S. Recla et al. / World Journal of Cardiovascular Diseases 3 (2013) 1-4 3
avoiding an additional open-heart surgery. Transcatheter
valve implantation was performed under general anes-
thesia with fluoroscopic and 3D-TEE guidance. An 18 F
Cook sheath was placed in femoral vein, and after sizing
of the Carpentier-band, a 39 mm Andra XXL stent
mounted on 30 × 60 mm balloon catheter (Balt) was po-
sitioned in the tricuspid-annulus and slight diabolo shape
created by expanding to 30 mm at both stent-ends fol-
lowed by using a 30 × 40 mm nucleus balloon (PFM).
The already prepared Edwards-valve was immediately
implanted within the previous Andra-stent and in the
metal band-marked tricuspid valve annulus. However,
the abrupt afterload increase after placement of the com-
petent Edwards-valve caused the right ventricular func-
tion to deteriorate. Balloon dilatation of the Atrium stent
within the Potts shunt to almost 9 mm diameter and In-
creasing dosages of catecholamines in addition to the
baseline inotropic therapy with milrinone and levo-
simendan failed to stabilize his circulation. Therefore,
the patient was put on ECMO (Extra-Corporal Mem-
brane Oxygenation) by utilizing the percutaneous femo-
ral vein (22 Fr sheath) and artery (16 Fr sheath) access.
Bi-ventricular and in particular right ventricular function
recovered over 5-days of ECMO therapy, and 7 days
ventilatory support. The “hybrid orchestra” of interven-
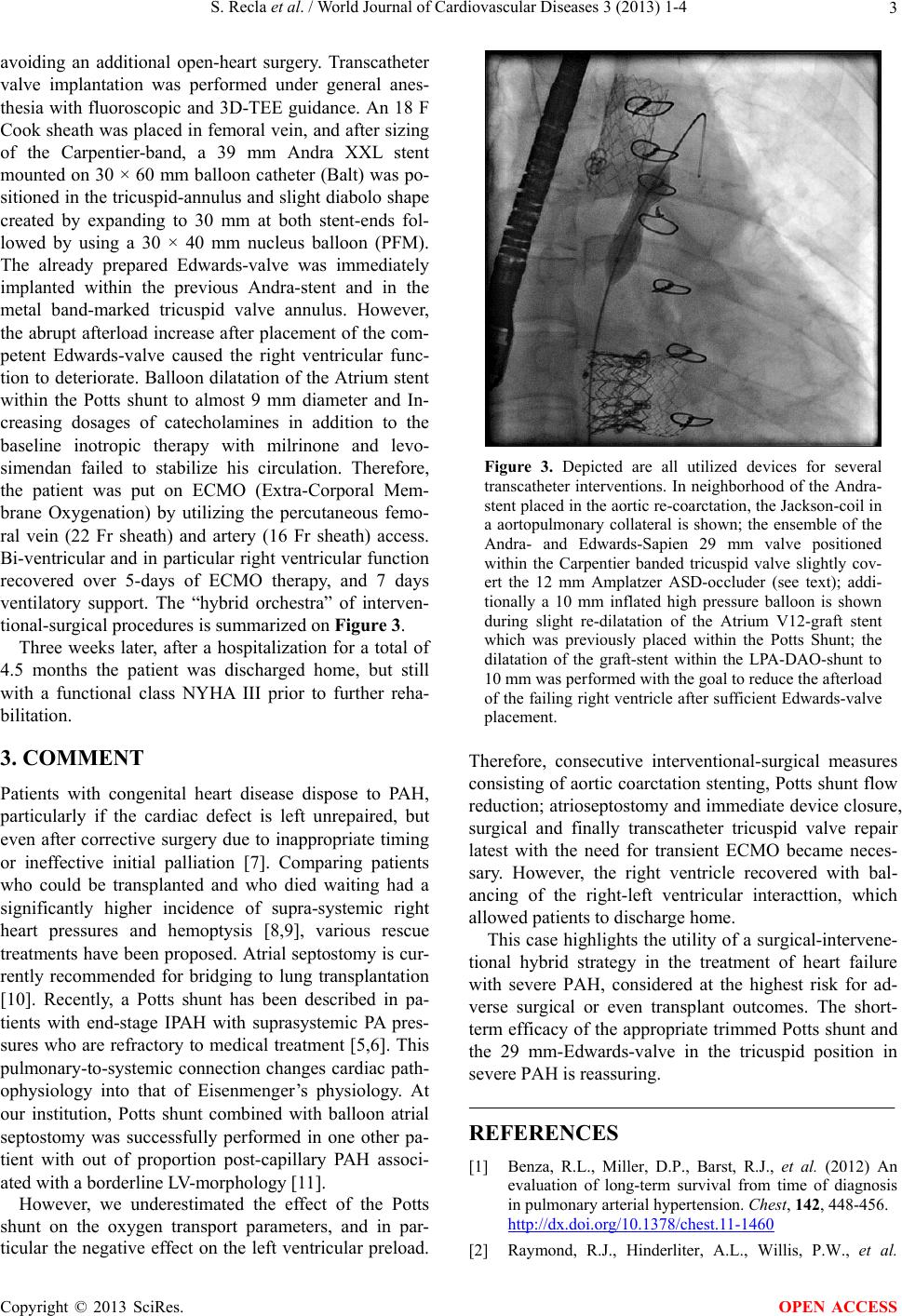
tional-surgical procedures is summarized on Figur e 3.
Three weeks later, after a hospitalization for a total of
4.5 months the patient was discharged home, but still
with a functional class NYHA III prior to further reha-
bilitation.
3. COMMENT
Patients with congenital heart disease dispose to PAH,
particularly if the cardiac defect is left unrepaired, but
even after corrective surgery due to inappropriate timing
or ineffective initial palliation [7]. Comparing patients
who could be transplanted and who died waiting had a
significantly higher incidence of supra-systemic right
heart pressures and hemoptysis [8,9], various rescue
treatments have been proposed. Atrial septostomy is cur-
rently recommended for bridging to lung transplantation
[10]. Recently, a Potts shunt has been described in pa-
tients with end-stage IPAH with suprasystemic PA pres-
sures who are refractory to medical treatment [5,6]. This
pulmonary-to-systemic connection changes cardiac path-
ophysiology into that of Eisenmenger’s physiology. At
our institution, Potts shunt combined with balloon atrial
septostomy was successfully performed in one other pa-
tient with out of proportion post-capillary PAH associ-
ated with a borderline LV-morpholo gy [11].
However, we underestimated the effect of the Potts
shunt on the oxygen transport parameters, and in par-
ticular the negative effect on the left ventricular preload.
Figure 3. Depicted are all utilized devices for several
transcatheter interventions. In neighborhood of the Andra-
stent placed in the aortic re-coarctation, the Jackson-coil in
a aortopulmonary collateral is shown; the ensemble of the
Andra- and Edwards-Sapien 29 mm valve positioned
within the Carpentier banded tricuspid valve slightly cov-
ert the 12 mm Amplatzer ASD-occluder (see text); addi-
tionally a 10 mm inflated high pressure balloon is shown
during slight re-dilatation of the Atrium V12-graft stent
which was previously placed within the Potts Shunt; the
dilatation of the graft-stent within the LPA-DAO-shunt to
10 mm was performed with the goal to reduce the afterload
of the failing right ventricle after sufficient Edwards-valve
placement.
Therefore, consecutive interventional-surgical measures
consisting of aortic coarctation stenting, Potts shunt flow
reduction; atrioseptostomy and immediate device closure,
surgical and finally transcatheter tricuspid valve repair
latest with the need for transient ECMO became neces-
sary. However, the right ventricle recovered with bal-
ancing of the right-left ventricular interacttion, which
allowed patients to discharge home.
This case highlights the utility o f a surgical-intervene-
tional hybrid strategy in the treatment of heart failure
with severe PAH, considered at the highest risk for ad-
verse surgical or even transplant outcomes. The short-
term efficacy of the appropriate trimmed Potts shunt and
the 29 mm-Edwards-valve in the tricuspid position in
severe PAH is reassuring.
REFERENCES
[1] Benza, R.L., Miller, D.P., Barst, R.J., et al. (2012) An
evaluation of long-term survival from time of diagnosis
in pulmonary arterial hypertension. Chest, 142, 448-456.
http://dx.doi.org/10.1378/chest.11-1460
[2] Raymond, R.J., Hinderliter, A.L., Willis, P.W., et al.
Copyright © 2013 SciRes. OPEN ACCESS