 Pharmacology & Pharmacy, 2013, 4, 535-541 http://dx.doi.org/10.4236/pp.2013.47077 Published Online October 2013 (http://www.scirp.org/journal/pp) 535 Anticonvulsant, Anxiolytic and Neurotoxicity Profile of Aqarqarha (Anacyclus pyrethrum) DC (Compositae) Root Ethanolic Extract Syed Mohd Abbas Zaidi1*, Shadab Ahmad Pathan2, Surender Singh3, Shakir Jamil4, Farhan Jalees Ahmad2, Roop Krishen Khar2 1Department of Moalajat, HSZH Govt. Unani Medical College, Bhopal, India; 2Department of Pharmaceutics, Faculty of Pharmacy, Jamia Hamdard, New Delhi, India; 3Department of Pharmacology, All India Institute of Medical Sciences, New Delhi, India; 4Department of Moalijat, Faculty of Medicine (Unani), Jamia Hamdard & CCRUM, New Delhi, India. Email: *drsymab@gmail.com Received July 27th, 2013; revised September 6th, 2013; accepted September 17th, 2013 Copyright © 2013 Syed Mohd Abbas Zaidi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Ethnopharmacological relevance: Aqarqarha (Anacyclus pyrethrum) DC root has long been used as a traditional an- tiepileptic remedy in Unani system of medicine over centuries. Aim of the Study: To rationalize the ethnomedical claim and screen for anxiolytic and neurotoxicity profile of ethanolic extract of Aqarqarha (Anacyclus pyrethrum) root (APE). Materials and Methods: The anticonvulsant and anxiolytic potential of APE (100 - 800 mg/kg) was evaluated against Pentylenetetrazole (PTZ), Bicuculline (BCL), Increasing current electroshock (ICES) and Elevated plus maze(EPM) models. Rotarod test was employed as neurotoxicity model including an additional higher dose (1600 mg/kg). Results: The APE showed significant anticonvulsant activity (p < 0.001) against PTZ (70 mg/kg, i.p.) in a dose-dependent manner but against BCL (30 mg/kg, i.p.) at the dose 800 mg/kg only (p < 0.001). However, it did not protect animals against ICES induced seizures (p > 0.05). The extract also showed anxiolytic behaviour in EPM (p < 0.001) and impaired motor coordination only at 1600 mg/kg in rotarod performance. HPTLC of the extract confirmed the presence of eugenol in the extract. Conclusions: The results suggested significant anticonvulsant activity of APE against PTZ and BCL but failure against ICES. Moreover, APE also exhibited anx iolytic poten tial without an y ev idence of neurotoxicity at the effective dose level. We concluded that anticonvulsant effect of APE is probably mediated by enhancing GA B Aer gi c ne u ro transmission. Keywords: Aqarqarha; HPTLC; Epilepsy; Aqarqarha (Anacyclus pyrethru m) 1. Introduction Epilepsy is one of the most common neurological dis- orders with a prevalence of 5 to 8 cases per 1000 of po- pulation in developed countries, and with even higher rates in under-developed countries [1,2]. Currently avai- lable antiepileptic drugs are synthetic compounds and have dose-related and chronic toxicity, involving vir- tually every major organ system, adverse effects on cog- nition and behavior, reduced bone mineral density and te- ratogenic effects [3-8]. The very high costs of the new antiepileptic drugs have a major impact on the overall cost of epilepsy therapy in both developed and under- developed countries [9]. In the treasury of Unani system of medicine, there are many herbal drugs which are reported to possess anti- epileptic activity and are being used by the physicians since the Stone Age. These drugs are relatively much safer as evident by their long clinical use. The root of Aqarqarha (Anacyclus pyrethrum) DC, popularly known as “Aqarqarha” in the Unani system of medicine, is reported to be used by the Unani physicians to manage, control and treat epilepsy [10,11]. It is also used to stimulate the salivary glands, cure chronic catarrh of the head and nostrils, cure toothache, improve sexu a l debility and clear the brain by exciting a free flow of nasal mucus, tears and by stimulating blood flow to the tissues [12]. Recently it has been found to possess immunostimulating [13] and aphrodisiac [14] action in various rodent *Corresponding a uthor. Copyright © 2013 SciRes. PP  Anticonvulsant, Anxiolytic and Neurotoxicity Profile of Aqarqarha (Anacyclus pyrethrum) DC (Compositae) Root Ethanolic Extract 536 models. The herb contains an essential oil and a pungent alka- loid, pellitorin or pyrethrin. It has alkamides, lignane (in- cluding sesamine), inulin (fructosan) and tannins. Alka- mides include deca-2, 4-dien acid-isobutylamide, ana- cycline, and dehydroanacycline [15,16]. The presence of a polyacetylenic compound is also reported in the root [17]. It is also reported to contain eugenol [18,19]. In an effort to scientifically validate an d rationa lize the medicinal value of this drug, the present study was un- dertaken to examine the anticonvulsant, anxiolytic and neurotoxicity profile of this herb’s root ethanolic extract in various experimental paradigms. 2. Materials and Methods 2.1. Plant Material Fresh roots of Aqarqarha (Anacyclus pyrethrum) DC (Com- positae) were purcha sed fro m an author ized supp lier (Green Earth Products, G.K.-1, New Delhi) which was harv ested in October from the Katra region of Jammu & Kashmir. Identification and taxonomical authentication was per- formed by a renowned taxonomist of NISCAIR, New Delhi and a voucher specimen no. “NISCAIR/RHMD/CON- SULT/-2008-09/1099/130/Aqarqarha (Anacyclus pyre- thrum)/DATE 07NOV2008” was deposited in the her- barium. 2.2. Preparation of Aqarqarha (Anacyclus pyre- thrum) Root Ethanolic Extract (APE) One kilogram (1 kg) of crushed, air dried roots of Ana- cyclus pyrethrum was milled into a fine powder in a commercial blender. The powder was extracted twice by cold maceration on each occasion with 2.5 L of 99.9% ethanol for 48 h at room temperature, with occasional shaking. The combined extracts were concentrated to dryness under reduced pressure at 40˚C ± 1˚C in a rotary evaporator, finally giving 35.56 g (3.556% w/w yield) of a dark brown, powdery crude APE. Aliquot portions of the crude APE residue were weighed and dissolved in 0.5% w/v Tween 80 for use on each day of our experi- ments. 2.3. HPTLC Fingerprinting of the Prepared Extract TLC profile of the APE was developed prior to going for the HPTLC. Toluene: Ethyl acetate: Formic acid (3:2:0.5) was selected as the mobile phase in the UV-mode. The prepared extract was dissolved in ethanol and applied to the pre-coated Silica gel 60F254 TLC plates (E. Merck) using a CAMAG Linomat IV Automatic Sample Spotter. After development in mobile phase, th e plates were dried in air and scanned at 254 nm using a CAMAG TLC Scanner and winCATS software. Eugenol was also applied in the same manner to the HPTLC plates to find out the presence of eugenol in the extract. 2.4. Drugs The drugs used were sodium valproate (Wockhardt Ltd., Mumbai, India), Pentylenetetrazole (PTZ) and Bicucul- line (BCL) from Sigma Aldrich, USA. Drugs were freshly prepared by dissolving in distilled water. All i.p. injections were given in volumes of not more than 10 ml/kg of the body weight for mice. 2.5. Animals Swiss male albino mice weighing 20 - 35 g were pro- cured from the central animal house, Jamia Hamdard, New Delhi. Animals were housed in group s of 6 per cage and maintained at 20˚C - 30˚C and 50% - 55% humidity in a natural light an d dark cycle, with free access to food and water. The experiments were performed during the light cycle in awake, freely moving animals that were adjusted to laboratory conditions before proceeding with the experiments. The animals were divided into four groups of plant’s extract (100, 200, 400 & 800 mg/kg) , reference anticonvulsan t drug-treated “test”, and distilled water-treated “cont rol” groups of 6 animals per group. The study was performed with prior approval of the Institutional Animal Ethics Committee (IAEC) of CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals) with approval reg. no. JH/CPCSEA/31-01-2000/Project no.418/2007 2010. Utmost care was taken to ensure that animals were treated in the most humane and ethically acceptable manner. 2.6. Evaluation of Anticonvulsant Activity 2.6.1. PTZ and BCL Test Seizures were induced chemically with PTZ as described by Vohora et al. [20]. Standard convulsing agents, PTZ (70 mg/kg, i.p.), and BCL (30 mg/kg, i.p.) were used to induce convulsions in mice. Sodium valproate (300 mg/ kg, p.o.) was used as the reference anticonvulsant drug for comparison. In the test group, the seizures were in- duced after 60 min prior treatment with graded doses of the APE (100 - 800 mg/kg, p.o.) and sodium valproate (300 mg/kg, p.o.). The ability of the APE to prevent the seizures or delay the latency or onset of the hind-limb tonic extensions was considered as an indication of anti- convulsant activity. The onset and duration of convul- sions in the mice were noted and recorded, and percen- tage protection was determined. Distilled water (10 ml/kg, p.o.) treated mice were used as “control” animals. Copyright © 2013 SciRes. PP 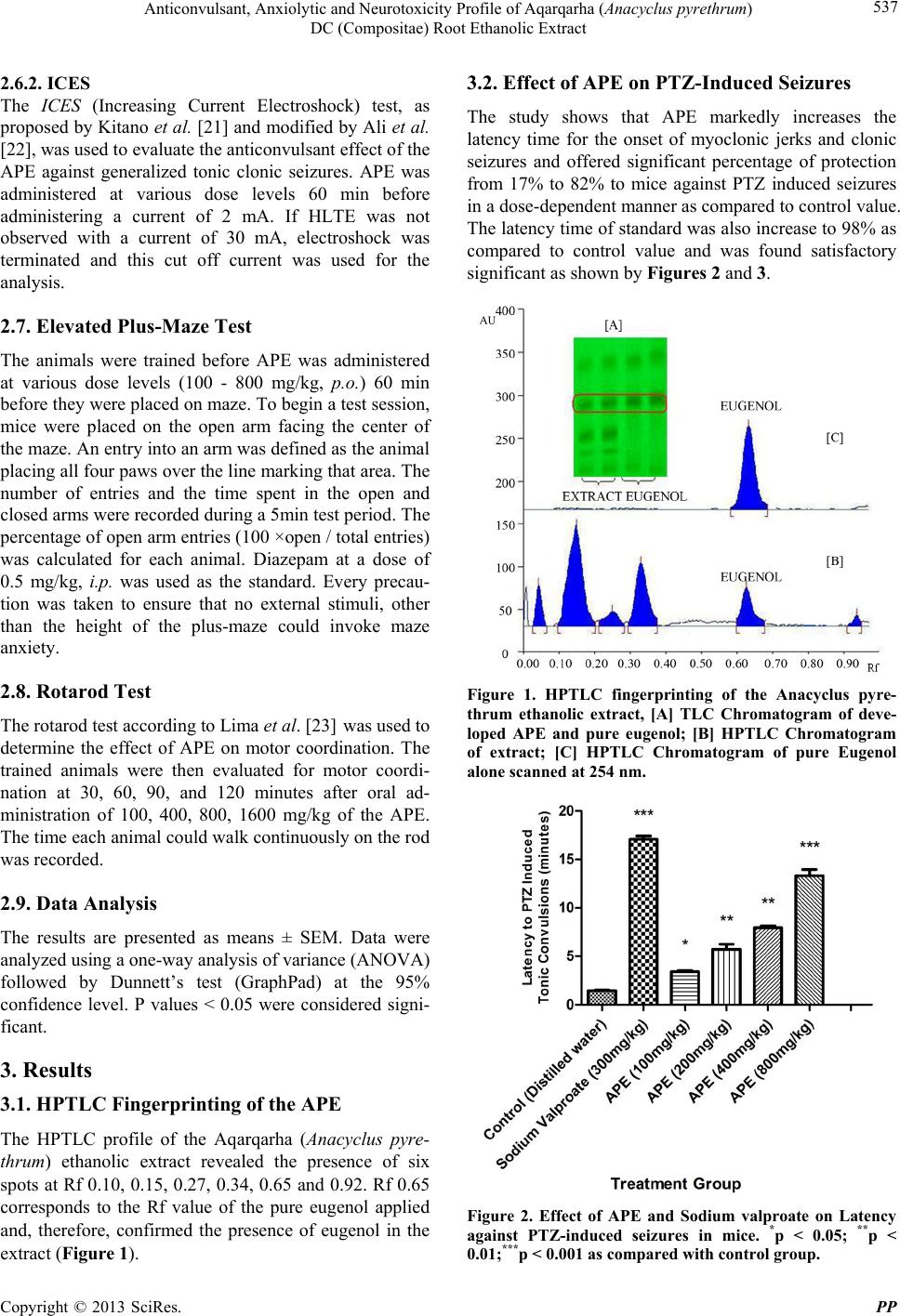 Anticonvulsant, Anxiolytic and Neurotoxicity Profile of Aqarqarha (Anacyclus pyrethrum) DC (Compositae) Root Ethanolic Extract 537 2.6.2. ICES The ICES (Increasing Current Electroshock) test, as proposed by Kitano et al. [21] and modified by Ali et al. [22], was used to evaluate the an ticonvulsant effect of the APE against generalized tonic clonic seizures. APE was administered at various dose levels 60 min before administering a current of 2 mA. If HLTE was not observed with a current of 30 mA, electroshock was terminated and this cut off current was used for the analysis. 2.7. Elevated Plus-Maze Test The animals were trained before APE was administered at various dose levels (100 - 800 mg/kg, p.o.) 60 min before they were placed on maze. To begin a test session, mice were placed on the open arm facing the center of the maze. An entry into an arm was defined as the animal placing all four paws over the line marking that area. The number of entries and the time spent in the open and closed arms were recorded during a 5min test period. The percentage of open arm entries (100 ×open / total entries) was calculated for each animal. Diazepam at a dose of 0.5 mg/kg, i.p. was used as the standard. Every precau- tion was taken to ensure that no external stimuli, other than the height of the plus-maze could invoke maze anxiety. 2.8. Rotarod Test The rotarod test according to Lima et al. [23] was used to determine the effect of APE on motor coordination. The trained animals were then evaluated for motor coordi- nation at 30, 60, 90, and 120 minutes after oral ad- ministration of 100, 400, 800, 1600 mg/kg of the APE. The time each animal could walk continuously on the rod was recorded. 2.9. Data Analysis The results are presented as means ± SEM. Data were analyzed using a one-way analysis of variance (ANOVA) followed by Dunnett’s test (GraphPad) at the 95% confidence level. P values < 0.05 were considered signi- ficant. 3. Results 3.1. HPTLC Fingerprinting of the APE The HPTLC profile of the Aqarqarha (Anacyclus pyre- thrum) ethanolic extract revealed the presence of six spots at Rf 0.10, 0.15, 0.27, 0.34, 0.65 and 0.92. Rf 0.65 corresponds to the Rf value of the pure eugenol applied and, therefore, confirmed the presence of eugenol in the extract (Figure 1). 3.2. Effect of APE on PTZ-Induced Seizures The study shows that APE markedly increases the latency time for the onset of myoclonic jerks and clonic seizures and offered significant percentage of protection from 17% to 82% to mice against PTZ induced seizures in a dose-dependent manner as compared to control value. The latency time of stan dard was also increase to 98% as compared to control value and was found satisfactory significant as shown by Figures 2 and 3. Figure 1. HPTLC fingerprinting of the Anacyclus pyre- thrum ethanolic extract, [A] TLC Chromatogram of deve- loped APE and pure eugenol; [B] HPTLC Chromatogram of extract; [C] HPTLC Chromatogram of pure Eugenol alone scanned at 254 nm. Figure 2. Effect of APE and Sodium valproate on Latency against PTZ-induced seizures in mice. *p < 0.05; **p < 0.01;***p < 0.001 as compared with control group. Copyright © 2013 SciRes. PP  Anticonvulsant, Anxiolytic and Neurotoxicity Profile of Aqarqarha (Anacyclus pyrethrum) DC (Compositae) Root Ethanolic Extract 538 Figure 3. Percentage anticonvulsant protective effect of APE on PTZ-induced seizures in mice. *p < 0.05; **p < 0.01 and ***p < 0.001 as compared with control group. 3.3. Effect of APE on BCL-Induced Seizures The study shows that 66% animals, treated with APE (800 mg/kg) produced significant protection (p < 0.001) against BCL induced seizures, while the animals treated with lower d oses of AP E (100 - 4 00 mg/k g ) d id n o t show any protection against BCL induced seizures. 100% animals showed significant (p < 0 .001) protectio n again st BCL induced seizures which were treated with standard (Sodium valproate). The latency of tonic convulsions was found 7.26%, 15.22 % 33.91% and 227.33% incr ease in APE (100-800 mg/kg) treated animals and 530.79% increase in sodium valproate treated animals as shown by Figures 4 and 5. 3.4. Effect of APE on ICES-Induced Seizures Aqarqarha (Anacyclus pyrethrum) root ethanolic extract (APE, 100 - 800 mg/kg, p.o.) did not show any protective effect against ICES-induced seizures. 3.5. Effect of APE on Elevated plus Maze In the elevated plus maze, the observed behavior con- firmed the anxiolytic activity of diazepam as reported. The APE at the dose of 800 mg/kg increased the per- centage of time spent and entries in the open arms (p < 0.001). However, APE (100 - 400 mg/kg) had no sig- nificant effect on any of the measured parameters (Fig- ures 6 and 7). 3.6. Effect of APE on Rotarod Test APE did not produce the statistically significant distur- Figure 4. Effects of APE and Sodium valproate on Latency against BCL induced seizures in mice ***p < 0.001 as compared wi t h control group. Figure 5. Percentage anticonvulsant protective effect of APE against BCL-induced seizures in mice. ***p < 0.001 as compared wi t h control group. bance in motor coordination or fall-off time up to the maximum dose of 1600 mg/kg p.o. at 60 min post- administration period. However, the dose of 1600 mg/kg of APE at 90 and 120 min post administration exhibited a significant impairment in motor coordination (Figure 8). 4. Discussion There are a number of synthetic anticonvulsant drugs currently available for use in the management, control Copyright © 2013 SciRes. PP  Anticonvulsant, Anxiolytic and Neurotoxicity Profile of Aqarqarha (Anacyclus pyrethrum) DC (Compositae) Root Ethanolic Extract 539 Figure 6. Number of open arm entries in Elevated plus maze. ***p < 0.001 as compared with control group. Figure 7. Percentage time spent in open arm in Elevated plus maze ***p < 0.001 as compared with control group. and/or treatment of individuals with epilepsy. Keeping in view of their inaccessibility, higher cost and increased toxicity, there is a dire need for the development of cheap, effective and relatively safer anticonvulsant agents from plants and other natural sources. Although Aqarqarha (Anacyclus pyrethrum) root is widely used by the practitioners of Unani system of medicine for epilepsy, relatively little scientific information ex ists in biomedical literature on the therapeutic efficacy of the plant. The data obtained in this study also indicate that relatively moderate and high doses of APE inhibited or attenuated PTZ-induced seizures, while the reference Figure 8. Effect of i.p. administration of APE on rotarod test endurance time in seconds at different time intervals: 30, 60, 90, and 120 min post administration. *p < 0.05 and **p < 0.01 as compared with control group. anticonvulsant drug used, i.e. sodium valproate, com- pletely abolished the seizures and protected all the ani- mals against PTZ-induced seizures. PTZ has been re- ported to produce seizures by inhibiting gamma amino- butyric acid (GABA) neurotransmission. Favoring the GABAergic hypothesis for sodium valproate is inhibition of the onset of seizures induced by GABA antagonists [24] which selectively enhances GABAergic inhib ition in the cerebral cortex [25]. It would appear, therefore, that the complete protection of the mice by the reference anticonvulsant drug used in this study against PTZ-indu ced seizures is in consonance with the above hypothesis. The findings of the present study, therefore, tend to suggest that APE might have inhibited and/or attenuated PTZ-induced seizures of the mice by inhibiting high-frequency firing of neurons and/or enhancing some ways interfering with GABA er- gic neurotransmission. BCL, a potent and selective GABAA-receptor antago- nist, produces seizures by blocking the effect of GABA at central GABAA-receptors, which have been associated with epilepsy. Sodium valproate, which also acts by enhancing GABA neurotransmission, antagonized BCL- induced seizures in the present study. However, APE only at dose 800 mg/kg, antagonized BCL-induced sei- zures, which was less effective in this regard com- pared with Sodium valproate. This observation also tends to suggest that APE interferes with GABAergic neuro- transmission. APE was also found to possess anxiolytic potential when examined on the elevated plus-maze test at the dose of 800 mg/kg. The magnitude of the anxio- lytic effects of this dose was very close to that observed with 0.5 mg/kg of diazepam. These anxiolytic properties could be mediated by some components in the extract interacting with the benzodiazepine/GABAA receptors as Copyright © 2013 SciRes. PP  Anticonvulsant, Anxiolytic and Neurotoxicity Profile of Aqarqarha (Anacyclus pyrethrum) DC (Compositae) Root Ethanolic Extract 540 agonists, with the 5-HT1A receptors agon ists, or with any other mechanisms. Furthermore, APE did not show any neurotoxic activities at the effective anticonvulsant dose level (800 mg/kg) in the rotarod test. However, it impaired motor coordination at 1600 mg/kg, 90 min after the APE administration. Our present state of knowledge of the chemical con- stituents of the extract is limited. It is, therefore, im- possible for us at this stage, to pinpoint and identify with certainty, the anticonvulsant principle of the extract. Nevertheless, eugenol (4-allyl-2-methoxy phenol, C 10H12O2, an aromatic terpene compound, whose presence was confirmed in the extract by HPTLC fingerprinting, is likely to account for the observed anticonvulsant activity of the APE. Szabadics and Erdelyi [26] have shown that eugenol blocks the synaptic transmission in the snail, Helix pomatia L., neurons both pre- and post-syn- aptically. There are some studies indicating that eugenol possesses an inhibitory effect on the events related to NMDA receptor activation and also protects neuronal cells from NMDA-induced excitotoxic and oxidative injury [27]. Furthermore, it protects hippocampal neu- rons from global ischemia through modulation of NMDA receptors and also delays NMDA-induced convulsions [28]. The experimental evidence obtained in the present laboratory animal study indicates that APE significantly delayed the onset of seizures induced by PTZ. Since PTZ-induced seizures have been shown to be due to the inhibition and/or attenuation of GABAergic neurotrans- mission, it is not unreasonable to speculate that APE probably produces its anticonvulsant activity by en- hancing GABAergic neurotransmission. In conclusion, the findings of the present laboratory animal study lend a pharmacological support to the sug- gested anecdotal, ethnomedical uses of Aqarqarha (Ana- cyclus pyrethrum) roots in the treatment of epilepsy in the Unani system of medicine. The results of the present laboratory animal study provide evidence in favor of the selective anticonvulsant activity of the herb, and show that APE also possesses significant anxiolytic activity without evidence of any neurotoxicity at the effective dose level used. The effectiveness of the plant’s extract in the experimental convulsion paradigm used probably suggests that the herb could be used in complex partial and absence types of epilepsy as supported by its failure to protect the animals against ICES test. However, extensive studies are needed to evaluate the precise mechanism(s) of the plant as a medicinal remedy for epilepsy. 5. Acknowledgements Authors are highly thankful to Dr Shadab Zafar (Medical Officer, Municipal Corporation of Delhi) for his useful suggestions. REFERENCES [1] J. W. Sander and S. D. Shorvon, “Epidemiology of the Epilepsies,” Journal of Neurology Neurosurgery & Psy- chiatry, Vol. 61, No. 5, 1996, pp. 433-443. http://dx.doi.org/10.1136/jnnp.61.5.433 [2] N. Senanayake and G. C. Roman, “Epidemiology of Epi- lepsies in Developing Countries,” WHO Bulletin, Vol. 71, No. 2, 1993, pp.247-258. [3] O. Devinsky, “Cognitive and Behavioral Effects of An- tiepileptic Drugs,” Epilepsia, Vol. 36, Suppl. s2, 1995, pp. S46-S65. http://dx.doi.org/10.1111/j.1528-1157.1995.tb05999.x [4] G. L. Holmes, “Critical Issues in the Treatment of Epi- lepsy,” American Journal of Hospital Pharmacist, Vol. 50, Suppl. 5, 1993, pp. S5-S16. [5] R. H. Mattson, “Efficacy and Adverse Effects of Estab- lished and New Antiepileptic Drugs,” Epilepsia, Vol. 36, Suppl. S2, 1995, S13- S26. http://dx.doi.org/10.1111/j.1528-1157.1995.tb05995.x [6] E. B. Samren, C. M. V. Duijn, G. C. M. L. Christiaens, A. Hofman and D. Lindhout, “Antiepileptic Drug Regimens and Major Congenital Abnormalities in the Offspring,” Annals of Neurology, Vol. 46, No. 5, 2001, pp. 739-746. http://dx.doi.org/10.1002/1531-8249(199911)46:5<739:: AID-ANA9>3.0.CO;2-2 [7] M. C. Smith and T. P. Bleck, “Convulsive Disorders: Toxicity of Anticonvulsants,” Clinical Neuropharmacol- ogy, Vol. 14, No. 2, 1991, pp. 97-115. http://dx.doi.org/10.1097/00002826-199104000-00001 [8] I. I. Ali, L. Schuh, G. L. Barkley and J. R. Gates, “An- tiepileptic Drugs and Reduced Bone Mineral Density,” Epilepsy & Behavior, Vol. 5, No. 3, 2004, pp. 296-300. http://dx.doi.org/10.1016/j.yebeh.2004.02.005 [9] D. Heaney, “The Pharmacoeconomics of the New An- tiepileptic Drugs,” Epilepsia, Vol. 40, Suppl. s8, 1999, pp. 25-31. http://dx.doi.org/10.1111/j.1528-1157.1999.tb00944.x [10] Z. Razi, “Al-Hawi-Fil-Tib (Urdu Translation),” CCRUM Publication, New Delhi, 1997. [11] I. Baitar, “Al-Jam-e-ul-Mufradat-Al-Adviah-Wal-Agh- ziya (Urdu translation),” CCRUM publication, New Delhi, 1999. [12] N. G. Khan, “Khazainul Adviah,” Matba Munshi Naval Kishore, Lucknow, 1921. [13] D. Bendjeddou, K. Lalaoui and D. Satta, “Immunostimu- lating Activity of the Hot Water-Soluble Polysaccharide Extracts of Anacyclus pyrethrum, Alpinia galanga and Citrullus colocynthis,” Journal of Ethnopharmacology, Vol. 88, No. 2-3, 2003, pp. 155-160. http://dx.doi.org/10.1016/S0378-8741(03)00226-5 [14] V. Sharma, M. Thakur, N. S. Chauhan and V. K. Dixit, “Evaluation of the Anabolic, Aphrodisiac and Reproduc- Copyright © 2013 SciRes. PP  Anticonvulsant, Anxiolytic and Neurotoxicity Profile of Aqarqarha (Anacyclus pyrethrum) DC (Compositae) Root Ethanolic Extract Copyright © 2013 SciRes. PP 541 tive Activity of Anacyclus pyrethrum DC in Male Rats,” Scientia Pharmaceutica, Vol. 77, 2009, pp. 97-110. http://dx.doi.org/10.3797/scipharm.0808-14 [15] J. M. Gulland and G. U. Hopton, “Pellitorine, the Pungent Principle of Anacyclus pyrethrum,” Journal of Chemical Society, 1930, pp. 6-11. http://dx.doi.org/10.1039/jr9300000006 [16] R. S. Burden and L. Crombie, “Amides of Vegetable Origin. Part XII. A New Series of Alka-2, 4-Dienoic Tyramine -Amides from Anacyclus pyrethrum D. C. (Co m- positae),” Journal of the Chemical Society C: Organic, No. 19, 1969, pp. 2477-2481. http://dx.doi.org/10.1039/j39690002477 [17] “The Wealth of India,” CSIR Publication, New Delhi, 1985. [18] K. Sukumaran and R. Kuttan, “Inhibition of Tobacco- Induced Mutagenesis by Eugenol and Plant Extracts,” Mutation Research/Genetic Toxicology, Vol. 343, No. 1, 1995, pp. 25-30. http://dx.doi.org/10.1016/0165-1218(95)90059-4 [19] A. Gorji and M. K. Ghadiri, “History of Epilepsy in Me- dieval Iranian Medicine,” Neuroscience & Biobehavoiral Reviews, Vol. 25, No. 5, 2001, pp. 455-461. http://dx.doi.org/10.1016/S0149-7634(01)00025-2 [20] D. Vohora, S. N. Pal and K. K. Pillai, “Thioperamide a Selective Histamine H3 Receptor Antagonist, Protects Against PTZ-Induced Seizures in Mice,” Life Science, Vol. 66, No. 22, 2000, pp. 297-301. http://dx.doi.org/10.1016/S0024-3205(00)00548-8 [21] Y. Kitano, C. Usui, K. Takasuna, M. Hirohashi and M. Nomura, “Increasing-Current Electroshock Seizure Test: A New Method for Assessment of Anti- and Pro-Con- vulsant Activities of Drugs in Mice,” Journal of Phar- macological and Toxicological Methods, Vol. 35, No. 1, 1996, pp. 25-29. http://dx.doi.org/10.1016/1056-8719(95)00115-8 [22] A. Ali, F. J. Ahmad, K. K. Pillai and D. Vohora, “Evi- dence of the Antiepileptic Potential of Amiloride with Neuropharmacological Benefits in Rodent Models of Epi- lepsy a n d Be ha vi o r , ” Epilepsy & Behaviour, Vol. 5, No. 3, 2004, pp. 322-328. http://dx.doi.org/10.1016/j.yebeh.2004.01.005 [23] T. C. M. Lima, G. S. Morato and R. N. Takahashi, “Eva- luation of the Control Properties of Artemisia Verlotu- rum,” Planta Medica, Vol. 59, No. 4, 1993, pp. 326-329. http://dx.doi.org/10.1055/s-2006-959692 [24] H. H. Frey and W. Loscher, “Di-n-Propyletic Acid-Pro- file of Anticonvulsant Activity in Mice,” Arzneimittelfor- schung, Vol. 26, 1976, pp. 299-301. [25] F. Baldino and H. M. Geller, “Sodium Valproate En- hancement of Gamma-Aminobutyric Acid (GABA) Inhi- bition: Electro-Physiological Evidence for Anticonvulsant Activity,” The Journal of Pharmacology and Experimen- tal Therapeutics, Vol. 217, No. 2, 1981, pp. 445-450. [26] J. Szabadics and L. Erdelyi, “Pre- and Postsynaptic Effect of Eugenol and Related Compounds on Helix pomatia L. Neurons,” Acta Biologica Hungarica, Vol. 51, No. 2-4, 2000, pp. 265-273. [27] M. B. Wie, M. H. Won, K. H. Lee, J. H. Shin, J. C. Lee, H. W. Suh, D. K. Song and Y. H. Kim, “Eugenol Protects Neuronal Cells from Excitotoxic and Oxidative Injury in Primary Cortical Cultures,” Neuroscience Letters, Vol. 225, No. 4, 1997, pp. 93-96. http://dx.doi.org/10.1016/S0304-3940(97)00195-X [28] M. H. Won, J. C. Lee, Y. H. Kim, D. K. Song, H. W. Suh, Y. S. Oh, J. H. Kim, T. K. Shin, Y. J. Lee and M. B. Wie, “Post-Ischemic Hypothermia Induced by Eugenol Pro- tects Hippocampal Neurons from Global Ischemia in Ger- bils,” Neuroscience Letters, Vol. 254, No. 2, 1998, pp. 101-104. http://dx.doi.org/10.1016/S0304-3940(98)00664-8
|