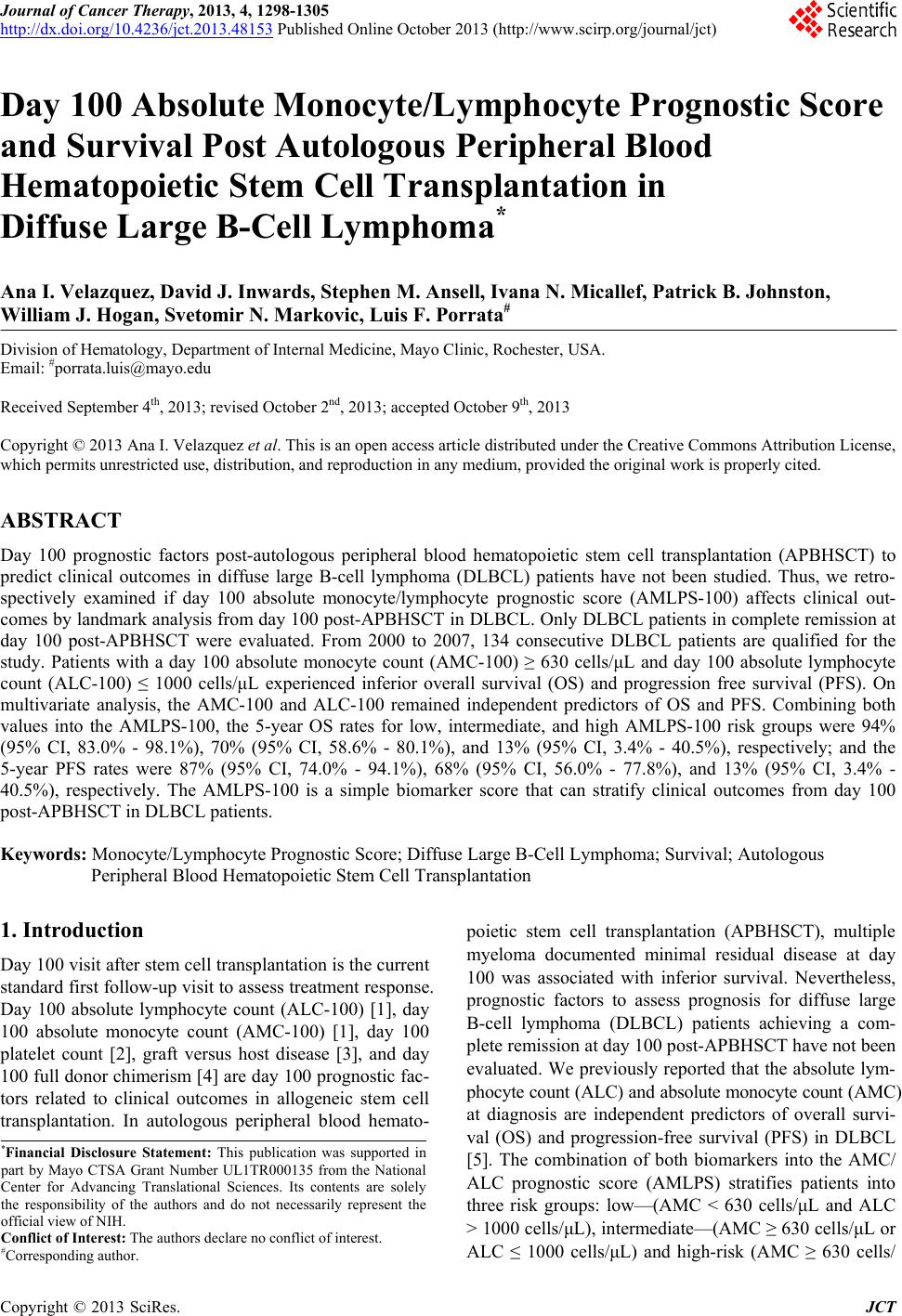
 Journal of Cancer Therapy, 2013, 4, 1298-1305 http://dx.doi.org/10.4236/jct.2013.48153 Published Online October 2013 (http://www.scirp.org/journal/jct) Day 100 Absolute Monocyte/Lymphocyte Prognostic Score and Survival Post Autologous Peripheral Blood Hematopoietic Stem Cell Transplantation in Diffuse Large B-Cell Lymphoma* Ana I. Velazquez, David J. Inwards, Stephen M. Ansell, Ivana N. Micallef, Patrick B. Johnston, William J. Hogan, Svetomir N. Markovic, Luis F. Porrata# Division of Hematology, Department of Internal Medicine, Mayo Clinic, Rochester, USA. Email: #porrata.luis@mayo.edu Received September 4th, 2013; revised October 2nd, 2013; accepted October 9th, 2013 Copyright © 2013 Ana I. Velazquez et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Day 100 prognostic factors post-autologous peripheral blood hematopoietic stem cell transplantation (APBHSCT) to predict clinical outcomes in diffuse large B-cell lymphoma (DLBCL) patients have not been studied. Thus, we retro- spectively examined if day 100 absolute monocyte/lymphocyte prognostic score (AMLPS-100) affects clinical out- comes by landmark analysis from day 100 post-APBHSCT in DLBCL. Only DLBCL patients in complete remission at day 100 post-APBHSCT were evaluated. From 2000 to 2007, 134 consecutive DLBCL patients are qualified for the study. Patients with a day 100 absolute monocyte count (AMC-100) ≥ 630 cells/μL and day 100 absolute lymphocyte count (ALC-100) ≤ 1000 cells/μL experienced inferior overall survival (OS) and progression free survival (PFS). On multivariate analysis, the AMC-100 and ALC-100 remained independent predictors of OS and PFS. Combining both values into the AMLPS-100, the 5-year OS rates for low, intermediate, and high AMLPS-100 risk groups were 94% (95% CI, 83.0% - 98.1%), 70% (95% CI, 58.6% - 80.1%), and 13% (95% CI, 3.4% - 40.5%), respectively; and the 5-year PFS rates were 87% (95% CI, 74.0% - 94.1%), 68% (95% CI, 56.0% - 77.8%), and 13% (95% CI, 3.4% - 40.5%), respectively. The AMLPS-100 is a simple biomarker score that can stratify clinical outcomes from day 100 post-APBHSCT in DLBCL patients. Keywords: Monocyte/Lymphocyte Prognostic Score; Diffuse Large B-Cell Lymphoma; Survival; Autologous Peripheral Blood Hematopoietic Stem Cell Transplantation 1. Introduction Day 100 visit after stem cell transplantation is the current standard first follow-up visit to assess treatment response. Day 100 absolute lymphocyte count (ALC-100) [1], day 100 absolute monocyte count (AMC-100) [1], day 100 platelet count [2], graft versus host disease [3], and day 100 full donor chimerism [4] are day 100 prognostic fac- tors related to clinical outcomes in allogeneic stem cell transplantation. In autologous peripheral blood hemato- poietic stem cell transplantation (APBHSCT), multiple myeloma documented minimal residual disease at day 100 was associated with inferior survival. Nevertheless, prognostic factors to assess prognosis for diffuse large B-cell lymphoma (DLBCL) patients achieving a com- plete remission at day 100 post-APBHSCT have not been evaluated. We previously reported that the absolute lym- phocyte count (ALC) and absolute monocyte count (AMC) at diagnosis are independent predictors of overall survi- val (OS) and progression-free survival (PFS) in DLBCL [5]. The combination of both biomarkers into the AMC/ ALC prognostic score (AMLPS) stratifies patients into three risk groups: low—(AMC < 630 cells/μL and ALC > 1000 cells/μL), intermediate—(AMC ≥ 630 cells/μL or ALC ≤ 1000 cells/μL) and high-risk (AMC ≥ 630 cells/ *Financial Disclosure Statement: This publication was supported in art by Mayo CTSA Grant Number UL1TR000135 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH. Conflict of Interest: The authors declare no conflict of interest. #Corresponding author. Copyright © 2013 SciRes. JCT  Day 100 Absolute Monocyte/Lymphocyte Prognostic Score and Survival Post Autologous Peripheral Blood Hematopoietic Stem Cell Transplantation in Diffuse Large B-Cell Lymphoma 1299 μL and ALC ≤ 1000 cells/μL) [5]. The AMLPS has been recently validated in several independent studies [6,7] confirming its utility as an assessment tool of prognosis in DLBCL. Post-transplant immunologic reconstitution, particularly ALC recovery (ALC ≥ 500 cells/µL) at day 15, has also been associated with prolonged PFS and OS in multiple hematological malignancies [8-19] and solid tumors [20-22]. However, recent reports suggest that the survival benefit obtained from early lymphocyte recovery post- stem cell transplant in DLBCL patients could be lost with long-term follow-up [10]. Therefore, the aim of this study was to evaluate if day 100 AMLPS (AMLPS-100) affects survival for DLBCL patients in complete remis- sion at day 100 post-APBHSCT. The value of AMLPS- 100 was also evaluated as a tool to identify high-risk patients for post-APBHSCT relapse that is simple and could be easily implemented in clinical practice. 2. Materials and Methods 2.1. Patient Population DLBCL patients achieving complete remission at day 100 post-APBHSCT at Mayo Clinic, Rochester, MN between 2000 and 2007 were considered for this study. Patients transplanted with bone marrow or combined bone marrow and peripheral blood stem cells and pa- tients with evidence of relapse or progression at day 100 post-APBHSCT were excluded. A total of 134 consecu- tive DLBCL patients in complete remission at day 100 post-APBHSCT qualified for the study. No patient re- fused authorization to use their medical records for re- search and none were lost to follow-up. Approval for the retrospective review of these patients’ records was ob- tained from the Mayo Clinic Institutional Review Board and the research was conducted in accordance with US federal regulations and the Declaration of Helsinki. 2.2. End Points The primary end point of the study was to assess the im- pact of AMLPS-100 on OS and PFS by landmark analy- sis from day 100 in DLBCL patients treated with APBHSCT. The AMC-100, ALC-100, and AMLPS-100 were obtained from a standard day 100 complete blood cell count (CBC). The secondary end point was to evalu- ate if the AMLPS-100 could stratify DLBCL patients into low-, intermediate- and high-risk groups for OS and PFS post-APBHSCT. 2.3. Conditioning Regimen All patients received carmustine (BCNU) 300mg/m2 on day-6; etoposide 100mg/m2 twice a day on days-5, -4, -3, and -2; cytarabine 100 mg/m2 twice a day on days-5, -4, -3, -2; and melphalan 140mg/m2 on day-1 (BEAM). 2.4. Prognostic Factors Prognostic factors evaluated include: age at day 100 (Age-100), ALC-100, AMC-100, absolute neutrophil count at day 100 (ANC-100), gender, International Prog- nostic Index (IPI) at diagnosis, infused CD34+ cell dose, lactate dehydrogenase at day 100 (LDH-100), hemog- lobin at day 100 (Hgb-100), platelets at day 100 (Plts- 100), day 15 absolute lymphocyte count post-APBHSCT (ALC-15), and white blood cell count at day 100 (WBC- 100). 2.5. Response and Survival Response criteria were based on the guidelines from the International Harmonization Project for Malignant Lym- phoma [23]. OS was measured from day 100 to the date of death, or last follow-up. PFS was defined as the time from day 100 to the time of progression, relapse, death, or last follow-up, whichever occurred first. 2.6. Statistical Analysis OS and PFS were analyzed using the approach of Kap- lan-Meier [24]. Differences between the survival curves were tested for statistical significance using the 2-tailed log-rank test. The Cox proportional hazard model was used for the univariate and multivariate analysis to evalu- ate the impact of the variables listed under the prognostic factors section for OS and PFS times [25]. The choice of the cut-off values for ALC-100 and AMC-100 was based on our previous AMLPS publication [5]. χ2 analysis was used to determine relationships between categorical variables. The Wilcoxon-rank test was used to determine associations between continuous variables and categories, and Spearman correlation coefficients were used to eva- luate associations for continuous variables. All two- sided p-values < 0.05 were determined to be statistically significant. 3. Results 3.1. Patients’ Characteristics For this cohort of 134 DLBCL patients, the median age at day 100 post-APBHSCT was 57.5 years (range: 23 - 77 years). Sixty-three percent of the patients were males, while 37% were females. The distribution of the patients’ baseline characteristics at day 100 is included in Table 1. The median follow-up period from day 100 post- APBHSCT for the cohort was 5.5 years (range: 0.1 - 12.7 years) and for living patients (N = 93) was 6.9 years (range: 2.5 - 12.7 years). Twenty-seven patients died Copyright © 2013 SciRes. JCT 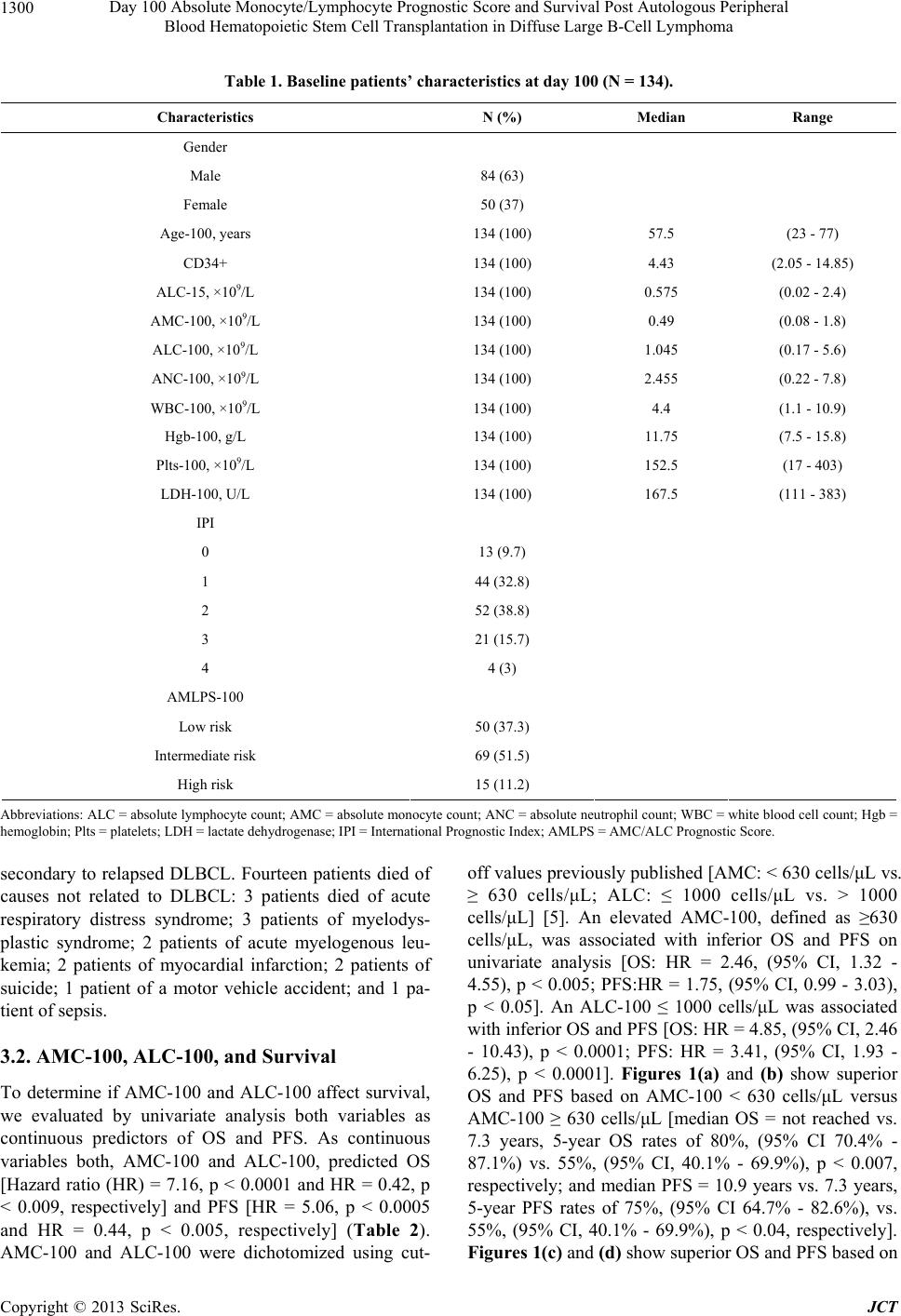 Day 100 Absolute Monocyte/Lymphocyte Prognostic Score and Survival Post Autologous Peripheral Blood Hematopoietic Stem Cell Transplantation in Diffuse Large B-Cell Lymphoma Copyright © 2013 SciRes. JCT 1300 Table 1. Baseline patients’ characteristics at day 100 (N = 134). Characteristics N (%) Median Range Gender Male 84 (63) Female 50 (37) Age-100, years 134 (100) 57.5 (23 - 77) CD34+ 134 (100) 4.43 (2.05 - 14.85) ALC-15, ×109/L 134 (100) 0.575 (0.02 - 2.4) AMC-100, ×109/L 134 (100) 0.49 (0.08 - 1.8) ALC-100, ×109/L 134 (100) 1.045 (0.17 - 5.6) ANC-100, ×109/L 134 (100) 2.455 (0.22 - 7.8) WBC-100, ×109/L 134 (100) 4.4 (1.1 - 10.9) Hgb-100, g/L 134 (100) 11.75 (7.5 - 15.8) Plts-100, ×109/L 134 (100) 152.5 (17 - 403) LDH-100, U/L 134 (100) 167.5 (111 - 383) IPI 0 13 (9.7) 1 44 (32.8) 2 52 (38.8) 3 21 (15.7) 4 4 (3) AMLPS-100 Low risk 50 (37.3) Intermediate risk 69 (51.5) High risk 15 (11.2) Abbreviations: ALC = absolute lymphocyte count; AMC = absolute monocyte count; ANC = absolute neutrophil count; WBC = white blood cell count; Hgb = hemoglobin; Plts = platelets; LDH = lactate dehydrogenase; IPI = International Prognostic Index; AMLPS = AMC/ALC Prognostic Score. secondary to relapsed DLBCL. Fourteen patients died of causes not related to DLBCL: 3 patients died of acute respiratory distress syndrome; 3 patients of myelodys- plastic syndrome; 2 patients of acute myelogenous leu- kemia; 2 patients of myocardial infarction; 2 patients of suicide; 1 patient of a motor vehicle accident; and 1 pa- tient of sepsis. 3.2. AMC-100, ALC-100, and Survival To determine if AMC-100 and ALC-100 affect survival, we evaluated by univariate analysis both variables as continuous predictors of OS and PFS. As continuous variables both, AMC-100 and ALC-100, predicted OS [Hazard ratio (HR) = 7.16, p < 0.0001 and HR = 0.42, p < 0.009, respectively] and PFS [HR = 5.06, p < 0.0005 and HR = 0.44, p < 0.005, respectively] (Table 2). AMC-100 and ALC-100 were dichotomized using cut- off values previously published [AMC: < 630 cells/μL vs. ≥ 630 cells/μL; ALC: ≤ 1000 cells/μL vs. > 1000 cells/μL] [5]. An elevated AMC-100, defined as ≥630 cells/μL, was associated with inferior OS and PFS on univariate analysis [OS: HR = 2.46, (95% CI, 1.32 - 4.55), p < 0.005; PFS:HR = 1.75, (95% CI, 0.99 - 3.03), p < 0.05]. An ALC-100 ≤ 1000 cells/μL was associated with inferior OS and PFS [OS: HR = 4.85, (95% CI, 2.46 - 10.43), p < 0.0001; PFS: HR = 3.41, (95% CI, 1.93 - 6.25), p < 0.0001]. Figures 1(a) and (b) show superior OS and PFS based on AMC-100 < 630 cells/μL versus AMC-100 ≥ 630 cells/μL [median OS = not reached vs. 7.3 years, 5-year OS rates of 80%, (95% CI 70.4% - 87.1%) vs. 55%, (95% CI, 40.1% - 69.9%), p < 0.007, respectively; and median PFS = 10.9 years vs. 7.3 years, 5-year PFS rates of 75%, (95% CI 64.7% - 82.6%), vs. 55%, (95% CI, 40.1% - 69.9%), p < 0.04, respectively]. Figures 1(c) and (d) show superior OS and PFS based on 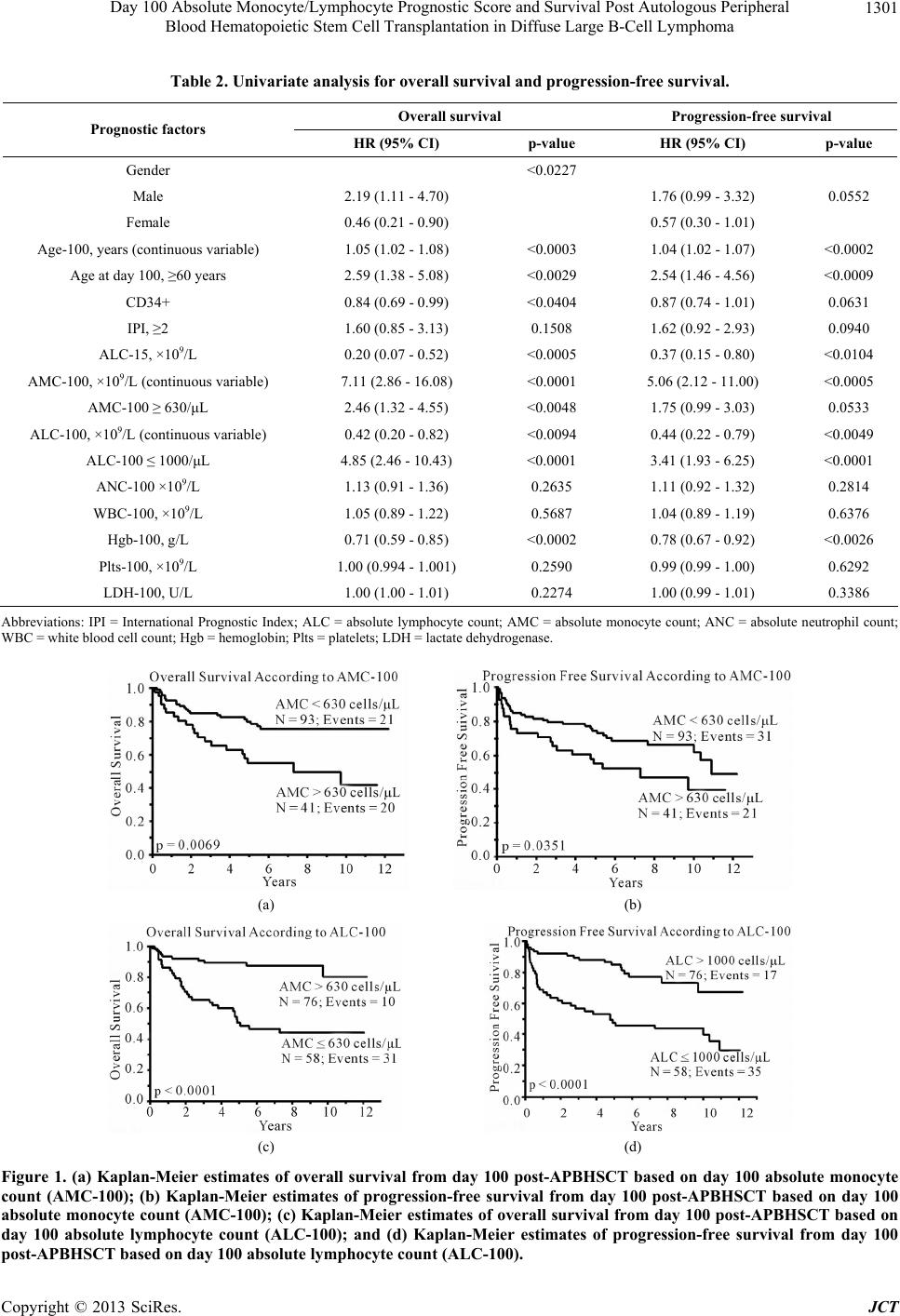 Day 100 Absolute Monocyte/Lymphocyte Prognostic Score and Survival Post Autologous Peripheral Blood Hematopoietic Stem Cell Transplantation in Diffuse Large B-Cell Lymphoma 1301 Table 2. Univariate analysis for overall survival and progression-free survival. Overall survival Progression-free survival Prognostic factors HR (95% CI) p-value HR (95% CI) p-value Gender <0.0227 Male 2.19 (1.11 - 4.70) 1.76 (0.99 - 3.32) Female 0.46 (0.21 - 0.90) 0.57 (0.30 - 1.01) 0.0552 Age-100, years (continuous variable) 1.05 (1.02 - 1.08) <0.0003 1.04 (1.02 - 1.07) <0.0002 Age at day 100, ≥60 years 2.59 (1.38 - 5.08) <0.0029 2.54 (1.46 - 4.56) <0.0009 CD34+ 0.84 (0.69 - 0.99) <0.0404 0.87 (0.74 - 1.01) 0.0631 IPI, ≥2 1.60 (0.85 - 3.13) 0.1508 1.62 (0.92 - 2.93) 0.0940 ALC-15, ×109/L 0.20 (0.07 - 0.52) <0.0005 0.37 (0.15 - 0.80) <0.0104 AMC-100, ×109/L (continuous variable) 7.11 (2.86 - 16.08) <0.0001 5.06 (2.12 - 11.00) <0.0005 AMC-100 ≥ 630/μL 2.46 (1.32 - 4.55) <0.0048 1.75 (0.99 - 3.03) 0.0533 ALC-100, ×109/L (continuous variable) 0.42 (0.20 - 0.82) <0.0094 0.44 (0.22 - 0.79) <0.0049 ALC-100 ≤ 1000/μL 4.85 (2.46 - 10.43) <0.0001 3.41 (1.93 - 6.25) <0.0001 ANC-100 ×109/L 1.13 (0.91 - 1.36) 0.2635 1.11 (0.92 - 1.32) 0.2814 WBC-100, ×109/L 1.05 (0.89 - 1.22) 0.5687 1.04 (0.89 - 1.19) 0.6376 Hgb-100, g/L 0.71 (0.59 - 0.85) <0.0002 0.78 (0.67 - 0.92) <0.0026 Plts-100, ×109/L 1.00 (0.994 - 1.001) 0.2590 0.99 (0.99 - 1.00) 0.6292 LDH-100, U/L 1.00 (1.00 - 1.01) 0.2274 1.00 (0.99 - 1.01) 0.3386 Abbreviations: IPI = International Prognostic Index; ALC = absolute lymphocyte count; AMC = absolute monocyte count; ANC = absolute neutrophil count; WBC = white blood cell count; Hgb = hemoglobin; Plts = platelets; LDH = lactate dehydrogenase. (a) (b) (c) (d) Figure 1. (a) Kaplan-Meier estimates of overall survival from day 100 post-APBHSCT based on day 100 absolute monocyte count (AMC-100); (b) Kaplan-Meier estimates of progression-free survival from day 100 post-APBHSCT based on day 100 absolute monocyte count (AMC-100); (c) Kaplan-Meier estimates of overall survival from day 100 post-APBHSCT based on day 100 absolute lymphocyte count (ALC-100); and (d) Kaplan-Meier estimates of progression-free survival from day 100 post-APBHSCT based on day 100 absolute lymphocyte count (ALC-100). Copyright © 2013 SciRes. JCT  Day 100 Absolute Monocyte/Lymphocyte Prognostic Score and Survival Post Autologous Peripheral Blood Hematopoietic Stem Cell Transplantation in Diffuse Large B-Cell Lymphoma 1302 ALC-100 > 1000 cells/μL versus ALC-100 ≤ 1000 cells/ μL [median OS = not reached vs. 5.1 years, 5-year OS rates of 89%, (95% CI, 80.2% - 94.6%), vs. 49%, (95% CI, 36.1% - 61.7%), p < 0.0001, respectively; and me- dian PFS = not reached vs. 4.8 years, 5-year PFS rates of 85%, (95% CI = 74.9% - 91.5%) vs. 46%, (95% CI, 33.6% - 58.9%), p < 0.0001, respectively]. 3.3. Univariate and Multivariate Analysis Gender, age-100 (continuous and dichotomized), CD34+, IPI, ALC-15, ALC-100 (continuous and dichotomized), AMC-100 (continuous and dichotomized), and Hgb-100 were identified as predictors for OS and PFS in the uni- variate analysis (Table 2). In the multivariate analysis, CD34+ and ALC-15 continue to be independent predic- tors of OS and PFS post-APBHSCT; age dichotomized as < or ≥60 years was associated with PFS. Both AMC-100 ≥ 630 cells/μL and ALC-100 ≤ 1000 cells/μL remained as independent predictors of OS after adjusting for several variables on multivariate analysis, with haz- ard ratios of 3.83 and 5.46 respectively (p < 0.0002; p < 0.0001); both day 100 variables were independent pre- dictors of PFS (Table 3). 3.4. Day 100 AMLPS (AMLPS-100) By univariate and multivariate analysis, the ALC-100 and AMC-100 were independent predictors for OS and PFS post-APBHSCT. Thus, we combinedALC-100 and AMC-100 into day 100 AMC/ALC prognostic score (AMLPS-100), using the same cut-off values from our previous publication of the AMLPS at diagnosis in DLBCL [5], to develop a simple scoring system that can be used to stratify by risk patients with DLBCL that are in complete disease remission at day 100 post-APBHSCT. According to the AMLPS-100, 37.3% (N = 50) of the patients were considered low risk (AMC < 630 cells/μL and ALC > 1000 cells/μL), 51.5% (N = 69) intermediate risk (AMC ≥ 630 cells/μL or ALC ≤ 1000 cells/μL), and 11.2% (N = 15) high risk (AMC ≥ 630 cells/μL and ALC ≤ 1000 cells/μL) (Table 4). Among the groups significant differences were seen in ALC-100, AMC-100, ANC-100, and HgB-100. Patients with a low-risk AMLPS-100 ex- perienced significantly superior OS and PFS compared to the other groups, with a 5-year OS rate of 94% (95% CI, 83.0% - 98.1%); median not reached; p < 0.0001 and a 5-year PFS rate of 87% (95% CI, 74.0% - 94.1%); me- dian not reached; p < 0.0001 (Figures 2(a) and (b)). The estimated 5-year OS among intermediate-risk patients was 70% (95% CI, 58.6% - 80.1%); median not reached (Figure 2(a)) and the 5-year PFS was 68% (95% CI, 56.0% - 77.8%) with a median PFS of 10.9 years (Figure 2(b)). The AMLPS-100 identified a group of high-risk patients with median OS of 2.18 years and an estimated 5-year OS of 13% (95% CI, 3.4% - 40.5%) (Figure 2(a)). Similarly, the median PFS for high-risk patients was 1 year with an estimated 5-year PFS rate of 13% (95% CI, 3.4% - 40.5%) (Figure 2(b)). 4. Discussion Currently, there are no studies available to advise DLBCL patients in complete remission at day 100 post- APBHSCT of their long-term prognosis starting at day 100 post-APBHSCT. We previously published the AMLPS at diagnosis for DLBCL stratifies patients into three risk groups in regard to clinical outcomes. This AMLPS has been validated as an independent prognostic indicator in DLBCL patients by other independent groups [6,7]. Hence, we sought to evaluate if the AMLPS- Table 3. Multivariate analysis for overall survival and progression-free survival. Overall survival Progression-free survival Prognostic factors HR (95% CI) p-value HR (95% CI) p-value Gender 0.4792 0.4949 Male 1.32 (0.62 - 2.96) 1.24 (0.67 - 2.40) Female 0.76 (0.34 - 1.60) 0.80 (0.42 - 1.49) Age-100, ≥60 years 1.57 (0.77 - 3.34) 0.2213 2.16 (1.17 - 4.09) <0.0142 CD34+ 0.83 (0.70 - 0.97) <0.0175 0.89 (0.76 - 1.01) 0.0751 IPI, ≥2 1.88 (0.95 - 3.88) 0.0720 1.90 (1.05 - 3.57) <0.0341 ALC-15, ×109/L 0.28 (0.09 - 0.074) <0.0094 0.54 (0.22 - 1.22) 0.1393 Hgb-100, g/L 3.83 (1.89 - 7.85) <0.0002 2.23 (1.19 - 4.15) <0.0132 AMC-100, ≥630/μL 3.83 (1.32 - 5.28) <0.0002 4.30 (2.31 - 8.31) <0.0001 ALC-100, ≤1000/μL 5.46 (2.99 - 14.10) <0.0001 5.04 (2.70 - 9.80) <0.0001 Abbreviations: IPI = International Prognostic Index; ALC = absolute lymphocyte count; AMC = absolute monocyte count; Hgb = hemoglobin. Copyright © 2013 SciRes. JCT 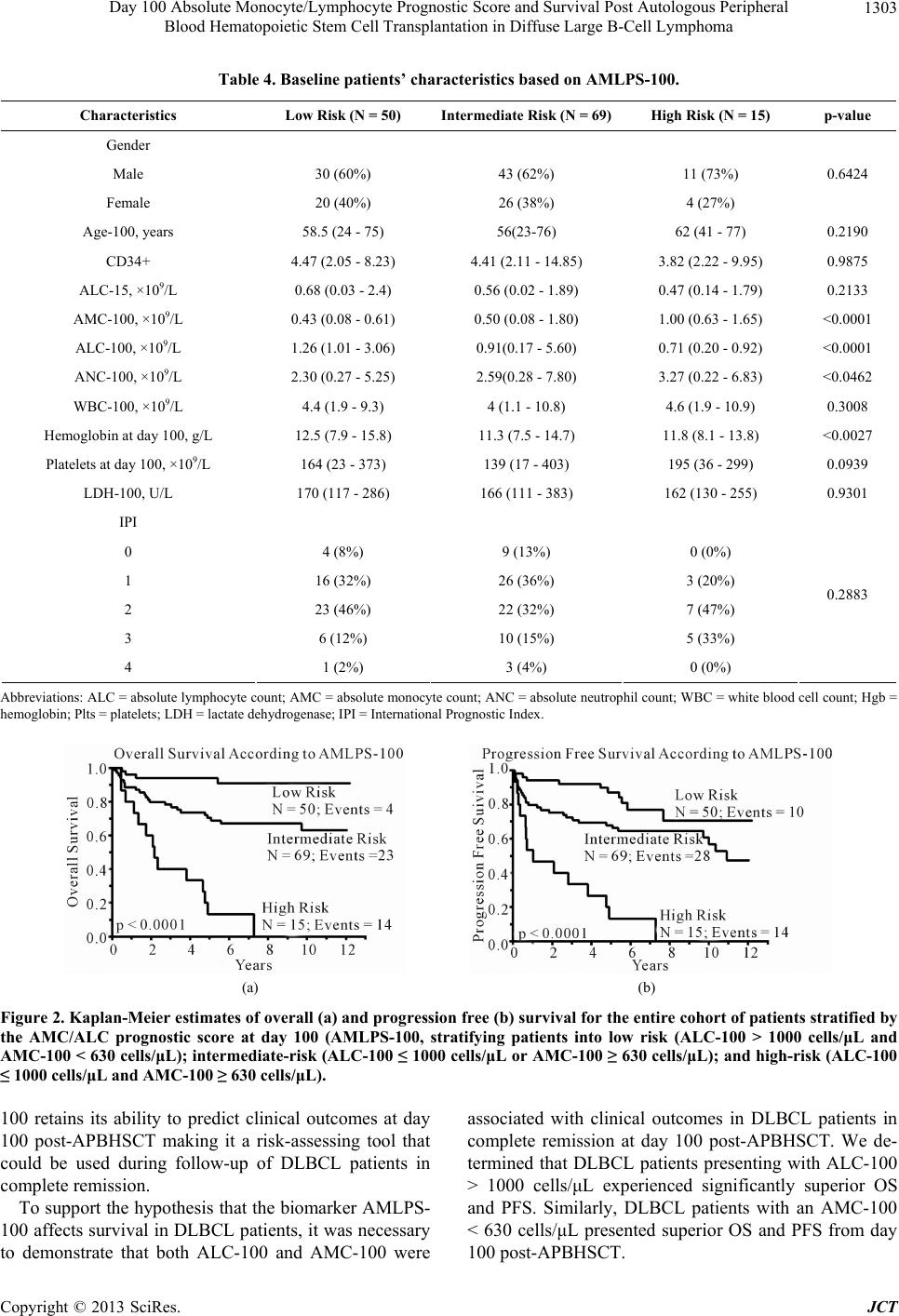 Day 100 Absolute Monocyte/Lymphocyte Prognostic Score and Survival Post Autologous Peripheral Blood Hematopoietic Stem Cell Transplantation in Diffuse Large B-Cell Lymphoma 1303 Table 4. Baseline patients’ characteristics based on AMLPS-100. Characteristics Low Risk (N = 50) Intermediate Risk (N = 69)High Risk (N = 15) p-value Gender Male 30 (60%) 43 (62%) 11 (73%) Female 20 (40%) 26 (38%) 4 (27%) 0.6424 Age-100, years 58.5 (24 - 75) 56(23-76) 62 (41 - 77) 0.2190 CD34+ 4.47 (2.05 - 8.23) 4.41 (2.11 - 14.85) 3.82 (2.22 - 9.95) 0.9875 ALC-15, ×109/L 0.68 (0.03 - 2.4) 0.56 (0.02 - 1.89) 0.47 (0.14 - 1.79) 0.2133 AMC-100, ×109/L 0.43 (0.08 - 0.61) 0.50 (0.08 - 1.80) 1.00 (0.63 - 1.65) <0.0001 ALC-100, ×109/L 1.26 (1.01 - 3.06) 0.91(0.17 - 5.60) 0.71 (0.20 - 0.92) <0.0001 ANC-100, ×109/L 2.30 (0.27 - 5.25) 2.59(0.28 - 7.80) 3.27 (0.22 - 6.83) <0.0462 WBC-100, ×109/L 4.4 (1.9 - 9.3) 4 (1.1 - 10.8) 4.6 (1.9 - 10.9) 0.3008 Hemoglobin at day 100, g/L 12.5 (7.9 - 15.8) 11.3 (7.5 - 14.7) 11.8 (8.1 - 13.8) <0.0027 Platelets at day 100, ×109/L 164 (23 - 373) 139 (17 - 403) 195 (36 - 299) 0.0939 LDH-100, U/L 170 (117 - 286) 166 (111 - 383) 162 (130 - 255) 0.9301 IPI 0 4 (8%) 9 (13%) 0 (0%) 1 16 (32%) 26 (36%) 3 (20%) 2 23 (46%) 22 (32%) 7 (47%) 3 6 (12%) 10 (15%) 5 (33%) 4 1 (2%) 3 (4%) 0 (0%) 0.2883 Abbreviations: ALC = absolute lymphocyte count; AMC = absolute monocyte count; ANC = absolute neutrophil count; WBC = white blood cell count; Hgb = hemoglobin; Plts = platelets; LDH = lactate dehydrogenase; IPI = International Prognostic Index. (a) (b) Figure 2. Kaplan-Meier estimates of overall (a) and progression free (b) survival for the entire cohort of patients stratified by the AMC/ALC prognostic score at day 100 (AMLPS-100, stratifying patients into low risk (ALC-100 > 1000 cells/μL and AMC-100 < 630 cells/μL); intermediate-risk (ALC-100 ≤ 1000 cells/μL or AMC-100 ≥ 630 cells/μL); and high-risk (ALC-100 ≤ 1000 cells/μL and AMC-100 ≥ 630 cells/μL). 100 retains its ability to predict clinical outcomes at day 100 post-APBHSCT making it a risk-assessing tool that could be used during follow-up of DLBCL patients in complete remission. To support the hypothesis that the biomarker AMLPS- 100 affects survival in DLBCL patients, it was necessary to demonstrate that both ALC-100 and AMC-100 were associated with clinical outcomes in DLBCL patients in complete remission at day 100 post-APBHSCT. We de- termined that DLBCL patients presenting with ALC-100 > 1000 cells/μL experienced significantly superior OS and PFS. Similarly, DLBCL patients with an AMC-100 < 630 cells/μL presented superior OS and PFS from day 100 post-APBHSCT. Copyright © 2013 SciRes. JCT  Day 100 Absolute Monocyte/Lymphocyte Prognostic Score and Survival Post Autologous Peripheral Blood Hematopoietic Stem Cell Transplantation in Diffuse Large B-Cell Lymphoma 1304 Since both the ALC-100 and AMC-100 were inde- pendent predictors for OS and PFS, we combined them into AMLPS-100. The AMLPS-100 was able to stratify patients into low-, intermediate-, and high-risk groups for OS and PFS post-APBHSCT. To minimize the inherent biases of a retrospective study, the following steps were taken. With regards to selection bias, we only included DLBCL patients that underwent APBHSCT. Patients infused with peripheral blood as well as bone marrow harvested stem cells were excluded. All patients were treated with the same condi- tioning regimen. All patients were required to be in com- plete remission at day 100 for the landmark analysis. With regards to confounding factors, our study included currently known prognostic factors, such as the IPI; in addition, we included Age-100, Hgb-100, ANC-100, LDH- 100, WBC-100, and Plts-100, all of which have been re- ported as prognostic factors at day 100 post-allogeneic stem cell transplantation [1-4]. On the other hand, strengths of this study include the long follow-up period of a well-defined group of DLBCL patients in complete remission at day 100 post- APBHSCT, with a median follow-up from day 100 post- APBHSCT for the cohort of 5.5 years and 6.9 years for living patients. Secondly, AMLPS-100 combines clinical biomarkers for the host immunity (i.e., ALC) [5] and tumor microenvironment (i.e., AMC) [26,27]. Thirdly, the AMLPS-100 is a simple biomarker score obtained from the day 100 CBC post-APBHSCT that can be used to assess clinical outcomes in DLBCL patients in com- plete remission at day 100 post-APBHSCT, whereas other prognostic techniques such as gene-expression pro- filing required fresh frozen tissue samples, limiting its use in complete remission DLBCL patients at day 100 post-APBHSCT. Further studies are warranted to vali- date the AMLPS-100. 5. Acknowledgements This publication was supported in part by Mayo CTSA Grant Number UL1TR000135 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not neces- sarily represent the official view of NIH. REFERENCES [1] L. J. DeCook, M. Thoma, T. Huneke, N. D. Johnson, R. A., M. M. Patnaik, et al., “Impact of Lymphocyte and Monocyte Recovery on the Outcomes of Allogeneic He- matopoietic SCT with Fludarabine and Melphalan Condi- tioning,” Bone Marrow Transplantation, Vol. 48, No. 5, 2013, pp. 708-714. http://dx.doi.org/10.1038/bmt.2012.211 [2] B. Bolwell, B. Pohlman, R. Sobecks, S. Andresen, S. Brown, L. Rybicki, et al., “ Prognostic Importance of the Platelet Count 100 Days Post Allogeneic Bone Marrow Transplant,” Bone Marrow Transplantation, Vol. 33, No. 4, 2004, pp. 419-423. http://dx.doi.org/10.1038/sj.bmt.1704330 [3] Z. Kuzmina, S. Eder, A Bohm, E. Pernicka, L. Vormittag, P. Kahs, et al., “Significant Worse Survival of Patients with NIH-Defined Chronic Graft-versus-Host Disease and Thrombocytopenia or Progressive Onset Type: Re- sults of a Prospective Study,” Leukemia, Vol. 26, No. 4, 2012, pp. 746-756. http://dx.doi.org/10.1038/leu.2011.257 [4] S. G. Holtan, W. J. Hogan, M. A. Elliott, S. M. Ansell, D. J. Inwards, L. F. Porrata, et al., “CD34+ Cell Dose and Establishment of Full Donor Chimerism at Day +100 Are Important Factors for Survival with Reduced-Intensity Conditioning with Fludarabine and Melphalan before Al- logeneic Hematopoietic SCT for Hematologic Malignan- cies,” Bone Marrow Transplantation, Vol. 45, No. 12, 2010, pp. 1699-1703. http://dx.doi.org/10.1038/bmt.2010.49 [5] R. A. Wilcox, K. Ristow, T. M. Habermann, D. J. In- wards, I. N. M. Micallef, P. B. Johnston, et al., “The Ab- solute Monocyte and Lymphocyte Prognostic Score Pre- dicts Survival and Identifies High-Risk Patients in Dif- fuse Large-B-Cell Lymphoma,” Leukemia, Vol. 25, No. 9, 2011, pp. 1502-1509. http://dx.doi.org/10.1038/leu.2011.112 [6] N. Batty, E. Ghonimi, L. Feng, L. Fayad, A. Younes, M. A. Rodriguez, et al., “The Absolute Monocyte and Lym- phocyte Prognostic Index for Patients with Diffuse Large- B-Cell Lymphoma Who Receive R-CHOP,” Clinical Lymphoma, Myeloma & Leukemia, Vol. 13, No. 1, 2013, pp. 15-18. http://dx.doi.org/10.1016/j.clml.2012.09.009 [7] C. Keane, D. Gill, F. Vari, D. Cross, L. Griffiths and M. Gandhi, “CD4 Tumor Infiltrating Lymphocytes Are Prog- nostic and Independent of R-IPI in Patients with DLBCL Receiving R-CHOP Chemo-Immunotherapy,” American Journal of Hematology, Vol. 88, No. 4, 2013, pp. 273- 276. http://dx.doi.org/10.1002/ajh.23398 [8] D. K. Hiwase, S. Hiwase, M. Bailey, G. Bollard and A. P. Schwarer, “Higher Infused Lymphocyte Dose Predicts Higher Lymphocyte Recovery, Which in Turn, Predicts Superior Overall Survival Following Autologous Hema- topoietic Stem Cell Transplantation for Multiple Mye- loma,” Biology of Blood and Marrow Transplantation, Vol. 14, No. 7, 2008, pp. 116-124. http://dx.doi.org/10.1016/j.bbmt.2007.08.051 [9] L. F. Porrata, D. J. Inwards, S. M. Ansell, I. N. Micallef, P. B. Johnston, D. A. Gastineau, et al., “Early Lympho- cyte Recovery Predicts Superior Survival after Autolo- gous Stem Cell Transplantation in Non-Hodgkin Lym- phoma: A Prospective Study,” Biology of Blood and Mar- row Transplantation, Vol. 14, No. 7, 2008, pp. 807-816. http://dx.doi.org/10.1016/j.bbmt.2008.04.013 [10] D. G. Stover, V. K. Reddy, Y. Shyr, B. N. Savani and N. Reddy, “Long-Term Impact of Prior Rituximab Therapy and Early Lymphocyte Recovery on Auto-SCT Outcome for Diffuse Large B-Cell Lymphoma,” Bone Marrow Tran- Copyright © 2013 SciRes. JCT  Day 100 Absolute Monocyte/Lymphocyte Prognostic Score and Survival Post Autologous Peripheral Blood Hematopoietic Stem Cell Transplantation in Diffuse Large B-Cell Lymphoma Copyright © 2013 SciRes. JCT 1305 splantation, Vol. 47, No. 1, 2012, pp. 82-87. http://dx.doi.org/10.1038/bmt.2011.29 [11] L. F. Porrata, M. A. Gertz, D. J. Inwards, M. R. Litzow, M. Q. Lacy, A. Tefferi, et al., “Early Lymphocyte Reco- very Predicts Superior Survival after Autologous Hema- topoietic Stem Cell Transplantation in Multiple Myeloma or Non-Hodgkin Lymphoma,” Blood, Vol. 98, No. 3, 2001, pp. 579-585. http://dx.doi.org/10.1182/blood.V98.3.579 [12] M. R. Boulassel, A. L. Herr, M. D. DebEdwards, A. Galal, S. Lachance, P. Laneuville, et al., “Early Lymphocyte Recovery Following Autologous Peripheral Stem Cell Transplantation Is Associated with Better Survival in Younger Patients with Lymphoproliferative Disorders,” Hematology, Vol. 11, No. 3, 2006, pp. 165-170. http://dx.doi.org/10.1080/10245330600667559 [13] L. N. Gordan, M. W. Sugrue, J. W. Lynch, K. D. Wil- liams, S. A. Khan and J. S. Moreb, “Correlation of Early Lymphocyte Recovery and Progression-Free Survival af- ter Autologous Stem-Cell Transplant in Patients with Hodgkin’s and Non-Hodgkin’s Lymphoma,” Bone Mar- row Transplantation, Vol. 31, No. 11, 2003, pp. 1009- 1013. http://dx.doi.org/10.1038/sj.bmt.1704050 [14] C. Joao, L. F. Porrata, D. J. Inwards, S. M. Ansell, I. N. Micallef, P. B. Johnston, et al., “Early Lymphocyte Re- covery after Autologous Stem Cell Transplantation Pre- dicts Superior Survival in Mantle-Cell Lymphoma,” Bone Marrow Transplantation, Vol. 37, No. 9, 2006, pp. 865- 871. http://dx.doi.org/10.1038/sj.bmt.1705342 [15] H. Kim, H. J. Sohn, S. Kim, J. S. Lee, W. K. Kim and C. Suh, “Early Lymphocyte Recovery Predicts Longer Sur- vival after Autologous Peripheral Blood Stem Cell Trans- plantation in Multiple Myeloma,” Bone Marrow Trans- plantation, Vol. 37, No. 11, 2006, pp. 1037-1042. http://dx.doi.org/10.1038/sj.bmt.1705373 [16] H. Kim, H. J. Sohn, S. E. Kim, H. J. Kang, S. Park, S. Kim, et al., “Lymphocyte Recovery Prolonged Survival after Autologous Peripheral Blood Stem Cell Transplan- tation in T-Cell Non-Hodgkin’s Lymphoma,” Bone Mar- row Transplantation, Vol. 34, No. 1, 2004, pp. 43-49. http://dx.doi.org/10.1038/sj.bmt.1704530 [17] L. F. Porrata, M. A. Gertz, M. R. Litzow, M. Q Lacy, A. Dispenzieri, D. J. Inwards, et al., “Early Lymphocyte Re- covery Predicts Superior Survival after Autologous He- matopoietic Stem Cell Transplantation for Patients with Primary Systemic Amyloidosis,” Clinical Cancer Re- search, Vol. 11, 2005, pp. 1120-1218. [18] L. F. Porrata, D. J. Inwards, I. N. Micallef, S. M. Ansell, S. M. Geyer and S. N. Markovic, “Early Lymphocyte Re- covery Post-Autologous Haematopoietic Stem Cell Tran- splantation Is Associated with Better Survival in Hodg- kin’s Disease,” British Journal of Haematology, Vol. 117, No. 3, 2006, pp. 629-633. http://dx.doi.org/10.1046/j.1365-2141.2002.03478.x [19] L. F. Porrata, M. R. Litzow, A. Tefferi, L. Letendre, S. Kumar, S. M. Gever, et al., “Early Lymphocyte Recovery Is a Predictive Factor for Prolonged Survival after Auto- logous Hematopoietic Stem Cell Transplantation for Acute Myelogenous Leukemia,” Leukemia, Vol. 16, No. 7, 2002, pp. 1311-1318. http://dx.doi.org/10.1038/sj.leu.2402503 [20] G. Ferrandina, L. Pierelli, A. Perillo, S. Rutela, M. Lu- dovisi, G. Leone, et al., “Lymphocyte Recovery in Ad- vanced Ovarian Cancer Patients after High-Dose Chemo- therapy and Peripheral Blood Stem Cell Plus Growth Factor Support: Clinical Implications,” Clinical Cancer Research, Vol. 9, 2003, pp. 195-200. [21] Y. Nieto, E. J. Shpall, I. K. McNiece, S. Nawaz, J. Beau- det, S. Rosinki, et al., “Prognostic Analysis Of Early Lymphocyte Recovery in Patients with Advanced Breast Cancer Receiving High-Dose Chemotherapy with an Autologous Hematopoietic Progenitor Cell Transplant,” Clinical Cancer Research, Vol. 10, 2004, pp. 5076-5086. [22] L. F. Porrata, J. N. Ingle, M. R. Litzow, S, Geyer and S. N. Markovic, “Prolonged Survival Associated with Early Lymphocyte Recovery after Autologous Hematopoietic Stem Cell Transplantation for Patients with Metastatic Breast Cancer,” Bone Marrow Transplantation, Vol. 28, No. 9, 2001, pp. 865-871. http://dx.doi.org/10.1038/sj.bmt.1703236 [23] B. D. Cheson, B. Pfistner, M. E. Juweid, R. D. Gascoyne, L. Specht, S. J. Horning, et al., “Revised Response Crite- ria for Malignant Lymphoma,” Journal of Clinical Onco- logy, Vol. 25, No. 5, 2007, pp. 579-586. http://dx.doi.org/10.1200/JCO.2006.09.2403 [24] E. L. Kaplan and P. Meier, “Nonparametric Estimation from Incomplete Observations,” Journal of the American Statistical Association, Vol. 53, No. 282, 1958, pp. 457- 481. http://dx.doi.org/10.1080/01621459.1958.10501452 [25] D. Cox, “Regression Models and Life Tables,” Journal of the Royal Statistical Society B, Vol. 34, 1972, pp. 187- 202. [26] C. M. Diaz-Montero, M. L. Salem, M. I. Nishimura, E. Garrett-Mayer, D. J. Cole and A. J. Montero, “Increased Circulating Myeloid-Derived Suppressor Cells Correlate with Clinical Cancer Stage, Metastatic Tumor Burden, and Doxorubicin-Cyclophosphamide Chemotherapy,” Can- cer Immunology, Immunotherapy: CII, Vol. 58, 2009, pp. 49-59. [27] D. I. Gabrilovich and S. Nagaraj, “Myeloid-Derived Sup- pressor Cells as Regulators of the Immune System,” Na- ture Reviews Immunology, Vol. 9, 2009, pp. 162-174.
|