 Energy and Power E ngineering, 2013, 5, 6-14 doi:10.4236/epe.2013.54B002 Published Online July 2013 (http://www.scirp .o rg/journal/epe) Copyright © 2013 SciRes. EPE Study of Alkali Metal Corrosion on Heating Surfaces and Bed Material Agglomerate in Biomass-fired Fluidized Bed Boiler Tuo Chen1, Yanfen Liao1, Shumei Wu1, Xiaoqian Ma1, Jinghui Song2 1School of Electric Power, South China University of Technology, Guangzhou, P.R.China 2Electric Power Research Institute of Guangdong Power Grid Corporation, Guangzhou, China Email: yfliao@scut. edu.cn Received April, 2013 ABSTRACT The bed material agglomeration and heating surface high-temperature Corrosion Problems of biomass-fired boiler in Sout h China were stud ied in t his wo rk. The inner and outer surfaces of the corrosion sample were investigated by scan- ning electron microscope (SEM) with Bruker EDX and XRD. Results showed that the outer side of the corrosion sam- ple was mainly composed of alkali chloride deposited ash, sulphide and a small amount of eutectoid; while the inner side of the corrosion sample was still mainly made up of the composition of SUS316, but added with alkali metal, oxy- gen, chlorine and sulphur elements, appearing as the corrosion products and eutectoid. It was thought that alkali chlo- ride deposit and the reaction with pipe metal to generate low melting point eutectoid on the outer surfaces, or the corro- sion reaction through the alkali metal sulphatization process was the main reasons leading to the damage of metal sur- face oxide film. Chlorine plays a role as haptoreaction in the corrosion process, and transports metal material as the form of chloride from the inner side to the outer side of the pipe surfaces by diffusion, accelerating the corrosion process. Meanwhile, the slag was studied by scanning electron microscope (SEM) with Br uker EDX, and the tran sfor- mation p rocess o f slage was c omputa tiona lly ana lyzed by FACT SAGE. Resul ts sho wed tha t the a mo unt of alkali metal in the agglomerates was little, however, caused a great impact on severe agglomerates. The increase of temperature en- hanced the conversion process of alkali metal to molten oxide, especially when the temperature was higher than 760℃, the amount of molten product increased sharply. Thus, the te mperature control of fluidized bed plays an important role in solving the prob lem of alkali metal agglo merates; it also r eliefs the volatile of alkali meta l into gas phase, benefiting the control of heating surface corrosion. Keywords: Biomass; Combustion Genera tion; High Temperature Corrosion; Agglomerate; Alkali Metal 1. Introduction With the concerns over energy shortage and CO2 emis- sions, biomas s is now bei ng consid ered a s an inexhau sti- ble and clean energy resource worldwide. As a large ag- ricultural country, China is producing high amounts of agricultural residue annually. The utilization of biomass resource would benefit alleviating the current energy shortage and environmental pollution. However, the raw biomass materials have the inherent characteristic of high alkali content, especially the high content of potassiu m [1,2], causing alkali metal p roblems during the process of burning utilization, such as the contaminating corrosion of heating surface, coking deposition and agglomeration [3,4]. Some efforts have been devoted onto the alkali metal corrosion problem of biomass boiler, and the study [5,6] shows that there are two main types of alkali metal cor- rosion problems. One was the alkali metal sulfate cor- rosion of fly ash sediment, which is caused by the reac- tion of alkali metal oxide K 2O , Na2O with SO3 in t he fl ue gas, generating alkali metal sulfate and releasing the HCl and Cl2, since these two substances can penetrate the metal oxidation protective film, thus react directly with internal metal to generated metal chloride. T he other was the eutectoid corrosion, induced by the eutectic reaction of the c hloride in sedi ment with metal surface, forming a kind of low melting point eutec to id. In sout h Chi na, there is rich resource of sugarcane and forestry residues, especially the sugar cane, which grow- ing in Guangdong, Guangxi, Hai’nan, Yunnan provinces in large-scale, accounting for more than 95% out- put of whole country. The inorganic elements in biomass are closely related to the growth area, and the alkali metal and chlorine content of the sugarcane is higher in south China for the inshore circumstance. Few research re-  T. CHEN ET AL. Copyright © 2013 SciRes. EPE garding alkali metal problems was reported about this typical southern plant. In the wor k, the heating surfa ce high-temperature cor- rosion problem, as well as the bed material agglomera- tion problem in a biomass-fired boiler was studied. The main reason for corrosion in the boiler heating surface was researched, and some corresponding control method s were put forward. 2. Introduce of the Boiler The circulating fluidized bed (CFB) combustion boiler was adopted in the biomass power plant, and was de- signed as high temperature and high pressure parameters, natural circulation, single furnace, balanced ventilation, solid slag discharge, open layout, and double row column steel suspension structure. The designed fuel was mixed biomass fuel, includin g the s kin and leave s of eucalyptus, abandoned sugarcane leaf and bagasse from the refine sugar industry. The residue ashes of biomass were used as the bed materials. Since the source of other biomass fuel was limited, the bagasse was used as the main burning material in the actual operation, with small amount of sugar cane leaf and eucalyptus processing waste. At rated conditions, the bed temperature was controlled between 750℃ ~ 850℃, and the outlet oxygen value in the flue gas was about 3.5% (excess air coefficient being 1.2). After put into operation for more than 140 days, the furnace heating surface pipe corrosion was occurred in large area, especially on the platen super-heater tube. The corrosion rate was much higher than the normal design value, reaching the magnitude of mm/thousa nd s o f ho ur s. The c o rro sive thi n was mor e t h an 2 mm after t wo mont hs operation, being 10 times highe r than the normal a mo unt of corrosion. 3. Biomass Fuel Proper ti es For the analysis of the corrosion of heating surface, the biomass fuel char acteristics were inspected. The biomass fuel utilized in this study was from the biomass power plant, which was grown in Zhanjian g. After being cut into 1-3cm pieces, the samples were dried in an oven at 100ºC for 5h, ground and sieved to less than 250µm. The samples were subsequently analyzed to determine the main parameters that influenced thermal conversion. Proximate determinations were made according to the Standard Practice for the Proximate Analysis of Biomass (E0870—82R98E01, ASTM). A GmbH VarioEL equip- ment model CHNS analyzer was used to determine the carbon, hydrogen, nitrogen and sulphur content. The Cl element content of samples was tested by ion chromato- graphy, as the Cl element of biomass exists in the form of ions, direct lea ching method [7,8] was use d as the pre- treatment methods . The results of the proximate and ultimate analysis are sho wn in Ta ble 1. It can be seen that the fuel ha s l ow ash content, while high volatile content, indicating a good ignition charac te ristics. In addition, an ash test was conducted according to the Standard Test Method for Ash in Biomass (E1755-01, ASTM). The ash was pretreated by the method of nitric acid - perchloric acid digestion in this work. The diges- tion solution is tested by atomic absorption spectrum (TAS-990 super F atomic absorption spectrophotometer) afte r constant volume and diluted. Converting the alkali metal as metallic oxide, the po- tassium oxide content in bagasse low temperature ash could reach 21.59%, and sodium oxide content could reach 8.05%, while reaches 14.38% and 17.35% respe c- tively in sugar cane leaf low-temperature ash. It was shown that the alkali metal contents both in bagasse and sugar cane leaf were very high. Although the potassium content in bagasse was slightly higher than the one in sugarcane leaves, the qualities of the alkali metal content of the two we re closer; the oxide quality content is around 30%. As alkali metal exists in the organism mainly in the form of water soluble salt, and partly exists in the car- boxyl and other functional groups or chemical adsorption material in the form of ionic adsorption. In almost all biomass, there are 90% of the potassium exists in the soluble in water or can be carried out ion exchange ma- terial in ion s tate, which has high mobilit y in the heating process[9]. Therefore, judging from the ash component analysis it can be foresee n that these kinds of bi omass fuel will produce alkali metal precipitation and the corres- ponding problem of alkali metal in the combustion process. Table 1. Ultimate and proximate analyses of biomas s fuels. Approximate Analysis /wt, % Elemental Analysis /wt, % type Mar % Aar % Var % Fcar % Car % Har % Oar % Nar % Sar % C lar % begass 10.37 2.63 72.60 14.39 41.28 7.84 33.83 0.43 0.06 0.0571 Sugar cane leaf 9.62 4.91 70.44 15.03 40.84 7.67 34.75 0.48 0.21 0.1769 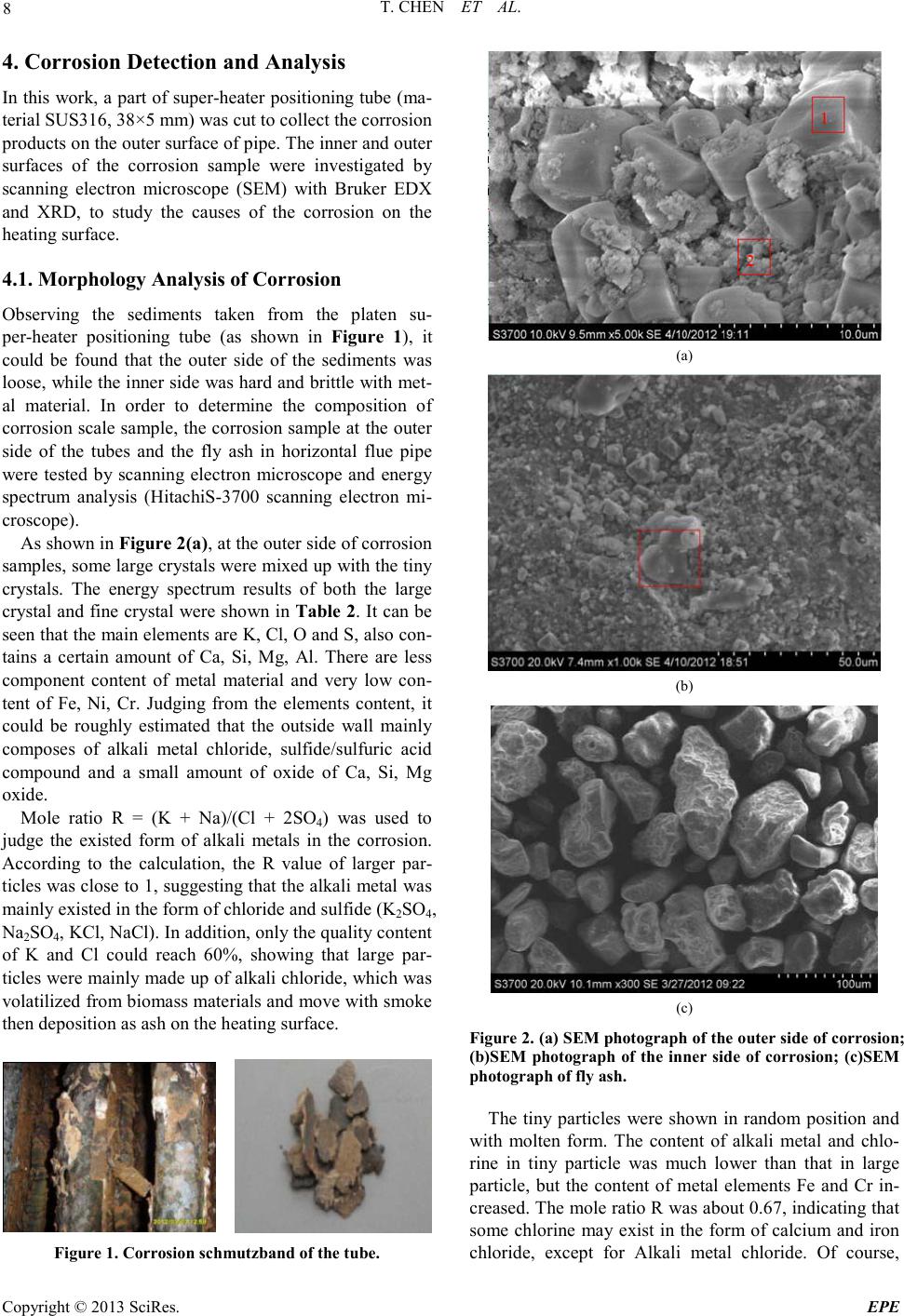 T. CHEN ET AL. Copyright © 2013 SciRes. EPE 4. Corrosi on Detection and Analysis In this wor k, a par t of super-hea ter positioning tube (ma- terial SUS316, 38×5 mm) was cut to collect the corrosion products o n the outer surface of pipe. The inner and o ute r surfaces of the corrosion sample were investigated by scanning electron microscope (SEM) with Bruker EDX and XRD, to study the causes of the corrosion on the heating surface. 4.1. Morphology Analysis of Corrosion Observing the sediments taken from the platen su- per-heater positioning tube (as shown in Figure 1), it could be found that the outer side of the sediments was loose, while the inner sid e was hard a nd brittle wi th met- al material. In order to determine the composition of corrosion scale sample, the corrosion sample at the out er side of the tubes and the fly ash in horizontal flue pipe were tested by scanning electron microscope and energy spectrum analysis (HitachiS-3700 scanning electron mi- croscope). As shown i n Figure 2(a), at the outer side of corrosion samples, so me large crystals we re mixe d up wit h the tiny crystals. The energy spectrum results of both the large crysta l and fi ne cryst al were s hown in Table 2. It can be seen that the main eleme nts are K, Cl, O and S, also con- tains a certain amount of Ca, Si, Mg, Al. There are less component content of metal material and very low con- tent of Fe, Ni, Cr. Judging from the elements content, it could be roughly estimated that the outside wall mainly composes of alkali metal chloride, sulfide/sulfuric acid compound and a small amount of oxide of Ca, Si, Mg oxide. Mole ratio R = (K + Na)/(Cl + 2SO4) was used to judge the existed form of alkali metals in the corrosion. According to the calculation, the R value of larger par- ticles was close to 1, suggesting that the alkal i metal wa s mainly existed in the form of chloride and sulfide (K2SO4, Na2SO4, KCl , NaCl) . In add ition, o nl y the q ua lit y conte nt of K and Cl could reach 60%, showing that large par- ticles wer e mai nly made up of alkali ch loride , which was volatilized fro m biomass mate rials and move wi th smoke then deposition as ash on the heating surface. Figure 1. Corrosion schmutzband of the tube. (a) (b) (c) Figure 2. (a) SEM phot ogra ph of the o uter s ide of c orrosio n; (b)SEM photograph of the inner side of corrosion; (c)SEM photog raph of f ly ash. The tiny particles were shown in random position and with molten form. The content of alkali metal and chlo- rine in tiny particle was much lower than that in large particle, but the content of metal elements Fe and Cr in- creased. The mole ratio R was about 0.67, indicating that some chlorine may exist in the form of calcium and iron chloride, except for Alkali metal chloride. Of course, 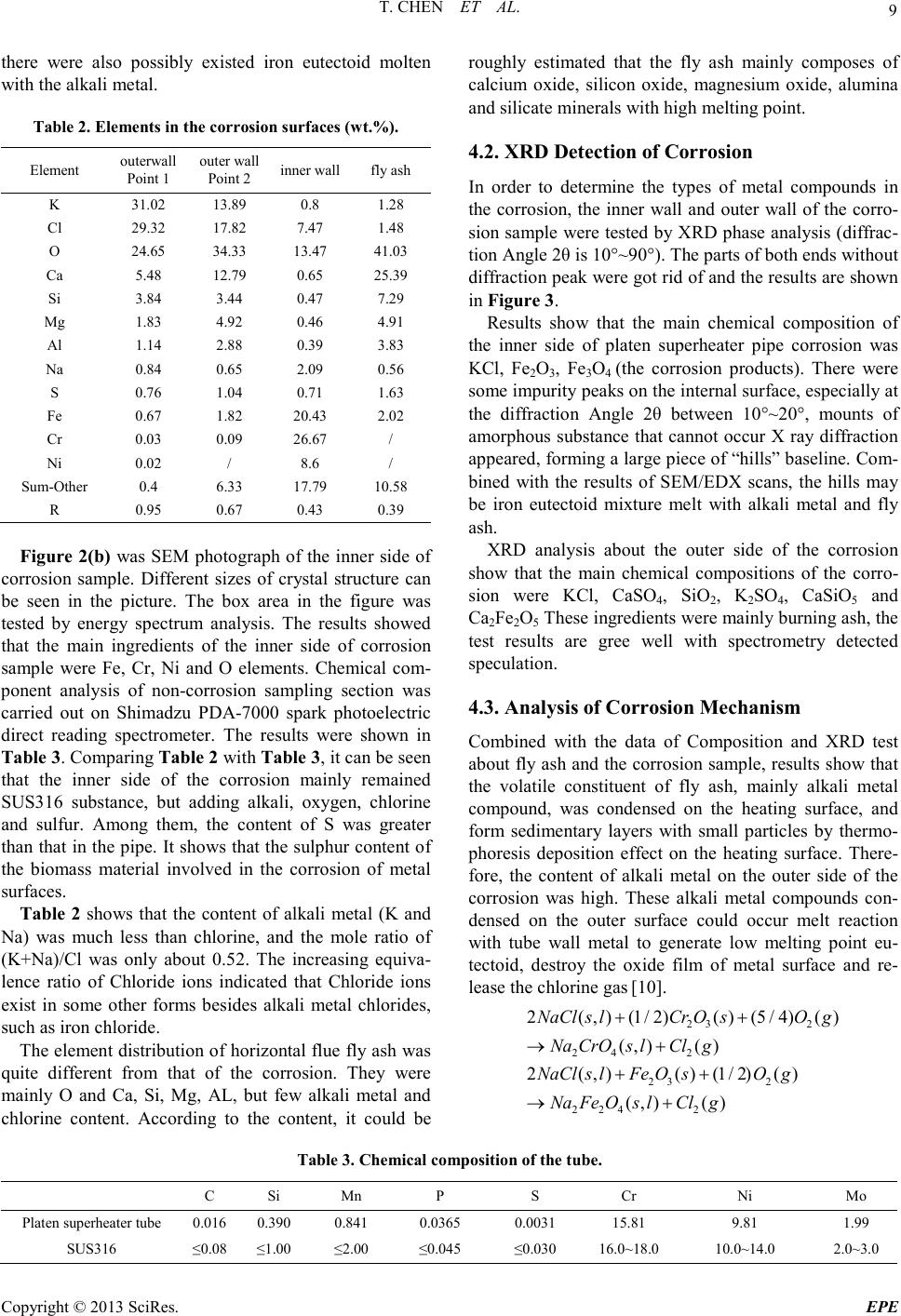 T. CHEN ET AL. Copyright © 2013 SciRes. EPE there were also possibly existed iron eutectoid molten with the alkali metal. Table 2. Elements in the c orrosion surfaces (wt.%). Element outerwall Point 1 outer wall Point 2 inner wall fly ash K 31.02 13.89 0.8 1.28 Cl 29.32 17.82 7.47 1.48 O 24.65 34.33 13.47 41.03 Ca 5.48 12.79 0.65 25.39 Si 3.84 3.44 0.47 7.29 Mg 1.83 4.92 0.46 4.91 Al 1.14 2.88 0.39 3.83 Na 0.84 0.65 2.09 0.56 S 0.76 1.04 0.71 1.63 Fe 0.67 1.82 20.43 2.02 Cr 0.03 0.09 26.67 / Ni 0.02 / 8.6 / Sum-Other 0.4 6.33 17.79 10.58 R 0.95 0.67 0.43 0.39 Figure 2(b) wa s SEM photograph of the inner side of corrosion sampl e. Different sizes of crystal structure can be seen in the picture. The box area in the figure was tested by energy spectrum analysis. The results showed that the main ingredients of the inner side of corrosion sampl e were Fe, Cr, Ni and O elements. Chemical com- ponent analysis of non-corrosion sampling section was carried out on Shimadzu PDA-7000 spark photoelectric direct reading spectrometer. The results we re shown in Table 3. Comparing Table 2 with Table 3, it can be seen that the inner side of the corrosion mainly remained SUS316 substance, but adding alkali, oxygen, chlorine and sulfur. Among them, the content of S was greater than t hat in t he pipe. It shows that the s ulphur c ontent o f the biomass material involved in the corrosion of metal surfaces. Table 2 shows that the content of alkali metal (K and Na) was much less than chlorine, and the mole ratio of (K+Na)/Cl was only about 0.52. The increasing equiva- lence ratio of Chloride ions indicated that Chloride ions exist in some other forms besides alkali metal chlorides, such as iro n chlo ride . The ele ment distr ibution of horiz ontal fl ue fl y ash was quite different from that of the corrosion. They were mainly O and Ca, Si, Mg, AL, but few alkali metal and chlorine content. According to the content, it could be roughly estimated that the fly ash mainly composes of calcium oxide, silicon oxide, magnesium oxide, alumina and silicate minerals with high melting point. 4.2. XRD Detection of Corrosion In order to determine the types of metal compounds in the corrosion, the inner wall and outer wall of the corro- sion sample were tested by XRD phase analysis (diffrac- tion Angle 2θ is 10°~90°). The parts of both ends without diffraction peak were got rid of and the results are shown in Figure 3. Results show that the main chemical composition of the inner side of platen superheater pipe corrosion wa s KCl, Fe2O3, Fe3O4 (the corrosion products). There wer e some imp ur it y p eaks o n the i n te rna l s urface , especially at the diffraction Angle 2θ between 10°~20°, mounts of amorphous substance that cannot occur X ray diffraction appeared, forming a large piece of “hills” baseline. Com- bined with the results of SEM/EDX scans, the hills may be iron eutectoid mixture melt with alkali metal and fly ash. XRD analysis about the outer side of the corrosion show that the main chemical compositions of the corro- sion were KCl, CaSO4, SiO2, K2SO4, CaSiO5 and Ca2Fe2O5 These ingredients were mainl y b ur ni n g as h, the test results are gree well with spectrometry detected speculatio n. 4.3. Analysis of Corrosion Mechanism Combined with the data of Composition and XRD test about fly ash and the corrosion sa mpl e, results show that the volatile constituent of fly ash, mainly alkali metal compound, was condensed on the heating surface, and form sedimentary layers with small particles by thermo- phoresis deposition effect on the heating surface. There- for e, the content of alkali metal on the outer side of the corrosion wa s high. These alkali metal compounds con- densed on the outer surface could occur melt reaction with tube wall metal to generate low melting point eu- tectoid, destroy the oxide film of metal surface and re- lease the chlorine gas [10]. 23 2 24 2 23 2 2 242 2(,)(1/ 2)()(5/4)() (,)( ) 2(,)()(1 /2)() (,)( ) NaClslCr OsOg Na CrOs lClg NaCls lFeOsOg NaFeOslClg ++ →+ ++ →+ Table 3. Chemical composition of the tube. C Si Mn P S Cr Ni Mo Platen superheater tube 0.016 0.390 0.841 0.0365 0.0031 15.81 9.81 1.99 SUS316 ≤0.08 ≤1.00 ≤2.00 ≤0.045 ≤0.030 16.0~18.0 10.0~14.0 2.0~3.0 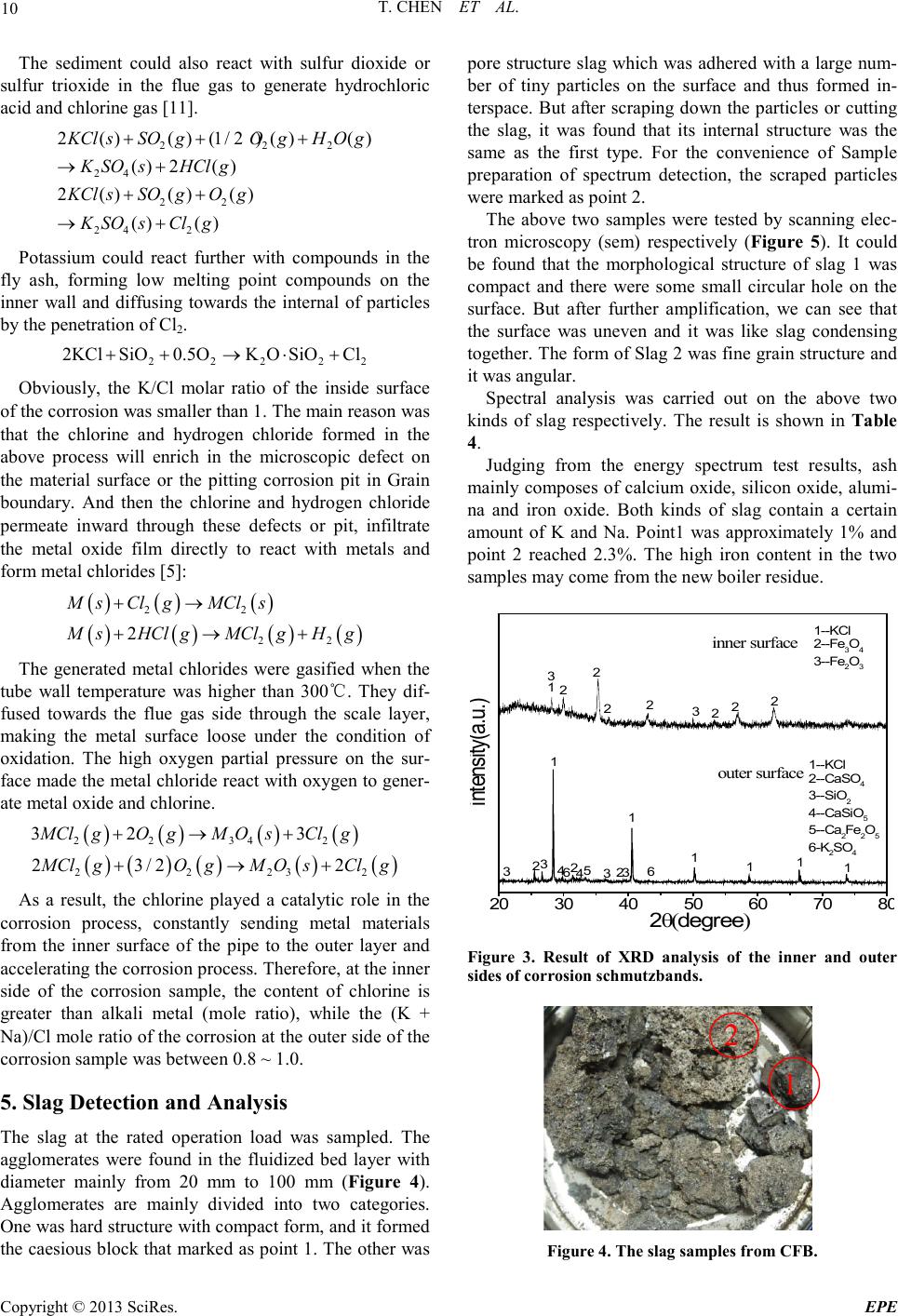 T. CHEN ET AL. Copyright © 2013 SciRes. EPE The sediment could also react with sulfur dioxide or sulfur trioxide in the flue gas to generate hydrochloric acid and chlorine gas [11]. 2 22 24 22 24 2 2()() (1/2)()() ()2( ) 2 ()()() ()() KCl sSOgOgH O g K SOsHCl g KCl sSOgOg K SOsClg ++ + →+ ++ →+ Potassium could react further with compounds in the fly ash, forming low melting point compounds on the inner wall and diffusing towards the internal of particles by the penetratio n of Cl2. 22 222 2KClSiO 0.5OKOSiO Cl++ →⋅+ Obviously, the K/Cl molar ratio of the inside surface of the corrosion was smaller than 1. The main reason was that the chlorine and hydrogen chloride formed in the above process will enrich in the microscopic defect on the material surface or the pitting corrosion pit in Grain boundary. And then the chlorine and hydrogen chloride permeate inward through these defects or pit, infiltrate the metal oxide film directly to react with metals and form metal chlo rides [5]: ( )()( ) ()( )()( ) 22 22 2 M sClgMCls MsHCl gMClgHg +→ +→+ The generated metal chlorides were gasified when the tube wall temperature was higher than 300℃. They dif- fused towards the flue gas side through the scale layer, making the metal surface loose under the condition of oxidation. The high oxygen partial pressure on the sur- face made the metal chloride react with oxygen to gener- ate metal oxide and chlorine. ( )()( )()( ) 2 234 2 2223 2 32 3 2 3/22 MCl gOgMOsCl g MCl gOgMOsCl g +→ + + →+ As a result, the chlorine played a catalytic role in the corrosion process, constantly sending metal materials from the inner surface of the pipe to the outer layer and accelerating the corrosion process. Therefore, at the inner side of the corrosion sample, the content of chlorine is greater than alkali metal (mole ratio), while the (K + Na)/Cl mole r atio of the co rrosio n at the outer side of the corrosion sample was between 0.8 ~ 1.0. 5. Slag Detection and Analysis The slag at the rated operation load was sampled. The agglomerates were found in the fluidized bed layer with diameter mainly from 20 mm to 100 mm (Figure 4). Agglomerates are mainly divided into two categories. One was hard structure with co mpact for m, and it formed the caesious block that marked as point 1. The other was por e structur e slag whi ch was adhered with a large num- ber of tiny particles on the surface and thus formed in- terspace. But after scraping down the particles or cutting the slag, it was found that its internal structure was the same as the first type. For the convenience of Sample preparation of spectrum detection, the scraped particles wer e marked as point 2. The above two samples were tested by scanning elec- tron microscopy (sem) respectively (Figure 5). It could be found that the morphological structure of slag 1 was compact and there were some small circular hole on the surface. But after further amplification, we can see that the surface was uneven and it was like slag condensing toget her. T he form of Slag 2 was fi ne grai n str ucture and it was angular. Spectral analysis was carried out on the above two kinds of slag respectively. The result is shown in Table 4. Judging from the energy spectrum test results, ash mainly composes of calcium oxide, silicon oxide, alumi- na and iron oxide. Both kinds of slag contain a certain amount of K and Na. Point1 was approximately 1% and point 2 reached 2.3%. The high iron content in the two samples may come from the new boiler residue. 20 30 40 50 60 70 80 ou ter s urfa c e 6 6 inn er s urfa c e 1 1 1 1 1 intensity(a.u.) 2θ(degree) 11--KCl 2-- CaSO 4 3--SiO 2 4-- CaSi O 5 5--Ca 2 Fe 2 O 5 6-K 2 SO 4 22 3 32 5 4433 322 2 2 222 1--KCl 2--Fe 3 O 4 3--Fe 2 O 3 3 1 Figure 3. Result of XRD analysis of the inner and outer sides of corrosion schmutzbands. Figure 4. The slag samples fr om CFB. 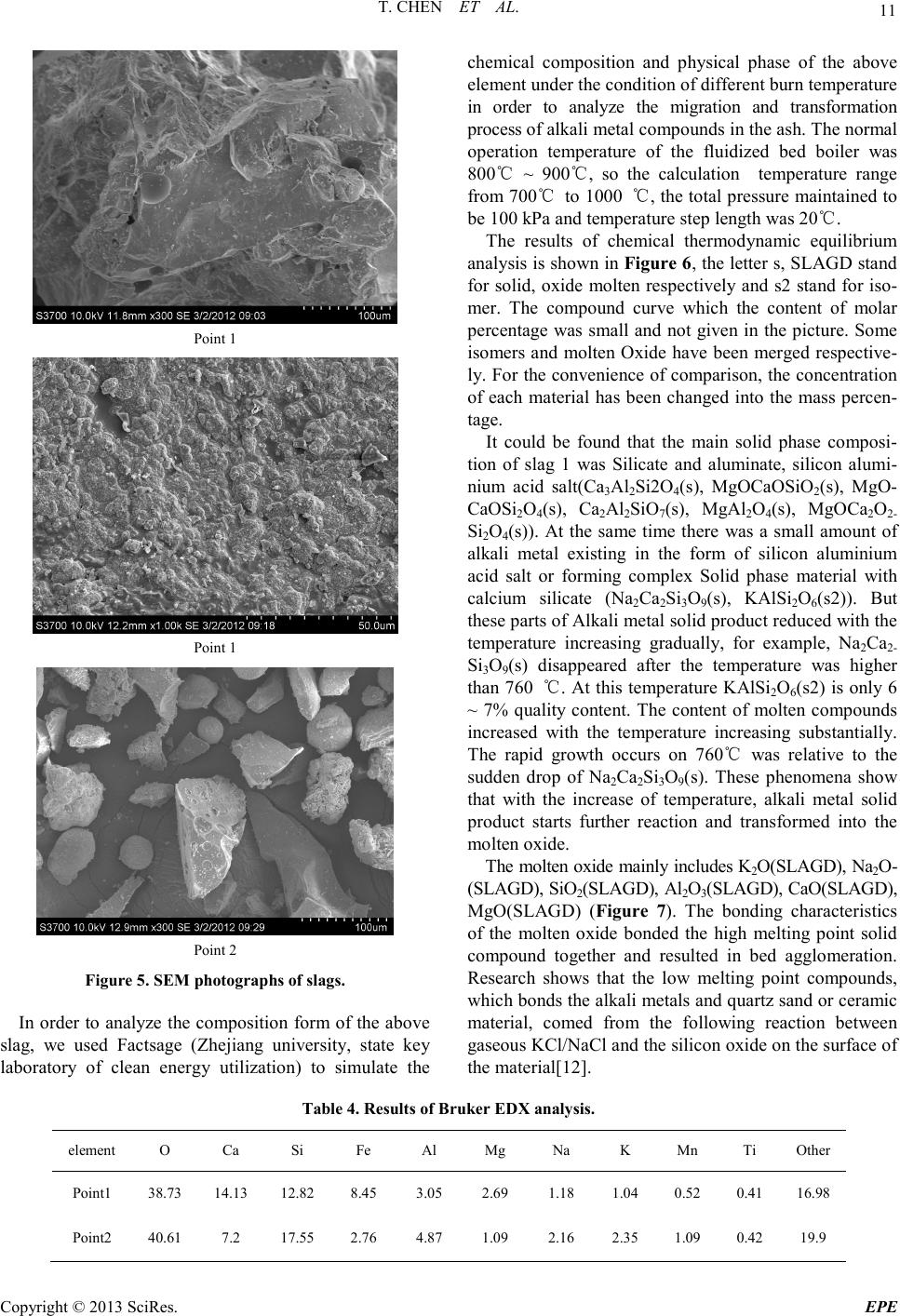 T. CHEN ET AL. Copyright © 2013 SciRes. EPE Point 1 Point 1 Point 2 Figure 5. SEM photogra phs of s lags. In order to analyze the composition form of the above slag, we used Factsage (Zhejiang university, state key laboratory of clean energy utilization) to simulate the chemical composition and physical phase of the above element under the condition of dif fere nt burn temperature in order to analyze the migration and transformation process of a lka l i metal compo und s i n t he a s h. The no rmal operation temperature of the fluidized bed boiler was 800℃ ~ 900℃, so the calculation temperature range from 700℃ to 1000 ℃, the total pressure maintained to be 100 kPa and temperature step length was 20℃. The results of chemical thermodynamic equilibrium anal ysis is s hown in Figure 6, the letter s, S LAGD stand for solid, oxide molten respectively and s2 stand for iso- mer. The compound curve which the content of molar percentage was small and not give n in the picture. So me isomers and molten Oxide have been merged respective- ly. For the convenience of comparison, the concentration of each material has been changed into the mass percen- tage. It could be found that the main solid phase composi- tion of slag 1 was Silicate and aluminate, silicon alumi- nium acid salt(Ca3Al2Si2O4(s), MgOCaOSiO2(s), MgO- CaOSi2O4(s), Ca2Al2SiO 7(s), MgAl2O4(s), MgOCa2O2- Si2O4(s)). At the same time there was a small amount of alkali metal existing in the form of silicon aluminium acid salt or forming complex Solid phase material with calcium silicate (Na2Ca2Si3O9(s), KAlSi 2O6(s2)). But these parts o f Alkali metal solid p ro duct re duced with the temperature increasing gradually, for example, Na2Ca2- Si3O9(s) disappeared after the temperature was higher than 760 ℃. At this temperature KAlSi2O6(s2) is only 6 ~ 7% quality content. The content of molten compounds increased with the temperature increasing substantially. The rapid growth occurs on 760℃ was relative to the sudden drop of Na2Ca2Si3O9( s). These phenomena show that with the increase of temperature, alkali metal solid product starts further reaction and transformed into the molten oxide. The molte n oxid e mai nly i nclu des K2O(SLAG D) , Na2O- (SLAGD), SiO 2(SLAGD), Al2O3(SLAG D) , C aO( S LAG D) , MgO(SLAGD) (Figure 7). The bonding characteristics of the molten oxide bonded the high melting point solid compound together and resulted in bed agglomeration. Research shows that the low melting point compounds, which bonds the alkali metals and quartz sand or ceramic material, come d from the following reaction between gaseous KCl/NaCl and the silicon oxide on the surface of the material[12]. Table 4. Results of Bruker EDX analysis. element O Ca Si Fe Al Mg Na K Mn Ti Other Point1 38.73 14. 13 12.82 8.45 3.05 2.69 1.18 1.04 0.52 0.41 16.98 Point2 40.61 7.2 17.55 2.76 4.87 1.09 2.16 2.35 1.09 0.42 19.9 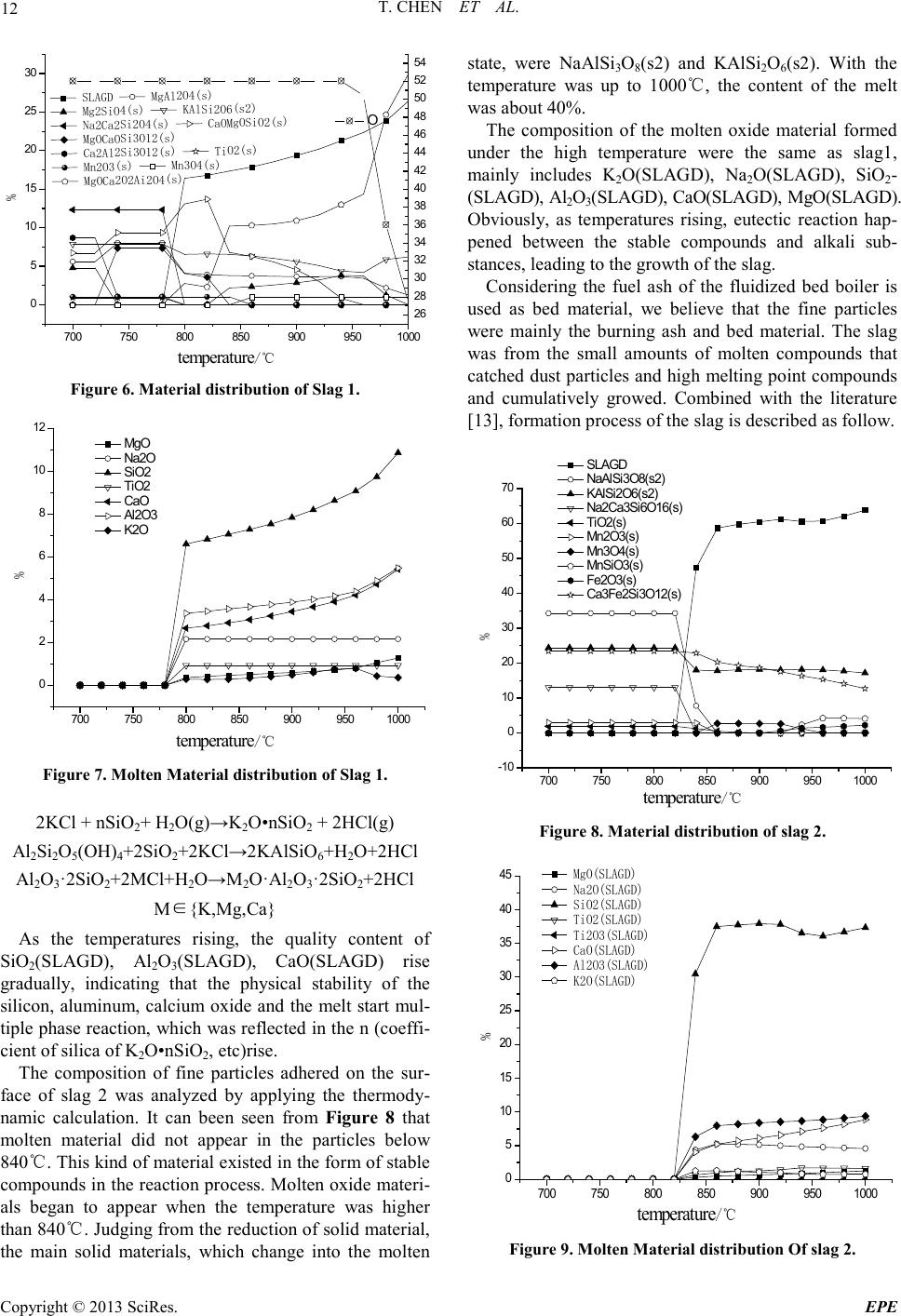 T. CHEN ET AL. Copyright © 2013 SciRes. EPE 700 750 800 850 900 9501000 0 5 10 15 20 25 30 26 28 30 32 34 36 38 40 42 44 46 48 50 52 54 % temperature/℃ SLAGD MgAl2O4(s) Mg2SiO4(s) KAlSi2O6(s2) Na2Ca2Si2O4(s) CaOMgOSiO2(s) MgOCaOSi3O12(s) Ca2Al2Si3O12(s) TiO2(s) Mn2O3(s) Mn3O4(s) MgOCa2O2Ai2O4(s) O Figure 6. Material distr ibution of Slag 1. 700 750 800 850 900 9501000 0 2 4 6 8 10 12 temperatur e/℃ % MgO Na2O SiO2 TiO2 CaO Al2O3 K2O Figure 7. Mol ten M a terial distr ibution o f Slag 1. 2KCl + nSiO 2+ H2O(g)→K 2O•nSiO2 + 2HCl(g) Al2Si2O5(OH)4+2SiO2+2K Cl →2 KAlSiO 6+H2O+2HCl Al2O3·2SiO2+2M Cl+H2O→M 2O·Al2O3·2SiO2+2 HC l M∈{K,Mg,Ca} As the temperatures rising, the quality content of SiO2(SLAGD), Al2O3(SLAGD), CaO(SLAGD) rise gradually, indicating that the physical stability of the silicon, aluminum, calcium oxide and the melt start mul- tiple phase reaction, which was reflected in the n (coeffi- cient of silica of K2O•nSiO2, etc)rise. The composition of fine particles adhered on the sur- face of slag 2 was analyzed by applying the thermo dy- namic calculation. It can been seen from Figure 8 that molten material did not appear in the particles below 840℃. This kind of material e xisted in the for m of stable compounds in the reaction process. Molten oxide materi- als began to appear when the temperature was higher than 840℃. Judging from the reduction of solid material, the main solid materials, which change into the molten state, were NaAlSi3O8(s2) and KAlSi2O6(s2). With the temperature was up to 1000℃, the content of the melt was about 40%. The composition of the molten oxide material formed under the high temperature wer e the same as slag1, mainly includes K2O(SLAGD), Na2O(SLAGD), SiO2- (SLAGD), Al2O3(SLAGD), CaO(SLAG D), MgO(SLAGD). Obviously, as temperatures rising, eutectic reaction hap- pened between the stable compounds and alkali sub- stances, l eading to t he growth of the slag. Considering the fuel ash of the fluidized bed boiler is used as bed material, we believe that the fine particles wer e mainly the burning ash and bed material. The slag was from the small amounts of molten compounds that catched dust particles and high melting point compounds and cumulatively growed. Combined with the literature [13], formation process of the slag is described as follow. 700 750 800 850 900 9501000 -10 0 10 20 30 40 50 60 70 temperatur e/℃ % SLAGD NaAlSi3O8(s2) KAlSi2O6(s2) Na2Ca3S i6O16(s) TiO2(s) Mn2O 3(s) Mn3O 4(s) MnS iO3(s) Fe2O3(s) Ca3Fe2Si3O12(s) Figure 8. Material distr ibution of slag 2. 700 750 800850 9009501000 0 5 10 15 20 25 30 35 40 45 temperatur e/℃ % MgO(SLAGD) Na2O(SLAGD) SiO2(SLAGD) TiO2(SLAGD) Ti2O3(SLAGD) CaO(SLAGD) Al2O3(SLAGD) K2O(SLAGD) Figure 9. Molten Material distribution Of slag 2.  T. CHEN ET AL. Copyright © 2013 SciRes. EPE In the combustion process, alkali metal compounds in the biomass materials and grey elements that accumu- lated to a certain degree such as Si, Al, Ca start reaction to generate low melting eutectic compounds. The melt catch dust particles and stability of high melting point compounds. After the completion of the viscous layer, the slags adhered slag particles in the furnace constantly and growed into a larger piece of slags. With the emer- genced a nd accumulation of agglomeration, the fluidized bed effect was deterior ating, leading to the emergence of local high temperature. When the local high temperature was higher than 840℃, the particles that adhere in the slag (point2) also changed into molten state, which make s the slag growing. Judging from the slag formation process, a small amount of the melt could be accumulated to form larger slags, which leaded to the deterioration of bed Fluidiza- tion effect. On the other hand, the formation of the melt mainly depended on the content of alkali metal and sili- con. Therefore the reasonable control of the content of silicon in the bed material, and operating temperature of fluidized bed would make a great contribution to the control of the bed material agglomeration. D ue to the lo w precipitation temperature of alkali metal, bed temperature control could relieve the heating surface corrosion prob- lems which we re caused by volatile alkali metal trans- fering into the gas phase. 6. Conclusions a) The ash of sugar cane bagasse and leaf produce in Gua ngdong ha ve a h igh co ntent o f alka li meta l, the q ual- ity content of the alkali metal oxide wa s around 30%, which was the important reasons for the serious corro- sion of heating surface in biomass direct combustion boiler. b) The main composition of the outer side of the cor- rosion was particles deposited ash condensed by alka- line metal chlorides as well as low melting point eutec- toid, sulfide, etc which wer e formed by alkali metal compound. The inner surface of the corrosion mainly remai n ed as SUS316 material composition, but adding the alkali, oxygen, chlorine and sulfur content. Among them, t he S c on tent o f the cor ros ion was greater tha n tha t of the pipe, showing that the sulphur content of a bio- mass material involved in the corrosion of metal surfaces. The alkali metal/chlorine mole ratio of Outside wall was close to 1. 0, and that of the inner wall was about 0.1 The content of chlorine was greater than that of alkali metal (mole ratio). c) Judging from the test of both inside and outside wall of the corrosion and the ho rizontal fl ue dust, the analysi s shows that Alkali metal deposited in the outer surface and tube wall metal started reaction to generate low melting point eutectoid, destroying the oxide film of the metal surface. Or started sulfuric acid salinization reac- tion with SO2 in the flue gas, released the chlorine and hydrogen chloride. The chlorine played a catalytic role in the corrosion process, constantly sending metal materials from the inner surface of the pipe to the outer layer and accelerated the corrosion process. d) The agglomeration in fluidized bed mainly com- poses of Silicate and aluminate, silicon aluminium acid salt which has high melting point, and the melt that was formed by alkali metal. A small amount of the melt can be accumulated and conglutinated to form a larger slag, which led to the deterioration of bed fluidization ef- fect. e) With the increase of temperature, alkali metal so- lidification material started further reaction to generate mo lten oxide material, especially when the temperature was higher than 760℃, the molten product content in- creased greatly. Therefore the reasonable control of flu- idized bed operating temperature made a great contribu- tion to the control of the bed material agglomeration. At the same time, due to the low precipitation temperature of alkali metal, be d temperatu re control could relieve the heating surface corrosion problems which wer e caused by volatile alkali metal tr a nsfering into the gas phase. 7. Acknowled gements This research was funded by National Basic Research Program of China(973 Program) (2011CB201500, 2013- CB228101); the National Natural Science Foundation of China (No. 50906025); Key Laboratory of Efficient and Clean Energy Utilization of Guangdong Higher Educa- tion Institutes (KLB10004); the Fundamental Research Funds for the Central Universities(2012ZZ0022). REFERENCES [1] I. Obernbergr, R. Biedermann , W. Widmann and R. Riedl, “Concentrations of Inorganic Elements in Biomass Fuels and Recovery in the Different Ash Fractions,” Biomass and Bioenergy, Vol. 12, No. 3, 1997, pp. 211-224. doi:10.1016/S0961-9534(96)00051-7 [2] K. Sal o, W. Mo jtahedi, “Fate o f Alkal is and Trace Metals in Biomass Gasification,” Biomass and Bioenergy, Vol. 15, No. 3, 1998, pp. 263- 267 doi:10.1016/S0961-9534(98)00019-1 [3] F. Lang, X. Q. Ma, J. J. Wang, “Study on the Ash Cha- racterist ics of Stalks,” Ren ewa bl e En ergy R eso urces, Vol. 25, No. 4, 2007, pp. 25-28. doi:10.3969/j.issn.1671-5292.2007.04.008 [4] J. Xu, “Experimental Research on Alkali Release from Biomass Combustion,” Master’s thesis, Zhejiang Univer- sity, Hangzhou, 2006. [5] H. P. Nielsena, F. J. Frandsena, K. Dam-Johansena, L. L. Baxter, “The Implications of Chlorine-associated Corro- sion on the Operatio n of Biomass-fired Boilers,” Progress in Energy and Combustion Science, V ol. 26 , No. 3, 2000,  T. CHEN ET AL. Copyright © 2013 SciRes. EPE pp. 283-298. doi:10.1016/S0360-1285(00)00003-4 [6] H. J. Grabke, E. R eese and M. Spiegel, “The Effects of Chlorides, Hydrogen Chloride, and Sulfur Dioxide in the Oxidation of Steels below Deposits,” Corrosion Science, Vol. 37, No. 7, 1995, pp. 1023-1043. doi:10.1016/0010-938X(95)00011-8 [7] M. Z. Shi, “Research on Sample Treatment of Chlorine Content in Solid Biofuel,” Goal Quality and Technology, No. 6, 2009, pp. 36-42. doi:10.3969/j.issn.1007-7677.2009.06.012 [8] M. L. Ye, Q. H. Shi and Y. Q. Wang, “Sample Pretreat- ment Technology of Ion Chromatography,” Modern Scientific Instruments, No. 2, 2004, pp. 49-53. doi:10.3969/j.issn.1003-8892.2004.02.011 [9] H. M ar s chner, “Mineral Nutrition of Higher Plants,” 2th Edition, Academic Press , London, 2002. [10] W. Wei, F. Huang, C. J. Yu and M. X. Fang,“Primary Investigation of High-temperature Corrosion Problems in Biomass Combustion Equipment,” Energy Engineering, No. 2, 2011, pp. 23-28. doi:10.3969/j.issn.1004-3950.2011.02.007 [11] O. H. Larsen, N. Henriksen and S. Inselmann, “The In- fluence of Boiler Design and Process Conditions on Fouling and Corrosion in Straw and Coal / strawfired Ul- tra Supercritical Power Plants,” Ninth European Bio- energy C onferen ce , Copenhagen, Denmark, 1996, p. 50. [12] M. Aho and E. Ferrer, “Importance of Coal Ash Compo- sition in Protecting the Boiler against Chlorine Deposition during Combustion of Chlorine-rich Biomass,” Fuel , Vol. 84, No. 2-3, 2005, pp. 201-212. doi:10.1016/j.fuel.2004.08.022 [13] M. Zevenhoven-Onderwater, M. Ohman, B. J. Skrifvars, R. Backman, A. Nordin and M. Hupa, “Bed Agglomera- tion Char acteristics of Wood-Derived Fuels in FBC,” Energ y & Fuels, Vol. 20, No. 2, 2006, pp. 818-824. doi:10.1021/ef050349d
|