 International Journal of Analytical Mass Spectrometry and Chromatography, 2013, 1, 48-54 http://dx.doi.org/10.4236/ijamsc.2013.11006 Published Online September 2013 (http://www.scirp.org/journal/ijamsc) The Combination of Coagulation-Flocculation Method and the SCWO in the Waste Water Tr eatment Problems Elmira Shakirovna Gayazova, Rustem Aytuganovich Usmanov, Farid Mukhamedovich Gumerov*, Sergey Vladimirovich Friedland, Zufar Ibrahimovich Zaripov, Farizan Rakibovich Gabitov, Rashid Zagitovich Musin Kazan National Research University, Kazan, Russia Email: *gum@kstu.ru Received July 18, 2013; revised August 22, 2013; accepted September 19, 2013 Academic Editor: Ilia Brondz1,2 1Department of Biosciences, University of Oslo, Oslo, Norway; 2R&D Department, Jupiter Ltd. Copyright © 2013 Elmira Shakirovna Gayazova et al. This is an open access article distributed under the Creative Commons Attribu- tion License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT The influence of the degree of wastewater coagulation-flocculation and supercritical water oxidation (SCWO) methods is considered. The regularities of changes in the composition of the purity of the reagents used and the parameters of SCWO are established. Based on the results of chromatographic analysis of the effluent after washing the mass rape, it is found that the achievement of the required parameters is achieved by treatment with a combination of coagulation- flocculation method and supercritical water oxidation (SCWO). The necessity of combining techniques is insufficient oxidation in SCWO lignin conducted at T = 400˚C and P = 25 MPa, T = 500˚C and P = 30 MPa. Effluent treatment of process of styrene and propylene oxide “Nizhnekamskneftekhim” conducted by the SCWO, using an oxidant (H2O2), and without an oxidant showed the possibility of cleaning without the use of an oxidizing agent in the process parame- ters T = 500˚C, P = 30 MPa. Keywords: Supercritical Water Oxidation (SCWO); Wastewater Treatment; Production of Pulp; Coagulation; Flocculation; Chemical Axigen Demand (COD); Chromato Graphic Analysis 1. Introduction In this paper, we have considered the possibility for super- critical water oxidation (SCWO) treatment of wastewater containing components, which are difficultly oxidizing. Oxidative methods are increasingly used in wastewater treatment. Most often ozone, chlorine, sodium hypochlo- rite, hydrogen peroxide, at least—Fenton’s reagent are used as oxidants. Each of these reagents has its pros and cons, so the introduction of advanced oxidation processes in water treatment practices using new oxidants is very important. In recent years, a new method of industrial wastewater treatment is rapidly developing, based on the use of the supercritical water as an oxidant (T > 374˚C, P > 22.1 MPa) [1]. When the water is in supercritical conditions, it behaves like a nonpolar solvent unlimitedly dissolving organic substances and gases, but not dissolving the mineral salts. In SCW almost complete utilization of in- dustrial waste water in a reasonable time is achieved by varying the temperature and concentration of the reac- tants. The completeness and speed of reactions in super- critical water (SCW) are provided by a molecular disper- sion of the reactants. The kinetics and mechanisms of chemical reactions in SCW depends on the temperature and pressure (density) of the medium. Thus, even a slight change in pressure SCW is accompanied by a significant change in the density, essential for diffusion, viscosity, and dielectric properties of the solvent medium. The SCWO process goes with the release of heat if the initial reaction mixture contains enough organic sub- stances (10% - 25%) [2]. 2. Experimental Waste water after rapeseed mass washing that contains hardly degradable polymer components (lignin, cellulose Table 1) was selected as the object of the study. The composition of the industrial wastewater after washing is shown in Ta ble 1. *Corresponding author. C opyright © 2013 SciRes. IJAMSC 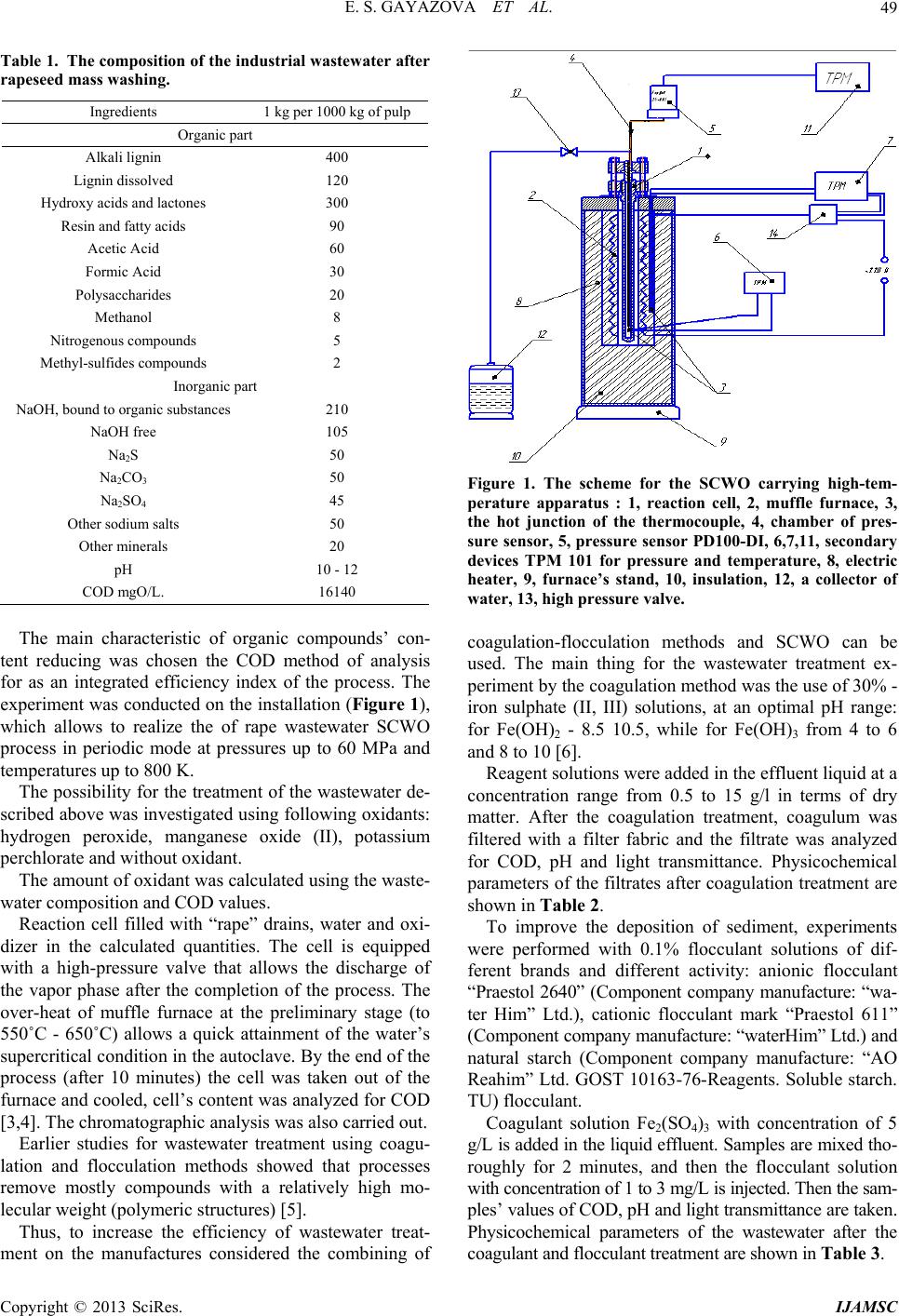 E. S. GAYAZOVA ET AL. 49 Table 1. The composition of the industrial wastew ater after rapeseed mass washing. Ingredients 1 kg per 1000 kg of pulp Organic part Alkali lignin 400 Lignin dissolved 120 Hydroxy acids and lactones 300 Resin and fatty acids 90 Acetic Acid 60 Formic Acid 30 Polysaccharides 20 Methanol 8 Nitrogenous compounds 5 Methyl-sulfides compounds 2 Inorganic part NaOH, bound to organic substances210 NaOH free 105 Na2S 50 Na2CO3 50 Na2SO4 45 Other sodium salts 50 Other minerals 20 рН 10 - 12 COD mgO/L. 16140 The main characteristic of organic compounds’ con- tent reducing was chosen the COD method of analysis for as an integrated efficiency index of the process. The experiment was conducted on the installation (Figure 1), which allows to realize the of rape wastewater SCWO process in periodic mode at pressures up to 60 MPa and temperatures up to 800 K. The possibility for the treatment of the wastewater de- scribed above was investigated using following oxidants: hydrogen peroxide, manganese oxide (II), potassium perchlorate and without oxidant. The amount of oxidant was calculated using the waste- water composition and COD values. Reaction cell filled with “rape” drains, water and oxi- dizer in the calculated quantities. The cell is equipped with a high-pressure valve that allows the discharge of the vapor phase after the completion of the process. The over-heat of muffle furnace at the preliminary stage (to 550˚C - 650˚C) allows a quick attainment of the water’s supercritical condition in the autoclave. By the end of the process (after 10 minutes) the cell was taken out of the furnace and cooled, cell’s content was analyzed for COD [3,4]. The chromatographic analysis was also carried out. Earlier studies for wastewater treatment using coagu- lation and flocculation methods showed that processes remove mostly compounds with a relatively high mo- lecular weight (polymeric structures) [5]. Thus, to increase the efficiency of wastewater treat- ment on the manufactures considered the combining of Figure 1. The scheme for the SCWO carrying high-tem- perature apparatus : 1, reaction cell, 2, muffle furnace, 3, the hot junction of the thermocouple, 4, chamber of pres- sure sensor, 5, pressure sensor PD100-DI, 6,7,11, secondary devices TPM 101 for pressure and temperature, 8, electric heater, 9, furnace’s stand, 10, insulation, 12, a collector of water, 13, high pressure valv e. coagulation-flocculation methods and SCWO can be used. The main thing for the wastewater treatment ex- periment by the coagulation method was the use of 30% - iron sulphate (II, III) solutions, at an optimal pH range: for Fe(OH)2 - 8.5 10.5, while for Fe(OH)3 from 4 to 6 and 8 to 10 [6]. Reagent solutions were added in the effluent liquid at a concentration range from 0.5 to 15 g/l in terms of dry matter. After the coagulation treatment, coagulum was filtered with a filter fabric and the filtrate was analyzed for COD, pH and light transmittance. Physicochemical parameters of the filtrates after coagulation treatment are shown in Table 2. To improve the deposition of sediment, experiments were performed with 0.1% flocculant solutions of dif- ferent brands and different activity: anionic flocculant “Praestol 2640” (Component company manufacture: “wa- ter Him” Ltd.), cationic flocculant mark “Praestol 611” (Component company manufacture: “waterHim” Ltd.) and natural starch (Component company manufacture: “AO Reahim” Ltd. GOST 10163-76-Reagents. Soluble starch. TU) flocculant. Coagulant solution Fe2(SO 4)3 with concentration of 5 g/L is added in the liquid effluent. Samples are mixed tho- roughly for 2 minutes, and then the flocculant solution with concentration of 1 to 3 mg/L is injected. Then the sam- ples’ values of COD, pH and light transmittance are taken. Physicochemical parameters of the wastewater after the coagulant and flocculant treatment are shown in Table 3. Copyright © 2013 SciRes. IJAMSC 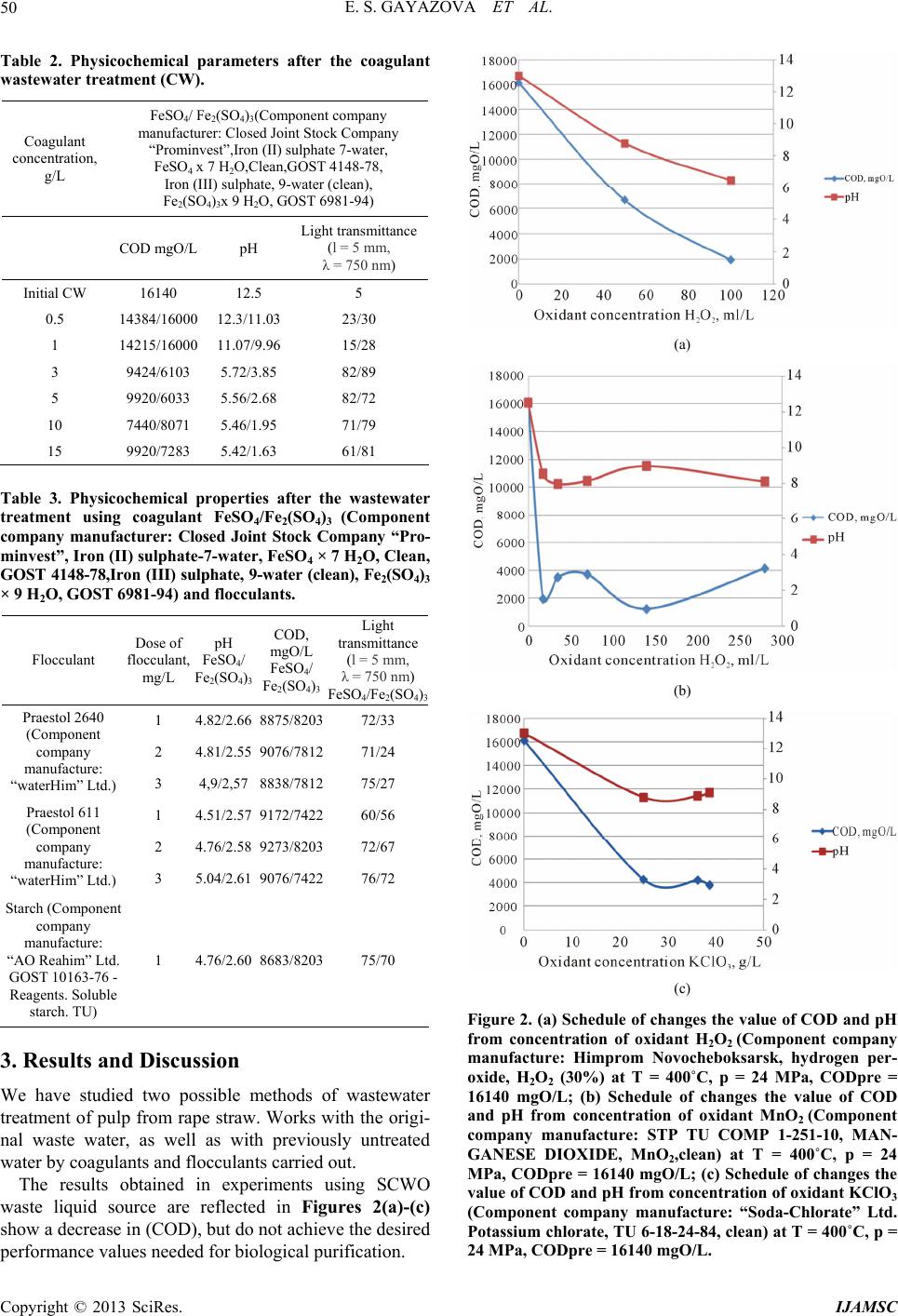 E. S. GAYAZOVA ET AL. 50 Table 2. Physicochemical parameters after the coagulant wastewater treatment (CW). Coagulant concentration, g/L FeSO4/ Fe2(SO4)3(Component company manufacturer: Closed Joint Stock Company “Prominvest”,Iron (II) sulphate 7-water, FeSO4 x 7 H2O,Clean,GOST 4148-78, Iron (III) sulphate, 9-water (clean), Fe2(SO4)3x 9 H2O, GOST 6981-94) COD mgО/L рН Light transmittance (l = 5 mm, λ = 750 nm) Initial СW 16140 12.5 5 0.5 14384/16000 12.3/11.03 23/30 1 14215/16000 11.07/9.96 15/28 3 9424/6103 5.72/3.85 82/89 5 9920/6033 5.56/2.68 82/72 10 7440/8071 5.46/1.95 71/79 15 9920/7283 5.42/1.63 61/81 Table 3. Physicochemical properties after the wastewater treatment using coagulant FeSO4/Fe2(SO4)3 (Component company manufacturer: Closed Joint Stock Company “Pro- minvest”, Iron (II) sulphate-7-water, FeSO4 × 7 H2O, Clean, GOST 4148-78,Iron ( III) sulphate, 9-water (clean), Fe2(SO4)3 × 9 H2O, GOST 6981-94) and flocculants. Flocculant Dose of flocculant, mg/L рН FeSO4/ Fe2(SO4)3 COD, mgО/L FeSO4/ Fe2(SO4)3 Light transmittance (l = 5 mm, λ = 750 nm) FeSO4/Fe2(SO4)3 1 4.82/2.66 8875/8203 72/33 2 4.81/2.55 9076/7812 71/24 Praestol 2640 (Component company manufacture: “waterHim” Ltd.) 3 4,9/2,57 8838/7812 75/27 1 4.51/2.57 9172/7422 60/56 2 4.76/2.58 9273/8203 72/67 Praestol 611 (Component company manufacture: “waterHim” Ltd.) 3 5.04/2.61 9076/7422 76/72 Starch (Component company manufacture: “AO Reahim” Ltd. GOST 10163-76 - Reagents. Soluble starch. TU) 1 4.76/2.60 8683/8203 75/70 3. Results and Discussion We have studied two possible methods of wastewater treatment of pulp from rape straw. Works with the origi- nal waste water, as well as with previously untreated water by coagulants and flocculants сarried out. The results obtained in experiments using SCWO waste liquid source are reflected in Figures 2(a)-(c) show a decrease in (COD), but do not achieve the desired performance values needed for biological purification. (a) (b) (c) Figure 2. (a) Schedule of changes the value of COD and pH from concentration of oxidant H2O2 (Component company manufacture: Himprom Novocheboksarsk, hydrogen per- oxide, H2O2 (30%) at T = 400˚C, p = 24 MPa, CODpre = 16140 mgO/L; (b) Schedule of changes the value of COD and pH from concentration of oxidant MnO2 (Component company manufacture: STP TU COMP 1-251-10, MAN- GANESE DIOXIDE, MnO2,clean) at T = 400˚C, p = 24 MPa, CODpre = 16140 mgO/L; (c) Schedule of changes the value of COD and pH from concentration of oxidant KClO3 (Component company manufacture: “Soda-Chlorate” Ltd. Potassium chlorate, TU 6-18-24-84, clean) at T = 400˚C, p = 24 MPa, CODpre = 16140 mgO/L. Copyright © 2013 SciRes. IJAMSC  E. S. GAYAZOVA ET AL. 51 In previously conducted studies to analyze the com- pleteness of organics removal were held chromatogra- phy-mass spectrometric studies of the decomposition of lignin-water-hydrogen peroxide by number of Japanese scientists, which shows that the water-soluble part con- tains the monomeric and dimeric lignin degradation pro- ducts. Analysis using GC-MS was found 31 products [7]. These results clearly showed that the soluble portion in- cludes various monomeric and dimeric compounds re- sulting from the destruction and elimination of ester bonds lignin propyl radical [2-4]. Reported studies indicate that more complete oxida- tion are monomeric components, polymers reacting oxi- dation SCWO conditions formed hydroxyl, carbonyl and carboxyl groups without causing extensive oxidation to gaseous components (CO2, CO) [8]. As a result discussed above, it would be logical to combine the process of SCWO by coagulation-floccula- tion treatment in the first stage. From the data in Table 3 can be seen that the best cleaning when FeSO4 (Component company manufac- turer: Closed Joint Stock Company “Prominvest”, Iron (II) sulphate-7-water, FeSO4 x 7 H2O, Clean, GOST 4148- 78, Iron (III) sulphate, 9-water (clean), Fe2(SO4)3x 9 H2O, GOST 6981-94) used as a coagulant observed using this reagent in an amount of 10 g/L. In the case of application of Fe2(SO4)3 best cleaning result is achieved by using a reagent concentration of 5 g/L. Increasing the concentration of the reagent solution is to move the waste water from acidic to alkaline medium at a pH lower than 6 is dissolved precipitate, and consequently leads to an increase of COD values. As it is shown in the Table 4 data, the best clarifica- tion of the wastewater occurs with the use of starch (Component company manufacture: “AO Reahim” Ltd. GOST 10163-76 - Reagents Soluble starch. TU) as floc- culating additive with a concentration of 1 - 2 mg/L. The efficiency of wastewater from pulp rape straw sig- nificant influence raw water pH: at values close to neu- tral, the degree of purification considerably lower than in Table 4. The composition of industrial wastewater produc- tion of styrene and propylene oxide of “Nizhnekamsk- neftekhim”. ingredients % mass. ethylbenzene 2.5 acetophenone 1 methylphenylcarbinol 6.5 phenol 2.5 propylene glycol 12 molybdenum 0.2 other 35.3 COD 225000 mg О/L the processing water with pH > 8. This effect is achieved when higher dosages coagulant flocculation occurs slowly precipitate bad precipitated and the supernatant liquid is present finely dispersed suspension. This prob- lem can be solved by increasing the initial pH of the waste water to an optimum. In general, waste water treatment, presented in this paper is the best iron sulfate (III). Use of this reagent results in a well-structured and floc sludge settling and effective brightening of treated water is not required to neutralize the water. Ferrous sulfate (II) also allows to obtain satisfactory results, but at doses of 1.5 - 2 times larger. After the pre-treatment of wastewater by coagulation- flocculation methods, waste liquid was subjected to the same treatment in the North Caucasus Military District installation. Processing results are presented in Figures 3(a)-(c). The liquid portion after the presented experiments was analyzed by gas chromatography-mass spectrometry. The study was conducted on the device (DFS) Thermo Elec- tron Corporation (Germany). The method of ionization: electron impact. The energy of the ionizing electrons was 70 eV, the ion source temperature 290˚C. Used capillary column ID-BPX5, length—60 m, diameter—0.32-mm. Carrier Gas-helium. Processing mass spectral data was performed using the program “Xcalibur”. Purity of the sample prior to entering the device diluted in chromato- graphically pure acetone at a concentration about 1%wt. Conditions for obtaining chromatograms: 1. The injector temperature—280˚C, the flow division (split) —1:20. 2. Warming up the column was carried out in the pro- gram mode: Initial temperature—60˚C (3 min), heating rate 10˚C/min, final temperature—280˚C (30 min.) 3. The flow of carrier gas through the column—2 mL / min 4. Temperature communication devices with a mass spectrometer—280˚C. 5. The sample volume of 0.1 mL. Below is a gas chromatography-mass spectrometry analysis of the products SCWO Figures 4(a) and (b). Analyzing data of chromatogram Figure 3a, can be concluded that the above substances are degradation pro- ducts of lignin not removed by coagulation and floccula- tion in particular (2,3-dimethyl 2-cyclopentenone-1, ace- tophenone, biphenyl, 4,5-dimethyl-1,3-dioxane-4-metha- nol), fatty acid esters are believed to be the reaction products of fatty and resin acids present in the composi- tion of the initial waste water. According to Figure 4(b) also detected decomposition products of lignin and complex esters of polyhydric acids, but their number is found in a smaller proportion, there- fore, decreases the amount of organic matter in the sam- Copyright © 2013 SciRes. IJAMSC 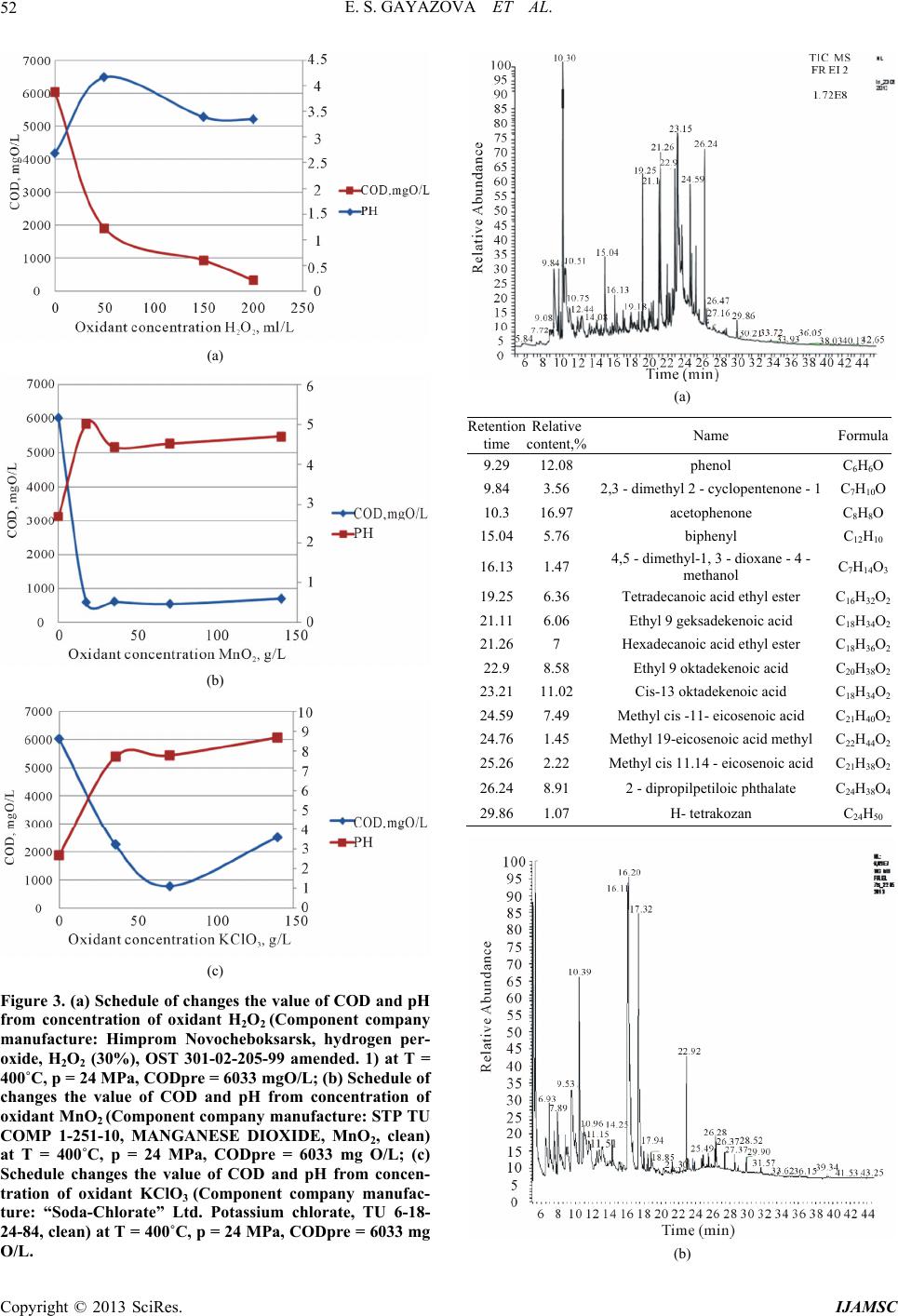 E. S. GAYAZOVA ET AL. 52 (a) (b) (c) Figure 3. (a) Schedule of changes the value of COD and pH from concentration of oxidant H2O2 (Component company manufacture: Himprom Novocheboksarsk, hydrogen per- oxide, H2O2 (30%), OST 301-02-205-99 amended. 1) at T = 400˚C, p = 24 MPa, CODpre = 6033 mgO/L; (b) Schedule of changes the value of COD and pH from concentration of oxidant MnO2 (Component company manufacture: STP TU COMP 1-251-10, MANGANESE DIOXIDE, MnO2, clean) at T = 400˚C, p = 24 MPa, CODpre = 6033 mg O/L; (c) Schedule changes the value of COD and pH from concen- tration of oxidant KClO3 (Component company manufac- ture: “Soda-Chlorate” Ltd. Potassium chlorate, TU 6-18- 24-84, clean) at T = 400˚C, p = 24 MPa, CODpre = 6033 mg O/L. (a) Retention time Relative content,% Name Formula 9.29 12.08phenol С6Н6О 9.84 3.56 2,3 - dimethyl 2 - cyclopentenone - 1С7Н10О 10.3 16.97acetophenone С8Н8О 15.045.76 biphenyl С12Н10 16.131.47 4,5 - dimethyl-1, 3 - dioxane - 4 - methanol С7Н14О3 19.256.36 Tetradecanoic acid ethyl ester С16Н32О2 21.116.06 Ethyl 9 geksadekenoic acid С18Н34О2 21.267 Hexadecanoic acid ethyl ester С18Н36О2 22.9 8.58 Ethyl 9 oktadekenoic acid С20Н38О2 23.2111.02Cis-13 oktadekenoic acid С18Н34О2 24.597.49 Methyl cis -11- eicosenoic acid С21Н40О2 24.761.45 Methyl 19-eicosenoic acid methyl С22Н44О2 25.262.22 Methyl cis 11.14 - eicosenoic acid С21Н38О2 26.248.91 2 - dipropilpetiloic phthalate С24Н38О4 29.861.07 Н- tetrakozan С24Н50 (b) Copyright © 2013 SciRes. IJAMSC 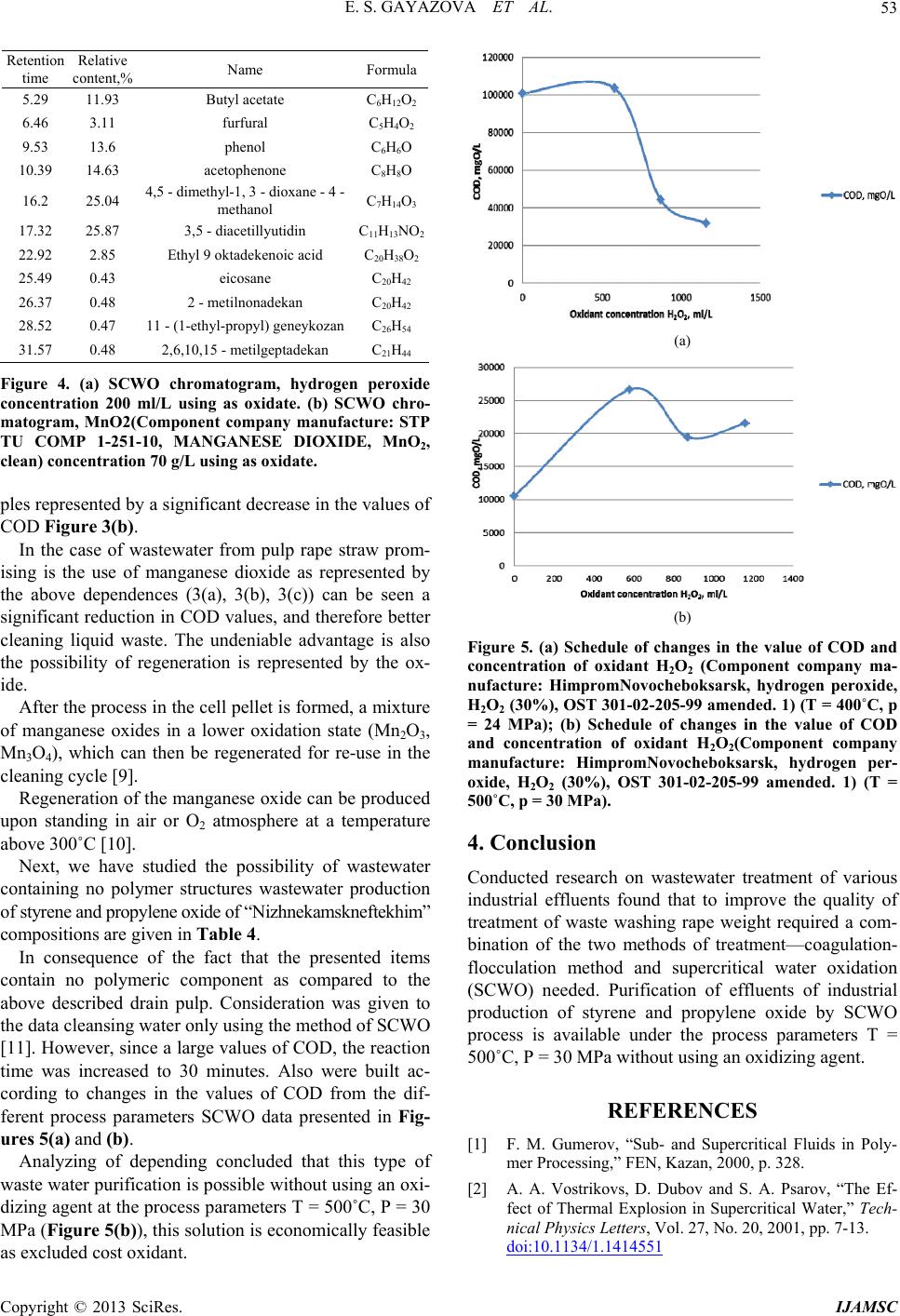 E. S. GAYAZOVA ET AL. 53 Retention time Relative content,% Name Formula 5.29 11.93 Butyl acetate С6Н12О2 6.46 3.11 furfural С5Н4О2 9.53 13.6 phenol С6Н6О 10.39 14.63 acetophenone С8Н8О 16.2 25.04 4,5 - dimethyl-1, 3 - dioxane - 4 - methanol С7Н14О3 17.32 25.87 3,5 - diacetillyutidin С11Н13NO2 22.92 2.85 Ethyl 9 oktadekenoic acid С20Н38О2 25.49 0.43 eicosane С20Н42 26.37 0.48 2 - metilnonadekan С20Н42 28.52 0.47 11 - (1-ethyl-propyl) geneykozan С26Н54 31.57 0.48 2,6,10,15 - metilgeptadekan С21Н44 Figure 4. (a) SCWO chromatogram, hydrogen peroxide concentration 200 ml/L using as oxidate. (b) SCWO chro- matogram, MnO2(Component company manufacture: STP TU COMP 1-251-10, MANGANESE DIOXIDE, MnO2, clean) concentration 70 g/L using as oxidate. ples represented by a significant decrease in the values of COD Figure 3(b). In the case of wastewater from pulp rape straw prom- ising is the use of manganese dioxide as represented by the above dependences (3(a), 3(b), 3(c)) can be seen a significant reduction in COD values, and therefore better cleaning liquid waste. The undeniable advantage is also the possibility of regeneration is represented by the ox- ide. After the process in the cell pellet is formed, a mixture of manganese oxides in a lower oxidation state (Mn2O3, Mn3O4), which can then be regenerated for re-use in the cleaning cycle [9]. Regeneration of the manganese oxide can be produced upon standing in air or O2 atmosphere at a temperature above 300˚C [10]. Next, we have studied the possibility of wastewater containing no polymer structures wastewater production of styrene and propylene oxide of “Nizhnekamskneftekhim” compositions are given in Table 4. In consequence of the fact that the presented items contain no polymeric component as compared to the above described drain pulp. Consideration was given to the data cleansing water only using the method of SCWO [11]. However, since a large values of COD, the reaction time was increased to 30 minutes. Also were built ac- cording to changes in the values of COD from the dif- ferent process parameters SCWO data presented in Fig- ures 5(a) and (b). Analyzing of depending concluded that this type of waste water purification is possible without using an oxi- dizing agent at the process parameters T = 500˚C, P = 30 MPa (Figure 5(b)), this solution is economically feasible as excluded cost oxidant. (a) (b) Figure 5. (a) Schedule of changes in the value of COD and concentration of oxidant H2O2 (Component company ma- nufacture: HimpromNovocheboksarsk, hydrogen peroxide, H2O2 (30%), OST 301-02-205-99 amended. 1) (T = 400˚C, p = 24 MPa); (b) Schedule of changes in the value of COD and concentration of oxidant H2O2(Component company manufacture: HimpromNovocheboksarsk, hydrogen per- oxide, H2O2 (30%), OST 301-02-205-99 amended. 1) (T = 500˚C, p = 30 MPa). 4. Conclusion Conducted research on wastewater treatment of various industrial effluents found that to improve the quality of treatment of waste washing rape weight required a com- bination of the two methods of treatment—coagulation- flocculation method and supercritical water oxidation (SCWO) needed. Purification of effluents of industrial production of styrene and propylene oxide by SCWO process is available under the process parameters T = 500˚C, P = 30 MPa without using an oxidizing agent. REFERENCES [1] F. M. Gumerov, “Sub- and Supercritical Fluids in Poly- mer Processing,” FEN, Kazan, 2000, p. 328. [2] A. A. Vostrikovs, D. Dubov and S. A. Psarov, “The Ef- fect of Thermal Explosion in Supercritical Water,” Tech- nical Physics Letters, Vol. 27, No. 20, 2001, pp. 7-13. doi:10.1134/1.1414551 Copyright © 2013 SciRes. IJAMSC  E. S. GAYAZOVA ET AL. Copyright © 2013 SciRes. IJAMSC 54 [3] S. V. Yakovlev, J. A. Karelin, Y. M. Tender and V. Voro- nov, “Process Wastewater Treatment,” Stroyizdat, Mos- kow, 1979, p. 320. [4] E. J. Buslaeva, K. G. Kravchuk, Y. F. Kargin and S. P. Gubin, “Reactions of MnO2, Mn2O3, α-Bi2O3, and Bi12Ti1–x MnxO20 with Supercritical Isopropanol,” Inor- ganic materials, Vol. 38, No. 6, 2002, pp. 706-710. doi:10.1023/A:1015813502466 [5] S. Timonin, “Engineering and Environment Book,” Pub- lisher N. Botchkareva, Kaluga, 2003, p. 884. [6] S. Saka and K. Ehara, “Biomass Research for Post-Pe- trochemistry by Supercritical Water,” Proceedings of the International Symposium on Highly Efficient Use of En- ergy and Reduction of Its Environmental Impact, Special Panel Session on Biomass Utilization, Osaka, 22 January 2002. [7] J. Tsujino, H. Kawamoto and S. Saka, “Reactivity of Lig- nin in Supercritical Methanol Studied with Various Lig- nin Model Compounds,” Journal of Wood Science, Vol. 49, No. 2, 2003, pp. 158-165. doi:10.1007/s100860300025 [8] D. Takada, K. Ehara and S. Saka, ”Gas Chroma Togra- phic and Mass Spectrometric (GC-MS) Analysis of Lig- nin-Derivedproducts from Cryptomeria Japonica Treated in Supercritical Water,” Journal of Wood Science, Vol. 50, No. 3, 2004, pp. 253-259. doi:10.1007/s10086-003-0562-6 [9] K. Ehara, D. Takada and S. Saka, ”GC-MS and IR Spec- troscopic Analyses of the Lignin-Derived Products from Softwood and Hardwood Treated in Supercritical Water,” Journal of Wood Science, Vol. 51, No. 3, 2005, pp. 256- 261. doi:10.1007/s10086-004-0653-z [10] M. E. Posin, “Technology of Mineral Salts (Fertilizers, Pesticides, Industrial Salts, Oxides and Acids),” Publish- ing House of the “Chemistry”, 1974, p. 792. [11] F. M. Gumerov, R. A. Kayumov, R. A. Usmanov, A. A. Sagdeev, I. Sh. Abdullin and R. F. Sharafeev, “Waste Management in Propylene Epoxidation Process with the Use of Supercritical Fluid Media,” American Journal of Analytical Chemistry, Vol. 3, No. 12A, 2012, pp. 950- 957.
|