion. It is well established in the dermatology literature that a rapid freeze of skin tumors with liquid nitrogen is much more effective than a slow freeze (using alternative cryogens) in eradicating tumor cells [35-40]. This is true in the eye as well, where a number of case series have shown the tumoricidal nature of liquid nitrogen [41-44]. The method of cryotherapy and types of cryogens used in the treatment of eye disease are not standardized within ophthalmology. In addition, the physiochemical and biological effects of the different cryogens on the ocular tissues have not been fully elucidated. Lastly, the lack of familiarity with the use and storage of liquid ni- trogen further limits the exposure of ophthalmologists- in-training to this cryogen. Thus, the practice of oph- thalmic cryotherapy is likely limited by the type of train- ing the ophthalmologist received during the residency and fellowship years. 5. Safety of Cryotherapy Although vertebrate cells and tissue are highly suscepti- ble to the destructive effects of cryotherapy, not all pathogenic organisms are fully destroyed by exposure to liquid nitrogen or other cryogens. In fact, a slow freezing method followed by storage in liquid nitrogen allows for excellent cryopreservation of viruses, microorganisms, and cells. Using a cryoprobe (which is a closed system), Open Access OJOph  Cryotherapy in Ophthalmology 105 involves no risk of contamination as there is no exposure of vapor or sprays arising from the cryogen. However, with an open system such as a liquid nitrogen cryospray, precautions must be taken to prevent contamination from the freezing agent. This is achieved by passing the liquid nitrogen through a cryogenic filter (including the Gelman and Millipore filters) when the liquid nitrogen tank is being filled. In addition, cold filters (including Brymill filters) may be used when transferring liquid nitrogen from the storage tank to handheld cryotherapy units. These filtration methods have consistently been shown to prevent transfer of bacteria, fungi, and molds [24,45,46]. Most complications from ocular cryotherapy with liq- uid nitrogen are related to surgeon inexperience and pro- longed contact of a cryoprobe or cryospray with surface tissue. Many times, a novice cryosurgeon is unable to break the contact of the cryoprobe with the tissue, lead- ing to an over-freeze. Depending on the tissue undergo- ing cryotherapy, the most common complications from cryotherapy include transitory uveitis, temporary chemo- sis, subconjunctival hemorrhage, corneal endothelial da- mage, paralysis of extraocular muscles from cryotherapy over muscle insertion sites, and sector iris atrophy [47]. Rarely, there have been reports of scleral melting after liquid nitrogen cryotherapy [48]. Although the above- mentioned adverse events rarely have long-term conse- quences, cryosurgery with liquid nitrogen is not a benign procedure and requires practical experience. Novice cryosurgeons will need to observe and learn when and how to use cryotherapy to treat surface eye lesions and intraocular diseases prior to performing the procedure themselves. 6. Cellular Effects of Cryotherapy The mechanism of cellular destruction during the freeze phase of cryotherapy is multifactorial and not yet fully elucidated. Some effects are well known, including is- chemia through vascular stasis and the destruction of small caliber blood vessels, ice crystal formation inside cells leading to cell wall rupture, denaturing of lipid- protein complexes, osmotic stress, tissue necrosis, cellu- lar apoptosis after freezing injury, and the buildup of toxic concentrations of solutes inside cells [46,49,50]. The latter mechanism is explained by cell dehydration, which occurs when water is withdrawn to make ice crys- tals intra- and extracellularly. As cryotherapy freezes ex- tracellular fluid, pure water crystals form extracellularly, thus concentrating the remaining extracellular solutes. At the same time the extracellular water is forming ice, the intracellular water is cooling below its freezing point but not forming ice crystals. This is called supercooling. The cell membrane is permeable to supercooled water but not to ice crystals. The supercooled water will tend to flow out of the cell and freeze externally. The net result of this process is cellular dehydration and solute concentra- tion intracellularly, which further destabilizes the cell [51]. The thaw phase of cryotherapy may be just as crucial as the freeze phase in cell destruction. A slow thaw al- lows for longer vascular stasis and longer exposure to toxic solute levels within the cell. The effect is enhanced by repeat freeze-thaw cycles, usually performed 2 - 3 times. The depth of freeze is related to the contact time (the longer the application, the deeper the freeze) [46]. Which of the aforementioned mechanism leads to cell death during cryosurgery depends on the following fac- tors: the cryogen employed (and thus the rate of freeze and the final temperature achieved), the method and length of application, the number of freeze-thaw cycles, the type of cells being frozen, the water content and vas- cularity of the tissue, and the rate of thaw. In regards to the type of cells undergoing cryotherapy, it has been shown that melanocytes are very sensitive to freezing. Hence there is a risk of depigmentation of skin after cu- taneous cryosurgery [46]. Collagen is the most resilient tissue, and cartilage necrosis is extremely rare with cryo- therapy. Thus, cryosurgery is particularly suitable in ar- eas where maintenance of elasticity and structure are important. The ability of a cryogen to freeze depends not only on its boiling point, but also upon the method by which it is applied to biologic tissue. If a thermocouple is placed within a living tissue at a location next to the application of a cryoprobe, a rapid and precipitous temperature fall will be appreciated in the beginning. This will be fol- lowed by a slow fall in the temperature until a point is reached where the temperature equilibrates despite con- tinuous cryogen application. This is because the cryogen acts as a heat sink while the surrounding tissues, reheated by blood vessels, resupply heat. The heat sink essentially loses efficiency with distance so that it is less effective in removing heat from the tissues at increasing distances from the cryoprobe application point. Eventually a point is reached when heat is being renewed as fast as it is be- ing extracted, a steady state condition occurs and the final temperature remains constant [52]. We have simu- lated the above scenario in the lab using non-living ani- mal tissue, a liquid nitrogen cryospray, and a thermocou- ple (Figure 1). Research by Wilkes and Fraunfelder nicely illustrated the salient factors in cellular and clinical ophthalmic cryosurgery [46]. The ability of a cryogen to freeze is dependent on its ability to remove heat, which is deter- mined by its boiling point. The ice ball produced by a cryoprobe becomes warmer as distance from the cryo- probe is increased. The broad categories of in vivo cryo- injury include intracellular and extracellular ice forma- tion and ischemic infarction. A rapid freeze and a slow Open Access OJOph 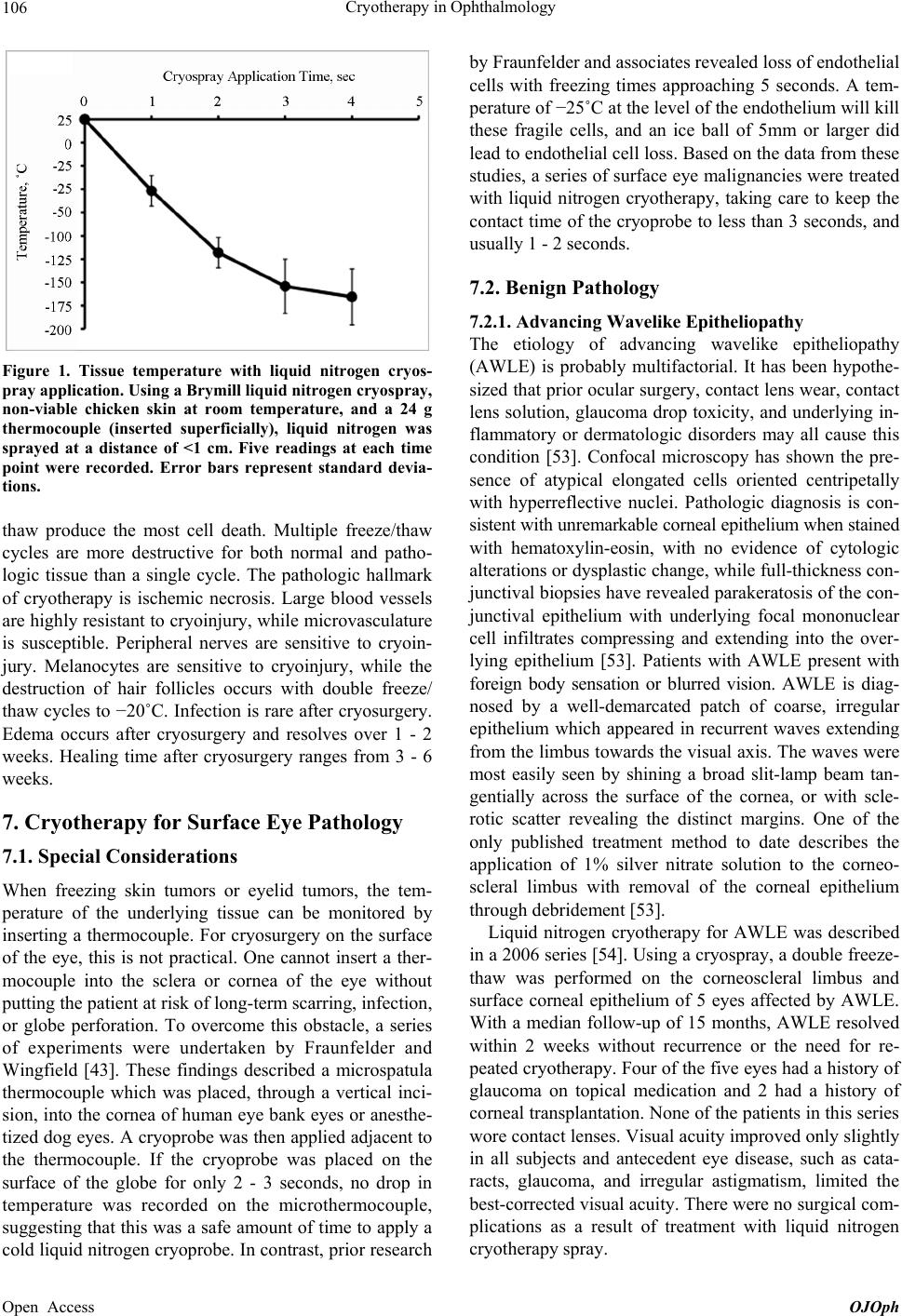 Cryotherapy in Ophthalmology 106 Figure 1. Tissue temperature with liquid nitrogen cryos- pray application. Using a Brymill liquid nitrogen cryospray, non-viable chicken skin at room temperature, and a 24 g thermocouple (inserted superficially), liquid nitrogen was sprayed at a distance of <1 cm. Five readings at each time point were recorded. Error bars represent standard devia- tions. thaw produce the most cell death. Multiple freeze/thaw cycles are more destructive for both normal and patho- logic tissue than a single cycle. The pathologic hallmark of cryotherapy is ischemic necrosis. Large blood vessels are highly resistant to cryoinjury, while microvasculature is susceptible. Peripheral nerves are sensitive to cryoin- jury. Melanocytes are sensitive to cryoinjury, while the destruction of hair follicles occurs with double freeze/ thaw cycles to −20˚C. Infection is rare after cryosurgery. Edema occurs after cryosurgery and resolves over 1 - 2 weeks. Healing time after cryosurgery ranges from 3 - 6 weeks. 7. Cryotherapy for Surface Eye Pathology 7.1. Special Considerations When freezing skin tumors or eyelid tumors, the tem- perature of the underlying tissue can be monitored by inserting a thermocouple. For cryosurgery on the surface of the eye, this is not practical. One cannot insert a ther- mocouple into the sclera or cornea of the eye without putting the patient at risk of long-term scarring, infection, or globe perforation. To overcome this obstacle, a series of experiments were undertaken by Fraunfelder and Wingfield [43]. These findings described a microspatula thermocouple which was placed, through a vertical inci- sion, into the cornea of human eye bank eyes or anesthe- tized dog eyes. A cryoprobe was then applied adjacent to the thermocouple. If the cryoprobe was placed on the surface of the globe for only 2 - 3 seconds, no drop in temperature was recorded on the microthermocouple, suggesting that this was a safe amount of time to apply a cold liquid nitrogen cryoprobe. In contrast, prior research by Fraunfelder and associates revealed loss of endothelial cells with freezing times approaching 5 seconds. A tem- perature of −25˚C at the level of the endothelium will kill these fragile cells, and an ice ball of 5mm or larger did lead to endothelial cell loss. Based on the data from these studies, a series of surface eye malignancies were treated with liquid nitrogen cryotherapy, taking care to keep the contact time of the cryoprobe to less than 3 seconds, and usually 1 - 2 seconds. 7.2. Benign Pathology 7.2.1. Advancing Wavelike E pi theli opathy The etiology of advancing wavelike epitheliopathy (AWLE) is probably multifactorial. It has been hypothe- sized that prior ocular surgery, contact lens wear, contact lens solution, glaucoma drop toxicity, and underlying in- flammatory or dermatologic disorders may all cause this condition [53]. Confocal microscopy has shown the pre- sence of atypical elongated cells oriented centripetally with hyperreflective nuclei. Pathologic diagnosis is con- sistent with unremarkable corneal epithelium when stained with hematoxylin-eosin, with no evidence of cytologic alterations or dysplastic change, while full-thickness con- junctival biopsies have revealed parakeratosis of the con- junctival epithelium with underlying focal mononuclear cell infiltrates compressing and extending into the over- lying epithelium [53]. Patients with AWLE present with foreign body sensation or blurred vision. AWLE is diag- nosed by a well-demarcated patch of coarse, irregular epithelium which appeared in recurrent waves extending from the limbus towards the visual axis. The waves were most easily seen by shining a broad slit-lamp beam tan- gentially across the surface of the cornea, or with scle- rotic scatter revealing the distinct margins. One of the only published treatment method to date describes the application of 1% silver nitrate solution to the corneo- scleral limbus with removal of the corneal epithelium through debridement [53]. Liquid nitrogen cryotherapy for AWLE was described in a 2006 series [54]. Using a cryospray, a double freeze- thaw was performed on the corneoscleral limbus and surface corneal epithelium of 5 eyes affected by AWLE. With a median follow-up of 15 months, AWLE resolved within 2 weeks without recurrence or the need for re- peated cryotherapy. Four of the five eyes had a history of glaucoma on topical medication and 2 had a history of corneal transplantation. None of the patients in this series wore contact lenses. Visual acuity improved only slightly in all subjects and antecedent eye disease, such as cata- racts, glaucoma, and irregular astigmatism, limited the best-corrected visual acuity. There were no surgical com- plications as a result of treatment with liquid nitrogen cryotherapy spray. Open Access OJOph  Cryotherapy in Ophthalmology 107 As the liquid nitrogen spray creates a superficial freeze when applied for less than 1 second, the unwanted cor- neal epithelium can be frozen without damage to the un- derlying corneal stroma or endothelium. In addition, lo- calized cryotherapy spares the non-treated corneal sur- face and limbus, preserving the regenerative potential of the corneal epithelium. Liquid nitrogen cryotherapy, us- ing the spray technique described, appears to be an effec- tive means of eradicating AWLE and may be used as an alternative to silver nitrate 1% solution. 7.2.2. C onjunctival Amyloidosis Conjunctival amyloidosis is rarely associated with sys- temic disease [55,56]. The etiology of primary ocular conjunctival amyloidosis has not been fully elucidated. However, it likely involves production of precursor im- munoglobulin light-chains by a population of benign, localized B-lymphocytes, followed by deposition of amy- loid in the conjunctiva [57]. Patients may present with painless swellings of the conjunctiva or eyelids, pseu- doptosis, or epiphora [58]. Clinically, the conjunctival le- sions appear as small, pink-red or yellow-red nodules which are well-vascularized, and involving the palpebral and forniceal conjunctiva [58]. Histologically, conjunc- tival amyloidosis appears as an acellular, amorphous, eosinophilic material with the characteristic staining with Congo-red of dichroic birefringence. The traditional treat- ment of localized conjunctival amyloidosis has been sur- gical debulking, with repeat debulking surgery should the amyloidosis recur [55,57]. Liquid nitrogen cryotherapy of primary ocular con- junctival amyloidosis was recently reported in a series of 4 patients [59]. Liquid nitrogen cryospray was applied to the lesions, either directly or after excisional biopsy to debulk the amyloid lesions. With a median follow-up of 24.5 months, 2 of the 4 patients had post-treatment re- currence of conjunctival amyloidosis after the first treat- ment, at 14 and 10 months respectively. Conjunctival amyloidosis was eradicated in all subjects after repeat cryotherapy. Surgical debulking prior to cryotherapy may be beneficial in allowing the freezing to reach the deeper blood supply of the lesions. 7.2.3. Conjun cti v al Lym phangiectasia Conjunctival lymphangiectasia is characterized by di- lated and prominent lymphatic channels within the con- junctiva. The condition is usually unilateral, however bi- lateral cases may be seen in Turner syndrome or Nonne- Milroy-Miege disease. Symptoms include ocular irrita- tion, dryness, epiphora, blurred vision, and pain [60]. The terms lymphangiectasia and lymphangioma are used in- terchangeably, and if there is bleeding into the lymph channels, the condition is called hemorrhagic lymphan- giectasia [61]. The etiology of lymphangiectasia remains unknown. Simple excision or marsupialization, or both, have been the traditional therapeutic options for this con- dition. The use of cryosurgery to treat hemorrhagic lym- phangiectasia was first reported in 1975 [62]. A case series of conjunctival lymphangiectasia treated with liq- uid nitrogen cryotherapy was reported in 2009 [63]. Us- ing a cryoprobe, cryotherapy was applied in a double freeze-thaw fashion after an incisional biopsy of a por- tion of the conjunctiva in 4 patients with conjunctival lymphangiectasia. With a mean follow-up of 24.5 months, 2 patients remained lesion free after therapy, while the other 2 patients had recurrence. The average time to re- currence was 18 months after therapy. Repeat cryother- apy led to resolution of recurrent conjunctival lymphan- giectasia in all patients treated. 7.2.4. Conjun cti v al Sarcoi dosis Sarcoidosis is a chronic, multisystem, granulomatous dis- order of uncertain etiology. Organ systems involved in- clude the lungs, skin, lymph nodes, and eyes. Ocular symptoms are present in a significant portion of patients with systemic sarcoidosis, with the most common of these symptoms being uveitis and conjunctival nodules [64]. Biopsy of conjunctival nodules is sometimes per- formed to aid in the diagnosis of sarcoidosis. These nod- ules are typified by the presence of non-infectious, non- caseating granulomas. The nodules can create ocular irri- tation and foreign body sensation. Treatment of these no- dules include lubrication, topical cyclosporine [65], or excision. Liquid nitrogen cryotherapy has recently been shown to treat conjunctival sarcoidosis in a 54-year-old woman [66]. In this case, multiple, bilateral, bi-psy- proven conjunctival sarcoid nodules were treated with liquid nitrogen cryospray. The patient remained free of conjunctival sarcoidosis 6 months after cryotherapy. 7.2.5. Conjunctival Vascular Tumors Vascular tumors of the conjunctiva include capillary he- mangiomas, varices, and hemangiopericytomas. De- pending on the type, management of these tumors typi- cally includes local excision, CO2 laser, cautery, topical corticosteroids, or oral propanolol. Given their inherent vascular nature, these tumors may be especially suscepti- ble to cryotherapy if easily accessible. A case report of a liquid cryotherapy to treat a conjunctival vascular tumor was reported in 2005 [44]. This report was a case of pre- sumptive acquired conjunctival capillary hemangioma as the patient declined an excisional biopsy. Using a cryos- pray method, the lesion underwent liquid nitrogen cryo- therapy, with significant regression of the lesion over 6 weeks. The lesion remained at its minimal size 1 year after therapy, although it had not completely regressed. It remains to be seen if certain vascular tumors would Open Access OJOph  Cryotherapy in Ophthalmology 108 benefit from adjuvant cryotherapy if traditional therapies prove to be ineffective. 7.2.6. Pterygia Pterygia are benign, wing-shaped folds of conjunctival and fibrovascular tissue extending over the limbus and encroaching onto the superficial cornea. Proliferation is thought to arise from activated limbal epithelial stem cells. The etiology of pterygia is unknown, but epidemi- ologic studies have implicated ultraviolet light, exposure to the environment, and chronic irritation as causative factors [67-69] These external factors may disrupt the apoptotic and anti-proliferative signals of epithelial stem cells [70,71]. The current definitive therapy for pterygia is surgical removal. Without additional treatment of the surgical bed after excision, pterygia excision is often complicated by recurrence. Various post-excision, adju- vant treatments have been described in the past, with recurrence rates of 6% with conjunctival autograft, 13% with beta-irradiation, and 29% with mitomycin C com- pared to 53% with excision alone after more than 5 years of follow-up [72]. The use of adjuvant liquid nitrogen cryotherapy of the surgical site after de novo and recurrent pterygia excision was recently described [73]. In this series, after excision of the pterygia, cryotherapy with a cryoprobe was per- formed, with the tip of the cryoprobe in contact with the corneoscleral limbus for approximately 1 second. A dou- ble freeze-thaw technique was used. In the de novo pterygia group (median follow up of 24.5 months), only 1 out of 15 patients had a recurrent pterygium after exci- sion and cryotherapy, resulting in a recurrence rate of 3.3% per year. In the recurrent pterygia group (median follow up of 27 months), 4 out of 6 patients had a recur- rent pterygium after excision and cryotherapy, resulting in a recurrence rate of 29.6% per year. Thus, liquid ni- trogen cryotherapy appears to be an appropriate adjuvant treatment after de novo pterygia excision to minimize recurrence (Figure 2(a) and (b)). However, recurrent ptery- gia have not been shown to be susceptible to adjuvant liquid nitrogen cryotherapy, with high rates of recurrence despite cryotherapy [73]. 7.2.7. Superior Limbic Keratoconjunctivitis The etiology of superior limbic keratoconjunctivitis (SLK) remains uncertain. One proposed mechanism is soft tis- sue microtrauma between the superior palpebral and su- perior bulbar conjunctival surfaces from normal repeti- tive eye blinking in susceptible individuals [74-76]. An- other hypothesis indicated an insufficient local tear sup- ply [77-79]. A multitude of treatments are suggested for SLK, including thermocautery, chemocautery, conjunc- tival resection, punctual occlusion, topical application of autologous serum, topical cyclosporine, topical ketotifen (a) (b) Figure 2. (a) Pterygium before excision and cryotherapy; (b) Appearance of eye 1 year after excision and cryotherapy. fumarate, bandage contact lenses, topical lodoxamide tromethamine, botulinum toxin, and topical vitamin A eyedrops [77-84]. The fact that there are so many treat- ments frequently means that no single treatment is ade- quate, and that the disease may be a result of a combina- tion of factors including dry eyes, mechanical trauma, local inflammation, and the effect of Graves disease on the eyes [85]. SLK typically presents with one or more of the fol- lowing clinical symptoms: unilateral or bilateral ocular burning, foreign body sensation, ocular pain, epiphora, photophobia, blepharospasm, and decreased vision. Some patients have mucus discharge and corneal filaments. Examination findings include superior conjunctival in- flammation with fine punctate staining by rose bengal of the upper cornea, superior limbus, and adjacent conjunc- tiva. Filaments are sometimes present at the superior lim- bus, with some patients exhibiting pseudoptosis. There is usually a fine papillary reaction of the upper eyelid palpebral conjunctiva. A case report of liquid nitrogen cryotherapy for the treatment of SLK refractive to medical therapy was re- ported in 2009 [86]. Four patients underwent liquid ni- Open Access OJOph  Cryotherapy in Ophthalmology 109 trogen cryospray with a double freeze-thaw technique to the superior limbus and inflamed conjunctiva (Figure 3(a) and (b)). Resolution of symptoms and signs oc- curred within 2 weeks in all cases. The SLK recurred in 2 of 4 patients with a median follow-up of 10 months. Re- currences were treated with another round of liquid ni- trogen cryotherapy (mean time to recurrence 3.6 months), while no eyes required a third treatment. Liquid nitrogen cryotherapy of SLK may act by removing the redundant superior conjunctiva by causing a scar to form between the superior bulbar conjunctiva and the underlying Te- non’s capsule and sclera [86]. It remains to be seen if the effects of cryotherapy on SLK are permanent. 7.2.8. Trichiasis Trichiasis is a misdirection of eyelashes that is acquired. In-turning if eyelashes and their subsequent contact with the cornea and conjunctiva leads to foreign body sensa- tion by the patient. Treatment modalities for trichiasis include manual epilation, electrolysis, cryotherapy, argon (a) (b) Figure 3. (a) Superior limbic keratoconjunctivitis (SLK); (b) Appearance of superior limbus 6 months after cryotherapy for SLK. laser, radiofrequency epilation and surgery. Cryotherapy for trichiasis was first reported in 1997, using a nitrous oxide cryoprobe [87]. In this series, local treatment of eyelids using a double freeze-thaw tech- nique and a microthermocouple achieved a low tempera- ture of 20˚C [87]. The use of liquid nitrogen for trichiasis cryotherapy was reported soon after, with success in greater than 90% of patients [88]. Subsequent use of cryotherapy to treat trichiasis in the setting of trachoma [89] and ocular cicatricial pemphigoid [90] has been re- ported. While highly successful, cryotherapy is not rec- ommended for trichiasis in the setting of paralytic lids, heavily pigmented patients, or trichiasis involving less than one-third of the eyelid margin [88]. 7.2.9. V er nal Kera to conjun ct ivitis Vernal keratoconjunctivitis (VKC) is an immune-medi- ated disorder affecting the ocular surface, with a combi- nation of environmental triggers and a pro-inflammatory state contributing to the pathogenesis. Clinical findings include the presence of upper tarsal giant papillae, cor- neal shield ulcers, or gelatinous limbal infiltrates (Horner- Trantas dots). The giant papillary changes in VKC are collections of neutrophils, eosinophils, lymphocytes, and other leukocytes surrounding a central vascular core [91]. Patients with VKC usually present with symptoms of ocular itching, tearing, mucus secretion, and photopho- bia. Cryotherapy for VKC has been reported by several groups, with the first report appearing in 1982 [92]. Singh reported a 22% recurrence rate with short-term follow up using a carbon dioxide cryogen [92]. Abiose and Merz, also using a carbon dioxide cryogen, had a 50% recurrence of giant papillae at just 4 weeks post treatment [93]. Mtanda and Sangawe studied 34 eyes with VKC treated with a carbon dioxide cryogen with disease recurrence in 2 eyes at 5 months [94]. Sank- arkumar et al. studied 30 eyes of 15 patients with VKC, who underwent treatment a carbon dioxide cryogen. Re- ported recurrence was only 3.3% at one year [95]. Jiang et al. combined resection, cryotherapy, and amniotic mem- brane transplantation for the treatment of VKC [96]. In this study of 16 eyes (follow-up ranging between 3 - 22 months), fourteen eyes (87.5%) were symptom-free 1 month after surgery with no evidence of VKC on exam. Recurrence of VKC was observed in 2 eyes (12.5%) after cryotherapy. Liquid nitrogen cryotherapy (using a cryoprobe) for VKC in 3 eyes was reported in 2008 [58]. The median follow-up was 24 months. Although clinical symptoms and visual acuity improved 1 month after therapy, giant papillae recurrence was noted after 1 month. Recurrent VKC was noted as early as 9 months after therapy. The median time to recurrence was 12 months. Open Access OJOph  Cryotherapy in Ophthalmology 110 Although cryotherapy may kill the central vascular core of giant papillae early on (resulting in some positive results after therapy), the high rate of recurrence may make cryosurgery an ineffective therapy for VKC. In addition, given the environmental and systemic factors that contribute to VKC, recurrence is likely in the ab- sence of continued anti-inflammatory and immunosup- pressive therapy. 7.3. Pre-Malignancy and Malignancy 7.3.1. Primar y Acqui red Melanosis and Mel anoma of the Conjunctiva Primary acquired melanosis (PAM) of the conjunctiva is a pre-malignant transformation of melanocytic cells. PAM occurs in up to 35% of adult Caucasians [97], while cases have been reported in highly pigmented indi- viduals [98]. The presentation of PAM is usually unilat- eral, although bilateral cases have been reported [98]. The majority of PAM conjunctival lesions is painless and asymptomatic, and may involve the cornea. However, a small portion of PAM lesions may give rise to conjunc- tival melanoma. In a large series of over 300 eyes with PAM, it was estimated that 8% of PAM cases evolved into melanoma at 5 years, 12% at 10 years, and 21% at 15 years [98]. The mainstay of PAM management includes observa- tion for malignant behavior (i.e. enlargement). Lesions with high suspicion for malignant transformation may be treated surgically, including excisional biopsy followed by intra-operative double freeze-thaw cryotherapy. Cryo- therapy for the treatment of PAM was first described in the early 1980’s [99]. Excision and cryotherapy of PAM has proven to be an effective treatment [100], although recurrences are not unusual. In a recent report of over 100 cases of PAM treated with excision and cryotherapy, and at least 3 years follow-up, the reported rate of PAM recurrence was 27%, with 3% progressing to melanoma [98]. Conjunctival melanoma can arise de novo, from a preexisting nevus, or from PAM. By far, PAM is the most common precursor to conjunctival melanoma, with up to 75% of conjunctival melanomas arising in associa- tion with PAM [101]. As in PAM, conjunctival mela- noma is often seen in lightly pigmented individuals. Con- junctival melanomas typically present as pigmented, fleshy, raised lesions, and may exhibit a prominent feeder vessel (Figure 4(a)). Malignant conjunctival melanoma metastasize to regional lymph nodes and other organs, including brain. Treatment of conjunctival melanoma varies according to the extent and location of involvement [100]. Adjuvant cryotherapy for conjunctival melanoma was described as early as 1980 [102,103]. Melanomas involving the bulbar conjunctiva and cornea may be treated by alcohol epithe- liectomy and partial scelroconjunctivectomy using the “no-touch” technique, followed by intra-operative double freeze-thaw cryotherapy (Figure 4(b) and (c)). In addi- tion to the above, melanomas involving the forniceal conjunctiva may require larger excision and mucous/ amniotic membrane grafts. Conjunctival melanotic le- (a) (b) (c) Figure 4. (a) Paralimbalconjunctival melanoma; (b) Para- limbal bed after removal of a conjunctival melanoma. Note the liquid nitrogen cryoprobe prior to application to the paralimbal region; (c) Paralimbal bed in (c) immediately after application of the liquid nitrogen cryoprobe. Note the frozen ice ball on the surface on the eye. Open Access OJOph  Cryotherapy in Ophthalmology 111 sions that extent into and beyond the globe may require enucleation and exenteration. Metastatic melanomas re- quire systemic therapy. 7.3.2. Conjunctival Intra epithelial Neopl asia and Squamous Cell Carcinoma Conjunctival intraepithelial neoplasia (CIN) is a local- ized squamous cell neoplasm that is minimally aggres- sive and confined to the surface epithelium. Classifica- tion and grading of CIN is based on the depth of in- volvement of dysplastic cells: mild CIN involves ~1/3 of the epithelial depth, moderate CIN involves ~1/2 of the epithelial depth, and severe CIN (previously called car- cinoma in situ) involves full thickness epithelium without invasion beyond the epithelial basement membrane. If the basement membrane is compromised and invaded by the abnormal cells, then the lesion has progressed to squamous cell carcinoma. Conjunctival intraepithelial neoplasia and squamous cell carcinoma occur more frequently in immunocom- promised individuals (including individuals with ac- quired immunodeficiency syndrome). Other possible risk factors include sun exposure and human papillomavirus infection [104]. Clinically, CIN and squamous cell car- cinoma may present as fleshy, elevated lesion at the lim- bus. Adjacent corneal epithelial involvement is not un- common. Although squamous cell carcinoma is locally invasive, it rarely metastasizes. Adjuvant cryotherapy for CIN and squamous cell car- cinoma was first proposed in 1977 [34]. Early studies using excision followed by nitrous oxide cryoprobes showed relatively good results with a 9% recurrence rate with at least 5 years of follow up [105]. More recently, an optimized technique for excision and cryotherapy has been described (similar to conjunctival PAM and mela- noma excision and cryotherapy), which includes treat- ment of the lesion by alcohol epitheliectomy and partial scelroconjunctivectomy using the “no-touch” technique, followed by intra-operative double freeze-thaw cryothe- rapy [100]. In a study of 60 patients with CIN and con- junctival squamous cell carcinoma treated with excision and cryotherapy, after a mean follow up of 56 months, the rate of recurrence was 4.5% for CIN and 5.3% for squamous cell carcinoma [106]. 7.3.3. Reactiv e Lymphoid Hyperpl as ia and Conjunctival Lymphoma Reactive lymphoid hyperplasia (RLH) is a benign prolif- eration of lymphoid tissue in the ocular adnexa, which may present unilaterally or bilaterally. Histologically, RLH lesions appear as polymorphic proliferations of small lymphocytes and intermixed plasma cells, immu- noblasts, and histiocytes. Conjunctival RLH is usually asymptomatic, with patients seeking medical attention because of cosmetic appearance or the fear of an uncer- tain diagnosis. The forniceal, bulbar, and palpebral con- junctivae can be affected, with a predilection for the for- nices. RLH lesions have a potential for malignant trans- formation and systemic lymphoma, necessitating an ex- cisional biopsy to rule out malignancy. Traditionally, conjunctival RLH has been treated with excision, while adjuvant radiation was reserved for biopsy-proven ma- lignant lesions. Cryotherapy for reactive lymphoid hyperplasia of the conjunctiva was first described in 1977 [33,34]. Eichler and Fraunfelder reported a retrospective case series of various conjunctival lymphoid tumors (including RLH) treated by liquid nitrogen cryospray [41]. With a mean follow up of 75 months, 5 biopsy-proven lymphoid hy- perplastic lesions were treated. Four out of five lesions were locally eradicated after only 1 treatment, while the fifth lesion was eradicated after repeat treatment. Thus, it appears that cryotherapy is a viable option in treating RLH. Conjunctival lymphoma is on the same spectrum as RLH, with the former being malignant. The great major- ity of conjunctival lymphomas are B-cell in origin, with T-cell lymphomas presenting in extremely rare cases [107]. Most conjunctival lymphomas are localized and not associated with systemic disease at presentation. However, systemic involvement may occur over time. Clinically, conjunctival lymphomas present in a similar fashion to RLH and may appear as a salmon pink, smooth, and soft lesion. Histopathologically, while RLH demonstrates a polycolonal expansion of lymphoid cells, malignant lymphoma is characterized by a diffuse sheet of monotonous, small, round lymphocytes, and may ex- hibit nuclear and mitotic features suggestive of higher malignant potential. Diagnosis and treatment of localized conjunctival lym- phoma includes excisional biopsy and adjuvant therapy, which has included external beam radiation, brachyther- apy, cryotherapy, intralesional interferon injections, and systemic rituximab [108]. Cryotherapy offers an advan- tage over radiation therapy in that there is very little risk to surrounding tissue. In addition, cryotherapy avoids possible local and systemic side-effects of interferon and rituximab administration, respectively. Cryotherapy for conjunctival lymphoma was first pro- posed in 1977 [34]. Subsequently, in a series of 37 le- sions of biopsy-proven conjunctival lymphomas treated with excisional biopsy and liquid nitrogen cryotherapy, the authors reported local eradication in 65% (24/37), 92% (34/37), and 97% (36/37) of lesions after the first, second, and third cryotherapy treatment, respectively (average follow up time of 75 months) [41]. Most re- cently, a rare case of isolated T-cell conjunctival lym- phoma in a 57-year-old female was treated with local Open Access OJOph  Cryotherapy in Ophthalmology 112 excision and triple-freeze cryotherapy, resulting in local eradication without systemic involvement after 24 months of follow up [107]. 8. Cryotherapy for Intraocular Pathology 8.1. Retinal Tears and Detachments Retinal tears and detachments arise for physical separa- tion of the neurosensory retina and the underlying retinal pigment epithelium (RPE). Retinal tears may arise from trauma or traction from the overlying vitreous. Retinal tears may then allow fluid influx between the neurosen- sory retina and the RPE, causing further extension of the separation and leading to a retinal detachment. Retinal detachments arising from retinal tears are referred to as rhegmatogenous retinal detachments. Retinal detachments arising from a tractional component without a tear are called tractional retinal detachments. Lastly, retinal de- tachments arising from a collection of exudate between the neurosensory retina and the RPE are called exudative retinal detachments. The treatment of retinal tears and detachments are multiple and depend on the patient, the size and location of the lesion, as well as surgeon preference. Prophylactic treatment of retinal tears without detachment may in- clude cryotherapy (Figure 5(a) and (b)) and/or laser therapy just outside of the tear to wall off the break. The goal of retinal detachment repair includes bringing the retina and RPE into contact to allow for reattachment and may include pneumatic retinopexy or pars plana vitrec- tomy with scleral buckle or silicone oil. In certain set- tings, further chorioretinal scarring, using cryotherapy or laser therapy, is used to adhere the detached retina to avoid repeat detachment. The early history of cryotherapy use in retinal tear and detachment repair is described in detail in Section III. Briefly, cryotherapy of the eye was first reported by Bi- etti in 1933 to seal a retinal hole [17]. Shortly after, Deutschmann used cryosurgery to treat retinal detach- ments [18]. Bellows and Kelman created retinal cryo- pexy instruments for the treatment of retinal tears [22, 109]. Cryoretinopexy in conjunction with scleral buck- ling for retinal detachments was also described [24-26]. Yanoff reported results from a series of 100 eyes treated with transconjunctival cryotherapy to seal pe- ripheral retinal breaks, with only 3 eyes developing reti- nal detachments and requiring additional procedures [110]. Wolfensberger and colleagues reported excellent results using prophylactic 360 degree peripheral retinal cryotherapy of fellow eyes after contralateral giant retinal tears [111]. Although generally safe, retinal cryopexy has been reported to cause cystoid macular edema [112], likely secondary to transient breakdown of the blood-re- tina barrier [113]. In addition, cases of retinal necrosis (a) (b) Figure 5. (a) Multiple retinal breaks; (b) Chorioretinal scarring after application of external cryotherapy to areas around retinal breaks in (a). with overtreatment using cryotherapy have been reported [114]. The safety and efficacy profile of cryotherapy for reti- nal tear repair is comparable to other techniques. In a recent randomized clinical trial of patients undergoing repair of rhegmatogenous retinal detachments with either intraoperative cryotherapy or postoperative (1 month later) laser retinopexy, the authors found that reattachment and postoperative complication rates were similar in both groups [115]. Although the study found that visual re- covery was faster in the retinopexy group, the difference in visual acuity after 6 months was not significant. In addition, cryotherapy intraoperatively during scleral buckl- ing procedure provided the advantage of one intervention with lower costs. 8.2. Retinopathy of Prematurity Retinopathy of prematurity (ROP, also called retrolental fibroplasia) is an ischemic retinopathy of premature and low birth weight infants. The incidence of ROP in the United States is about 1300 newborn children annually, Open Access OJOph  Cryotherapy in Ophthalmology 113 with severe visual impairment in a large percentage. The risk of ROP is inversely proportional to birth weight, with 37% of infants weighing less than 750 grams de- veloping severe ROP. The diagnosis of ROP is rarely made in infants with birth weights of greater than 2000 grams. The Preferred Practice Patterns of the American Academy of Ophthalmology recommends screening for newborns with less than 30 weeks of gestation and/or a birth weight of less than 1500 grams. The development of retinal vasculature begins during week 16 of gestation and can progress to the final weeks of gestation. Premature birth, in conjunction with subse- quent iatrogenic oxygen supplementation, halts and alters normal retinal vasculature development, leading to the onset of ROP and abnormal neovascularization. Severe ROP can progress to fibroglial proliferation, vitreoretinal traction, and retinal detachment. Hindle and Leyton reported the first use of cryother- apy to prevent progression of ROP in 1978 [116]. This revolutionized the treatment of ROP, as prior treatments were limited. In this technique, trans-scleral cryotherapy is used to ablate areas of avascular retina and thereby prevent further neovascularization. In 1988, the first multicenter randomized trial of cryotherapy for treatment of ROP (the CRYO-ROP study) was reported [117]. Three month [117] and 12 month [118] data from this trial were promising, with cryotherapy reducing unfa- vorable outcomes by half. Five-year data from the CRYO-ROP study supported the safety and efficacy of cryotherapy treatment of ROP [119]. Ten-year data from the CRYO-ROP study showed that 44.4% of treated eyes (versus 62.1% of untreated eyes) were legally blind, showing long-term value from cryotherapy in preserving visual acuity in eyes with ROP [120]. More recently, laser therapy has become the standard of care for ROP management, while anti-vascular endothelial growth fac- tor (anti-VEGF) therapy has shown promise as an addi- tional modality for the treatment of ROP [121]. 8.3. Retinal Capillary Hemangiomas Retinal capillary hemangiomas (RCHs) are benign vas- cular tumors that may occur sporadically (sometimes referred to as von Hippel lesions) or in the setting of von Hippel-Lindau (VHL) disease. In the setting of VHL dis- ease, RCHs are usually detected in the second or third decade of life, and seen in up to 60% of patients with VHL disease [122]. In a retrospective case series of 68 patients with RCHs, it was found that 46% of patients had VHL disease [123]. The majority of patients with RCHs have no significant vision loss from the lesion [124], likely due to the predominately superotemporal and midperipheral retinal location of most RCHs [123]. Treatment of RCHs is based on size, location, associ- ate edema, and effects of visual acuity. The majority of RCHs may be safely observed [125]. Singh et al. re- ported that 82% (68 total patients) of observed RCH le- sions remained stable with a mean follow up of 84 months [125]. For RCHs requiring treatment, laser pho- tocoagulation and cryotherapy have been shown to be equally effective in controlling the growth of the lesion, as well as the associated retinal edema. In the same study by Singh and colleagues, 74% of treated RCHs remained controlled with one session of laser photocoagulation or cryotherapy [125]. 9. Summary and Conclusion Cryotherapy in ophthalmology has a rich history and continues to be an important supplement in the treatment of ophthalmic pathology. The use of cryotherapy in oph- thalmology has helped advance maturing fields (such as cataract extraction), while in other instances revolution- ized patient care (including retinopathy of prematurity and ocular surface malignancies). Further applications of cryotherapy in eye disease continue to emerge. 10. Acknowledgements We’d like to thank Dr. Robert Watzke for his contribu- tion of photos documenting the use of cryotherapy for retinal tear repair. REFERENCES [1] R. R. Lubritz, “Cryosurgery,” Clinics in Dermatology, Vol. 5, No. 4, 1987, pp. 120-127. http://dx.doi.org/10.1016/0738-081X(87)90033-2 [2] S. M. Cooper and R. P. Dawber, “The History of Cryo- surgery,” Journal of the Royal Society of Medicine, Vol. 94, No. 4, 2001, pp. 196-201. [3] A. Freiman and N. Bouganim, “History of Cryotherapy,” Dermatology Online Journal, Vol. 11, No. 2, 2005, p. 9. [4] A. Squazzi and D. Bracco, “A Historical Account of the Technical Means Used in Cryotherapy,” Minerva Medica, Vol. 65, No. 70, 1974, pp. 3718-3722. [5] A. Brock, “Greek Medicine, Being Extracts Illustrative of Medical Writers from Hippocrates to Galen,” 2nd Edition, Dutton, New York, 1972. [6] J. Arnott, “On the Treatment of Cancer by the Regulated Application of an Anaesthetic Temperature,” Churchill, London, 1851. [7] A. White, “Liquid Air: Its Application in Medicine and Surgery,” Medication Reconciliation, Vol. 56, 1899, pp. 109-112. [8] A. White, “Possibilities of Liquid Air to the Physcian,” JAMA, Vol. 36, No. 7, 1901, pp. 426-429. http://dx.doi.org/10.1001/jama.1901.52470070012001d [9] H. G. Irvine and D. D. Turnacliff, “Liquid Oxygen in Dermatology,” Arch Dermatol Syphilol, Vol. 19, No. 2, 1929, pp. 270-280. http://dx.doi.org/10.1001/archderm.1929.02380200098007 Open Access OJOph  Cryotherapy in Ophthalmology 114 [10] H. Allington, “Liquid Nitrogen in the Treatment of Skin Disease,” California Medicine, Vol. 72, No. 3, 1950, p. 153. [11] I. S. Cooper and T. Hirose, “Application of Cryogenic Surgery to Resection of Parenchymal Organs,” The New England Journal of Medicine, Vol. 274, No. 1, 1966, pp. 15-18. http://dx.doi.org/10.1056/NEJM196601062740103 [12] S. Zacarian, “Cryogenics: The Cryolesion and the Patho- genesis of Cryonecrosis, in Cryosurgery for Skin Cancer and Cutaneous Disorders,” St. Louis, Mosby, 1985, pp. 1-30. [13] P. Lepivert, “Predictability of Cryonecrosis by Tissue Impedancemetry,” Low Temperature Medicine, Vol. 4, 1977, p. 129. [14] S. A. Zacarian, “Cryosurgery of Cutaneous Carcinomas. An 18-Year Study of 3,022 Patients with 4,228 Carcino- mas,” Journal of the American Academy of Dermatology, Vol. 9, No. 6, 1983, pp. 947-956. http://dx.doi.org/10.1016/S0190-9622(83)70213-6 [15] D. Bracco, “The Historic Development of Cryosurgery,” Clinics in Dermatology, Vol. 8, No. 1, 1990, pp. 1-4. http://dx.doi.org/10.1016/0738-081X(90)90061-5 [16] G. F. Graham, “Cryosurgery,” Clinics in Plastic Surgery, Vol. 20, No. 1, 1993, pp. 131-147. [17] G. Bietti, “Ricerche Sulle Variazione di Temperature di Alcune Zone del Bulbo Oculaire per Diatermocoagulax- ioni Episclerali, Termocauterizazioni e Criocausticazi- oni,” Bollettino d’Oculistica, Vol. 12, 1933, pp. 1427- 1457. [18] R. Deutschmann, “Die Behandlung der Netzhautablosung mit Jodtinkur und Kohlensaureschnee,” Klin Montasbl Augenh, Vol. 94, 1935, p. 349. [19] T. Krwawicz, “Intracapsular Extraction of Intumescent Cataract by Application of Low Temperature,” The Brit- ish Journal of Ophthalmology, Vol. 45, No. 4, 1961, pp. 279-283. http://dx.doi.org/10.1136/bjo.45.4.279 [20] S. P. Amoils, “The Joule Thomson Cryoprobe,” Archives of Ophthalmology, Vol. 78, No. 2, 1967, pp. 201-207. http://dx.doi.org/10.1001/archopht.1967.00980030203014 [21] J. G. Bellows, “Indications and Technique of Cryoextrac- tion of Cataracts,” Archives of Ophthalmology, Vol. 73, No. 4, 1965, pp. 476-481. http://dx.doi.org/10.1001/archopht.1965.00970030478006 [22] J. G. Bellows, “The Application of Cryogenic Techniques in Ophthalmology,” American Journal of Ophthalmology, Vol. 57, 1964, pp. 29-33. [23] C. D. Kelman, “Cryosurgery for Cataract Extracction and the Treatment of Other Eye Diseases,” Highlights Oph- thalmol, Vol. 7, 1964, pp. 181-209. [24] J. G. Bellows, “Cryotherapy of Ocular Disease,” J.B. Lippincott Co., Philadelphia, 1966. [25] H. A. Lincoff, J. M. McLean and H. Nano, “Cryosurgical Treatment of Retinal Detachment. Transactions,” Ameri- can Academy of Ophthalmology and Otolaryngology, Vol. 68, 1964, pp. 412-432. [26] C. L. Schepens, I. D. Okamura and R. J. Brockhurst, “The Scleral Buckling Procedures. I. Surgical Techniques and Management,” Archives of Ophthalmology, Vol. 58, No. 6, 1957, pp. 797-811. http://dx.doi.org/10.1001/archopht.1957.00940010819003 [27] J. I. Barraquer, “Method for Cutting Lamellar Grafts in Frozen Cornea, New Orientation for Refractive Surgery,” Arch Soc Am Ophthal Optom, Vol. 1, 1958, pp. 271-286. [28] Herbert E. Kaufman, “Preserving Corneas by Freezing,” Archives of Ophthalmology, Vol. 73, No. 6, 1965, pp. 907-908. http://dx.doi.org/10.1001/archopht.1965.00970030909029 [29] S. A. Zacarian, “Cancer of the Eyelid—A Cryosurgical Approach,” Annals of Ophthalmology, Vol. 4, No. 6, 1972, pp. 473-480. [30] H. E. Farris and F. T. Fraunfelder, “Cryosurgical Treat- ment of Ocular Squamous Cell Carcinoma of Cattle,” Journal of the American Veterinary Medical Association, Vol. 168, No. 3, 1976, pp. 213-216. [31] H. E. Farris, F. T. Fraunfelder and C. H. Frith, “A Simple Cryosurgical Unit for Treatment of Animal Tumors,” Veterinary Medicine, Small Animal Clinician, Vol. 70, No. 3, 1975, pp. 299-302. [32] F. T. Fraunfelder, H. Howard and M. L. Ray, “New Therapy Method for Cancer Eye in Cattle,” Southern Beef Producer, Vol. 5, 1974. [33] F. T. Fraunfelder, et al., “The Role of Cryosurgery in External Ocular and Periocular Disease,” Transactions. Section on Ophthalmology. American Academy of Oph- thalmology and Otolaryngology, Vol. 83, No. 4, 1977, pp. 713-724. [34] F. T. Fraunfelder, H. E. Farris Jr. and T. R. Wallace, “Cryosurgery for Ocular and Periocular Lesions,” The Journal of Dermatologic Surgery and Oncology, Vol. 3, No. 4, 1977, pp. 422-427. [35] F. T. Fraunfelder, et al., “Results of Cryotherapy for Eye- lid Malignancies,” American Journal of Ophthalmology, Vol. 97, No. 2, 1984, pp. 184-188. [36] E. G. Kuflik and A. A. Gage, “The Five-Year Cure Rate Achieved by Cryosurgery for Skin Cancer,” Journal of the American Academy of Dermatology, Vol. 24, No. 6, 1991, pp. 1002-1004. http://dx.doi.org/10.1016/0190-9622(91)70160-4 [37] P. J. Holt, “Cryotherapy for Skin Cancer: Results over a 5-Year Period Using Liquid Nitrogen Spray Cryosur- gery,” The British Journal of Dermatology, Vol. 119, No. 2, 1988, pp. 231-240. http://dx.doi.org/10.1111/j.1365-2133.1988.tb03205.x [38] E. G. Kuflik and A. A. Gage, “Recurrent Basal Cell Car- cinoma Treated with Cryosurgery,” Journal of the Ameri- can Academy of Dermatology, Vol. 37, No. 1, 1997, pp. 82-84. http://dx.doi.org/10.1016/S0190-9622(97)70215-9 [39] F. Jaramillo-Ayerbe, “Cryosurgery in Difficult to Treat Basal Cell Carcinoma,” International Journal of Derma- tology, Vol. 39, No. 3, 2000, pp. 223-229. http://dx.doi.org/10.1046/j.1365-4362.2000.00952.x [40] F. T. Fraunfelder, et al., “Cryosurgery for Malignancies of the Eyelid,” Ophthalmology, Vol. 87, No. 6, 1980, pp. 461-465. [41] M. D. Eichler and F. T. Fraunfelder, “Cryotherapy for Open Access OJOph  Cryotherapy in Ophthalmology 115 Conjunctival Lymphoid Tumors,” American Journal of Ophthalmology, Vol. 118, No. 4, 1994, pp. 463-467. [42] F. A. Jakobiec, et al., “Cryotherapy for Conjunctival Pri- mary Acquired Melanosis and Malignant Melanoma. Ex- perience with 62 Cases,” Ophthalmology, Vol. 95, No. 8, 1988, pp. 1058-1070. [43] F. T. Fraunfelder and D. Wingfield, “Management of Intraepithelial Conjunctival Tumors and Squamous Cell Carcinomas,” American Journal of Ophthalmology, Vol. 95, No. 3, 1983, pp. 359-363. [44] F. W. Fraunfelder and F. T. Fraunfelder, “Liquid Nitro- gen Cryotherapy of a Conjunctival Vascular Tumor,” Cornea, Vol. 24, No. 1, 2005, pp. 116-117. http://dx.doi.org/10.1097/01.ico.0000134185.55310.1d [45] C. D. Kelman and I. S. Cooper, “Cryogenic Ophthalmic Surgery,” American Journal of Ophthalmology, Vol. 56, 1963, pp. 731-739. [46] T. D. Wilkes and F. T. Fraunfelder, “Principles of Cryo- surgery,” Ophthalmic Surgery, Vol. 10, No. 8, 1979, pp. 21-30. [47] D. L. Wingfield and F. T. Fraunfelder, “Possible Com- plications Secondary to Cryotherapy,” Ophthalmic Sur- gery, Vol. 10, No. 8, 1979, pp. 47-55. [48] S. M. Tucker, et al., “Scleral Melt after Cryotherapy for Conjunctival Melanoma,” Ophthalmology, Vol. 100, No. 4, 1993, pp. 574-577. [49] J. H. Sullivan, “Cryosurgery in Ophthalmic Practice,” Ophthalmic Surgery, Vol. 10, No. 8, 1979, pp. 37-41. [50] W.-L. Yang, T. Addona, D. G. Nair, L. X. Qi and T. S. Ravikumar, “Apoptosis Induced by Cryoinjury in Human Colorectal Cancer Cells Is Associated with Mitochondrial Dysfunction,” International Journal of Cancer, Vol. 103, No. 3, 2003, pp. 360-369. http://dx.doi.org/10.1002/ijc.10822 [51] H. T. Mergman, “Mechanics of Freezing in Living Cells and Tissues,” Science, Vol. 124, 1956, pp. 15-30. [52] D. Torre, “Cryosurgical Instrumentation and Depth Dose Monitoring,” In: E. D.-S. Breitbart, Ed., Clinics in Der- matology: Advances in Cryosurgery, Elsevier, New York, 1990, p. 48. [53] G. D’Aversa, et al., “Advancing Wave-Like Epitheliopa- thy. Clinical Features and Treatment,” Ophthalmology, Vol. 104, No. 6, 1997, pp. 962-969. http://dx.doi.org/10.1016/S0161-6420(97)30199-7 [54] F. W. Fraunfelder, “Liquid Nitrogen Cryotherapy of Ad- vancing Wavelike Epitheliopathy,” Cornea, Vol. 25, No. 2, 2006, pp. 196-198. http://dx.doi.org/10.1097/01.ico.0000170691.67584.ec [55] I. Leibovitch, et al., “Periocular and Orbital Amyloidosis: Clinical Characteristics, Management, and Outcome,” Oph- thalmology, Vol. 113, No. 9, 2006, pp. 1657-1664. http://dx.doi.org/10.1016/j.ophtha.2006.03.052 [56] H. Demirci, et al., “Conjunctival Amyloidosis: Report of Six Cases and Review of the Literature,” Survey of Oph- thalmology, Vol. 51, No. 4, 2006, pp. 419-433. http://dx.doi.org/10.1016/j.survophthal.2006.04.007 [57] M. B. Pepys, “Amyloidosis,” Annual Review of Medicine, Vol. 57, 2006, pp. 223-241. http://dx.doi.org/10.1146/annurev.med.57.121304.131243 [58] F. W. Fraunfelder, “Liquid Nitrogen Cryotherapy for Surface Eye Disease (an AOS Thesis),” Transactions of the American Ophthalmological Society, Vol. 106, 2008, pp. 301-324. [59] F. W. Fraunfelder, “Liquid Nitrogen Cryotherapy for Conjunctival Amyloidosis,” Archives of Ophthalmology, Vol. 127, No. 5, 2009, pp. 645-648. http://dx.doi.org/10.1001/archophthalmol.2008.535 [60] H. D. Perry and A. J. Cossari, “Chronic Lymphangiecta- sis in Turner’s Syndrome,” The British Journal of Oph- thalmology, Vol. 70, No. 5, 1986, pp. 396-399. http://dx.doi.org/10.1136/bjo.70.5.396 [61] L. M. Jampol and K. C. Nagpal, “Hemorrhagic Lym- phangiectasia of the Conjunctiva,” American Journal of Ophthalmology, Vol. 85, 1978, pp. 419-420. [62] I. A. Wasfy, “Lymphangiectasia Haemorrhagica Con- junctivae: Report of Three Cases with a Note on Suc- cessful Treatment with Cryosurgery in One Case,” Bulle- tin of the Ophthalmological Society of Egypt, Vol. 68, 1975, pp. 37-44. [63] F. W. Fraunfelder, “Liquid Nitrogen Cryotherapy of Con- junctival Lymphangiectasia: A Case Series,” Archives of Ophthalmology, Vol. 127, No. 12, 2009, pp. 1686-1687. http://dx.doi.org/10.1001/archophthalmol.2009.306 [64] A. Rothova, “Ocular Involvement in Sarcoidosis,” The British Journal of Ophthalmology, Vol. 84, No. 1, 2000, pp. 110-116. http://dx.doi.org/10.1136/bjo.84.1.110 [65] J. Y. Oh and W. R. Wee, “Cyclosporine for Conjunctival Sarcoidosis,” Ophthalmology, Vol. 115, No. 1, 2008, p. 222. http://dx.doi.org/10.1016/j.ophtha.2007.08.024 [66] F. W. Fraunfelder and D. S. Dhoot, “Successful Treat- ment of Conjunctival Sarcoidosis with Local Cryother- apy,” Ophthalmic Surgery, Lasers & Imaging: The Offi- cial Journal of the International Society for Imaging in the Eye, 2010, pp. 1-4. [67] I. J. Wang, et al., “Mechanism of Abnormal Elastin Gene Expression in the Pinguecular Part of Pterygia,” The American Journal of Pathology, Vol. 157, No. 4, 2000, pp. 1269-1276. http://dx.doi.org/10.1016/S0002-9440(10)64642-1 [68] M. T. Coroneo, N. Di Girolamo and D. Wakefield, “The Pathogenesis of Pterygia,” Current Opinion in Ophthal- mology, Vol. 10, No. 4, 1999, pp. 282-288. http://dx.doi.org/10.1097/00055735-199908000-00011 [69] T. M. Nolan, et al., “The Role of Ultraviolet Irradiation and Heparin-Binding Epidermal Growth Factor-Like Growth Factor in the Pathogenesis of Pterygium,” The American Journal of Pathology, Vol. 162, No. 2, 2003, pp. 567-574. http://dx.doi.org/10.1016/S0002-9440(10)63850-3 [70] M. Gebhardt, et al., “Differential Expression of Vascular Endothelial Growth Factor Implies the Limbal Origin of Pterygia,” Ophthalmology , Vol. 112, No. 6, 2005, pp. 1023-1030. http://dx.doi.org/10.1016/j.ophtha.2005.01.023 [71] P. Sakoonwatanyoo, D. T. Tan and D. R. Smith, “Expres- Open Access OJOph  Cryotherapy in Ophthalmology 116 sion of p63 in Pterygium and Normal Conjunctiva,” Cor- nea, Vol. 23, No. 1, 2004, pp. 67-70. http://dx.doi.org/10.1097/00003226-200401000-00011 [72] Donaldson, K.E.A., E.C., “Recent Advances in Pterygium Excision,” Cont Ophthalmol, Vol. 2, 2003, pp. 1-7. [73] F. W. Fraunfelder, “Cryotherapy for Pterygia,” Ophthal- mology, Vol. 115, No. 12, 2008, pp. 2314-2314. [74] I. Cher, “Superior Limbic Keratoconjunctivitis: Multifac- torial Mechanical Pathogenesis,” Clinical & Experimental Ophthalmology, Vol. 28, No. 3, 2000, pp. 181-184. http://dx.doi.org/10.1046/j.1442-9071.2000.00284.x [75] N. Yokoi, et al., “New Surgical Treatment for Superior Limbic Keratoconjunctivitis and Its Association with Conjunctivochalasis,” American Journal of Ophthalmol- ogy, Vol. 135, No. 3, 2003, pp. 303-308. http://dx.doi.org/10.1016/S0002-9394(02)01975-X [76] J. D. Nelson, “Superior Limbic Keratoconjunctivitis (SLK),” Eye, Vol. 3, Pt. 2, 1989, pp. 180-189. http://dx.doi.org/10.1038/eye.1989.26 [77] H. Y. Yang, et al., “Lacrimal Punctal Occlusion for the Treatment of Superior Limbic Keratoconjunctivitis,” Ame- rican Journal of Ophthalmology, Vol. 124, No. 1, 1997, pp. 80-87. [78] E. Goto, et al., “Treatment of Superior Limbic Kerato- conjunctivitis by Application of Autologous Serum,” Cornea, Vol. 20, No. 8, 2001, pp. 807-810. http://dx.doi.org/10.1097/00003226-200111000-00006 [79] H. D. Perry, et al., “Topical Cyclosporine A 0.5% as a Possible New Treatment for Superior Limbic Keratocon- junctivitis,” Ophthalmology, Vol. 110, No. 8, 2003, pp. 1578-1581. http://dx.doi.org/10.1016/S0161-6420(03)00538-4 [80] I. J. Udell, A. C. Guidera and J. Madani-Becker, “Ke- totifen Fumarate Treatment of Superior Limbic Kerato- conjunctivitis,” Cornea, Vol. 21, No. 8, 2002, pp. 778- 780. http://dx.doi.org/10.1097/00003226-200211000-00009 [81] S. Watson, A. B. Tullo and F. Carley, “Treatment of Su- perior Limbic Keratoconjunctivitis with a Unilateral Band- age Contact Lens,” The British Journal of Ophthalmology, Vol. 86, No. 4, 2002, pp. 485-486. http://dx.doi.org/10.1136/bjo.86.4.485 [82] R. D. Grutzmacher, R. S. Foster and L. S. Feiler, “Lo- doxamide Tromethamine Treatment for Superior Limbic Keratoconjunctivitis,” American Journal of Ophthalmol- ogy, Vol. 120, No. 3, 1995, pp. 400-402. [83] I. A. Mackie, “Management of Superior Limbic Kerato- conjunctivitis with Botulinum Toxin,” Eye, Vol. 9, Pt. 1, 1995, pp. 143-144. http://dx.doi.org/10.1038/eye.1995.25 [84] Y. Ohashi, et al., “Vitamin A Eyedrops for Superior Lim- bic Keratoconjunctivitis,” American Journal of Ophthal- mology, Vol. 105, No. 5, 1988, pp. 523-527. [85] E. F. Kadrmas and G. B. Bartley, “Superior Limbic Kera- toconjunctivitis. A Prognostic Sign for Severe Graves Ophthalmopathy,” Ophthalmology , Vol. 102, No. 10, 1995, pp. 1472-1475. [86] F. W. Fraunfelder, “Liquid Nitrogen Cryotherapy of Su- perior Limbic Keratoconjunctivitis,” American Journal of Ophthalmology, Vol. 147, No. 2, 2009, pp. 234-238. [87] J. H. Sullivan, “The Use of Cryotherapy for Trichiasis. Transactions. Section on Ophthalmology,” American Aca- demy of Ophthalmology and Otolaryngology, Vol. 83, No. 4, Pt. 1, 1977, pp. 708-712. [88] F. T. Fraunfelder and G. J. Petursson, “The Use of Liquid Nitrogen Cryospray for Treatment of Trichiasis,” Oph- thalmic Surgery, Vol. 10, No. 8, 1979, pp. 42-46. [89] C. D. Rice, R. C. Kersten and S. Al-Hazzaa, “Cryother- apy for Trichiasis in Trachoma,” Archives of Ophthal- mology, Vol. 107, No. 8, 1989, pp. 1180-1182. http://dx.doi.org/10.1001/archopht.1989.01070020246033 [90] M. J. Elder and W. Bernauer, “Cryotherapy for Trichiasis in Ocular Cicatricial Pemphigoid,” The British Journal of Ophthalmology, Vol. 78, No. 10, 1994, pp. 769-771. http://dx.doi.org/10.1136/bjo.78.10.769 [91] N. Kumagai, et al., “Role of Structural Cells of the Cor- nea and Conjunctiva in the Pathogenesis of Vernal Kera- toconjunctivitis,” Progress in Retinal and Eye Research, Vol. 25, No. 2, 2006, pp. 165-187. http://dx.doi.org/10.1016/j.preteyeres.2005.09.002 [92] G. Singh, “Cryosurgery in Palpebral Vernal Catarrh,” Annals of Ophthalmology, Vol. 14, No. 3, 1982, pp. 252- 254. [93] A. Abiose and M. Merz, “Cryosurgery in the Manage- ment of Vernal Keratoconjunctivitis,” Annals of Oph- thalmology, Vol. 15, No. 8, 1983, pp. 744-747. [94] A. T. Mtanda and J. L. Sangawe, “Cryosurgery in Vernal Catarrh,” Tropical and Geographical Medicine, Vol. 35, No. 4, 1983, pp. 381-383. [95] T. Sankarkumar, A. Panda and S. K. Angra, “Efficacy of Cryotherapy in Vernal Catarrh,” Annals of Ophthalmol- ogy, Vol. 24, No. 7, 1992, pp. 253-256. [96] D. Jiang, M. Zhang and Y. Hu, “Resection and Cryother- apy Combined with Amniotic Membrane Transplantation for the Treatment of Vernal Keratoconjunctivitis with Giant Papillae,” Journal of Huazhong University of Sci- ence and Technology. Medical Sciences, Vol. 26, No. 5, 2006, pp. 618-620. [97] P. Gloor and G. Alexandrakis, “Clinical Characteriza- tion of Primary Acquired Melanosis,” Investigative Oph- thalmology & Visual Science, Vol. 36, No. 8, 1995, pp. 1721-1729. [98] J. A. Shields, et al., “Primary Acquired Melanosis of the Conjunctiva: Experience with 311 Eyes,” Transactions of the American Ophthalmological Society, Vol. 105, 2007, pp. 61-71, Discussion 71-72. [99] S. Brownstein, et al., “Cryotherapy for Precancerous Me- lanosis (Atypical Melanocytic Hyperplasia) of the Con- junctiva,” Archives of Ophthalmology, Vol. 99, No. 7, 1981, pp. 1224-1231. http://dx.doi.org/10.1001/archopht.1981.03930020098009 [100] J. A. Shields, C. L. Shields and P. De Potter, “Surgical Management of Conjunctival Tumors. The 1994 Lynn B. McMahan Lecture,” Archives of Ophthalmology, Vol. 115, No. 6, 1997, pp. 808-815. http://dx.doi.org/10.1001/archopht.1997.01100150810025 [101] R. Folberg, I. W. McLean and L. E. Zimmerman, “Con- Open Access OJOph  Cryotherapy in Ophthalmology Open Access OJOph 117 junctival Melanosis and Melanoma,” Ophthalmology, Vol. 91, No. 6, 1984, pp. 673-678. [102] A. S. Grove Jr., “Melanomas of the Conjunctiva,” Inter- national Ophthalmology Clinics, Vol. 20, No. 2, 1980, pp. 161-175. [103] F. A. Jakobiec, et al., “Combined Surgery and Cryother- apy for Diffuse Malignant Melanoma of the Conjunc- tiva,” Archives of Ophthalmology, Vol. 98, No. 8, 1980, pp. 1390-1396. http://dx.doi.org/10.1001/archopht.1980.01020040242005 [104] J. M. McDonnell, A. J. Mayr and W. J. Martin, “DNA of Human Papillomavirus Type 16 in Dysplastic and Ma- lignant Lesions of the Conjunctiva and Cornea,” The New England Journal of Medicine, Vol. 320, No. 22, 1989, pp. 1442-1446. http://dx.doi.org/10.1056/NEJM198906013202202 [105] G. Peksayar, M. K. Soyturk and M. Demiryont, “Long- Term Results of Cryotherapy on Malignant Epithelial Tu- mors of the Conjunctiva,” American Journal of Ophthal- mology, Vol. 107, No. 4, 1989, pp. 337-340. [106] M. Tunc, et al., “Intraepithelial and Invasive Squamous Cell Carcinoma of the Conjunctiva: Analysis of 60 Cases,” The British Journal of Ophthalmology, Vol. 83, No. 1, 1999, pp. 98-103. http://dx.doi.org/10.1136/bjo.83.1.98 [107] A. Al-Muammar, W. G. Hodge and J. Farmer, “Conjunc- tival T-Cell Lymphoma: A Clinicopathologic Case Re- port,” Ophthalmology, Vol. 113, No. 3, 2006, pp. 459- 461. http://dx.doi.org/10.1016/j.ophtha.2005.10.042 [108] P. S. Tsai and K. A. Colby, “Treatment of Conjunctival Lymphomas,” Seminars in Ophthalmology, Vol. 20, No. 4, 2005, pp. 239-246. http://dx.doi.org/10.1080/08820530500350845 [109] C. D. Kelman, “Cryosurgery for Cataract Extraction and the Treatment of Other Eye Diseases,” Highlights of Oph- thalmology, Vol. 7, 1964, pp. 181-209. [110] M. Yanoff, “Prophylactic Cryotherapy of Retinal Breaks,” Annals of Ophthalmology, Vol. 9, No. 3, 1977, pp. 283- 286. [111] T. J. Wolfensberger, G. W. Aylward and P. K. Leaver, “Prophylactic 360 Degrees Cryotherapy in Fellow Eyes of Patients with Spontaneous Giant Retinal Tears,” Oph- thalmology, Vol. 110, No. 6, 2003, pp. 1175-1177. http://dx.doi.org/10.1016/S0161-6420(03)00256-2 [112] R. W. Kimball, P. H. Morse and W. E. Benson, “Cystoid Macular Edema after Cryotherapy,” American Journal of Ophthalmology, Vol. 86, No. 4, 1978, pp. 572-573. [113] E. H. Jaccoma, B. P. Conway and P. A. Campochiaro, “Cryotherapy Causes Extensive Breakdown of the Blood- Retinal Barrier. A Comparison with Argon Laser Photo- coagulation,” Archives of Ophthalmology, Vol. 103, No. 11, 1985, pp. 1728-1730. http://dx.doi.org/10.1001/archopht.1985.01050110124039 [114] R. J. Bowman, et al., “Retinal Necrosis as a Complication of Cryotherapy,” Eye, Vol. 8, Pt. 5, 1994, pp. 600-601. http://dx.doi.org/10.1038/eye.1994.146 [115] R. P. Lira, et al., “Cryotherapy vs Laser Photocoagulation in Scleral Buckle Surgery: A Randomized Clinical Trial,” Archives of Ophthalmology, Vol. 128, No. 12, 2010, pp. 1519-1522. http://dx.doi.org/10.1001/archophthalmol.2010.271 [116] N. W. Hindle and J. Leyton, “Prevention of Cicatricial Retrolental Fibroplasia by Cryotherapy,” Canadian Jour- nal of Ophthalmology, Vol. 3, No. 4, 1978, pp. 277-282. [117] Cryotherapy for Retinopathy of Prematurity Cooperative Group, “Multicenter Trial of Cryotherapy for Retinopathy of Prematurity. Preliminary Results,” Archives of Oph- thalmology, Vol. 106, No. 4, 1988, pp. 471-479. http://dx.doi.org/10.1001/archopht.1988.01060130517027 [118] Cryotherapy for Retinopathy of Prematurity Cooperative Group, “The Natural Ocular Outcome of Premature Birth and Retinopathy. Status at 1 Year,” Archives of Ophthal- mology, Vol. 112, No. 7, 1994, pp. 903-912. http://dx.doi.org/10.1001/archopht.1994.01090190051021 [119] Cryotherapy for Retinopathy of Prematurity Cooperative Group, “Multicenter Trial of Cryotherapy for Retinopathy of Prematurity. Snellen Visual Acuity and Structural Out- come at 5 1/2 Years after Randomization,” Archives of Ophthalmology, Vol. 114, No. 4, 1996, pp. 417-424. http://dx.doi.org/10.1001/archopht.1996.01100130413008 [120] Cryotherapy for Retinopathy of Prematurity Cooperative Group, “Multicenter Trial of Cryotherapy for Retinopathy of Prematurity: Ophthalmological Outcomes at 10 Years,” Archives of Ophthalmology, Vol. 119, No. 8, 2001, pp. 1110-1118. http://dx.doi.org/10.1001/archopht.119.8.1110 [121] H. A. Mintz-Hittner, K. A. Kennedy and A. Z. Chuang, “Efficacy of Intravitreal Bevacizumab for Stage 3+ Reti- nopathy of Prematurity,” The New England Journal of Medicine, Vol. 364, No. 7, 2011, pp. 603-615. http://dx.doi.org/10.1056/NEJMoa1007374 [122] R. R. Lonser, et al., “Von Hippel-Lindau Disease,” Lan- cet, Vol. 361, No. 9374, 2003, pp. 2059-2067. http://dx.doi.org/10.1016/S0140-6736(03)13643-4 [123] A. D. Singh, et al., “Retinal Capillary Hemangioma: A Comparison of Sporadic Cases and Cases Associated with von Hippel-Lindau Disease,” Ophthalmology, Vol. 108, No. 10, 2001, pp. 1907-1911. http://dx.doi.org/10.1016/S0161-6420(01)00758-8 [124] E. Y. Chew, “Ocular Manifestations of von Hippel-Lin- dau Disease: Clinical and Genetic Investigations,” Trans- actions of the American Ophthalmological Society, Vol. 103, 2005, pp. 495-511. [125] A. D. Singh, et al., “Treatment of Retinal Capillary He- mangioma,” Ophthalmology, Vol. 109, No. 10, 2002, pp. 1799-1806. http://dx.doi.org/10.1016/S0161-6420(02)01177-6
|