 Journal of Biomaterials and Nanobiotechnology, 2013, 4, 343-350 http://dx.doi.org/10.4236/jbnb.2013.44043 Published Online October 2013 (http://www.scirp.org/journal/jbnb) 343 Impact of Biomechanical Forces on Antibiotics Release Kinetics from Hydroxyapatite Coated Surgical Fixation Pins Mirjam Lilja1,2, Jan H. Sörensen3, Torben C. Sörensen4, Maria Åstrand2, Philip Procter5,6, Hartwig Steckel3*, Maria Strømme1* 1Division for Nanotechnology and Functional Materials, Department of Engineering Sciences, The Ångström Laboratory, Uppsala University, Uppsala, Sweden; 2Sandvik Coromant AB, Stockholm, Sweden; 3Department of Pharmaceutics and Biopharmaceutics, Christian Albrecht University Kiel, Kiel, Germany; 4Stryker Trauma GmbH, Schönkirchen, Germany; 5Stryker Trauma AG, Selzach, Switzerland; 6School of Engineering and Design, Brunel University, Uxbridge, UK. Email: *hsteckel@pharmazie.uni-kiel.de, maria.stromme@angstrom.uu.se Received July 16th, 2013; revised August 16th, 2013; accepted September 12th, 2013 Copyright © 2013 Mirjam Lilja et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT This work investigates the impact of biomechanical wear and abrasion on the antibiotic release profiles of hydroxyapa- tite (HA) coated fixation pins during their insertion into synthetic bone. Stainless steel fixation pins are coated with crystalline TiO2 by cathodic arc evaporation forming the bioactive layer for b iomimetic deposition of Tobramycin con- taining HA. Tobramycin is either introduced by co-precipitation during HA formation or by adsorption-loading after HA deposition. The samples containing antibiotics are inserted into bone mimicking polyethylene foam after which the drug release is monitored using high performance liquid chromatography. This analysis shows that HA coating wear and delamination significantly decrease the amount of drug released during initial burst, but only marginally influence the sustained release period. Spalled coating fragments are fo und to remain within the synth etic bone material structu re. The presence of HA within this structure supports the assumption that the local release of Tobramycin is not only ex- pected to eliminate bacteria growth directly at the pin interface but as well at some distance from the implant. Further- more, no negative effect of gamma sterilization could be observed on the drug release profile. Overall, the observed results demonstrate the feasibility of a multifunctional implant coating that is simultaneously able to locally deliver clinically relevant doses of antibiotics and an HA coating capable of promoting osteoconduction. This is a potentially promising step toward orthopaedic devices that co mbine good fixation with the ability to treat an d prevent post-surgical infections. Keywords: Hydroxyapatite; Fixation Pin; Tobramycin; Coating Wear; Drug Release; Gamma Sterilizati on 1. Introduction External fixators are commonly used for fracture stabili- zation in certain trauma patients [1]. The bone-screw interface has been identified as “the common weak link in fracture fixation” [2]. Studies report that a rate of pin loosening up to 80% for standard metal pins and loose pins affect not only fracture fixation but also cause infec- tions [3-5]. The concept of applying a biocompatible and bioactive hydroxyapatite (HA) coating onto metallic im- plants as functional surface coating has been verified to be an effective way of improving the bone-pin interface strength [6-8]. Several animal and clinical studies con- firmed the osteoconductive properties of HA coatings resulting in enhanced stability at the pin-bone interface and hence reduced pin loosening [9-11]. Nanoporous HA coatings deposited biomimetically (HA-B) have shown promising properties as drug deliv- ery vehicles [12-20]. Antibacterial drugs eluting from such HA coatings provide a possibility to combat bacte- ria at the implant site and hence contribute towards mini- mizing the risk of implant related infections post-surgery. Despite excellent properties of HA as a biomaterial, defi- cient mechanical performance including brittleness, low tensile strength and impact resistance has restricted its application in many load-bearing applications [6,7,21]. *Corresponding a uthor. Copyright © 2013 SciRes. JBNB  Impact of Biomechanical Forces on Antibiotics Release Kinetics from Hydroxyapatite Coated Surgical Fixation Pins 344 Plasma sprayed HA coatings (HA-P) have been shown to delaminate from the implant substrate [22,23] or expel coating segments [24] due to low cohesive and adhesive strength. Th e bond strength of HA-P coatings is not only dependent on the coating characteristics but also on coating thickness and the type and design of the implant used [21]. For example, the use of a thin TiO2 bonding layer connecting the HA-P coating to the metallic im- plant has been shown to induce strong adhesion of such coatings to the implant [25]. Despite the poo r biomechanical properties, HA coating delamination has only been identified in some animal studies [26]. Delaminated particles were found to be sur- rounded by bone and not associated with a foreign-body cell reaction [27]. To date, only a few investigations on the insertion and wear characteristics of HA coatings are available [28,29]. While the use of HA-B coatings as drug delivery vehicle has been extensively studied, to the best of our knowl- edge no attempts have been made to establish a correla- tion between the insertion characteristics of HA-B coated implants and the drug release profiles from such implant surfaces. The aim of this study is to analyze the impact of bio- mechanical forces on the HA-B coating wear and corre- late the latter to the in vitro drug release properties of th e coatings. To bring the con cept of antibiotic loaded HA-B coatings on fixation pins further towards clin ical practice, the release properties of Tobramycin loaded HA-B coated fixations pins are also evaluated after gamma-steriliza- tion. 2. Material and Methods 2.1. Substrate Materials and Coating Deposition A crystalline, anatase phase dominated TiO2 coating was deposited on stainless steel fixation pins (Ø 4 mm, 90 mm × 30 mm), as reported earlier in detail [30]. The pins were either covered with a biomimetic coating [31] ob- tained through immersion for 6 days in Dulbecco’s Phosphate Buffered Saline (PBS), denot e d HA-B, or with a co-precipitated, drug containing HA coating as de- scribed by Söre nsen et al. [28] and denoted Co-4. 2.2. Preparation of Drug Loaded Samples Tobramycin was incorporated into HA-B coated pins using a previously described adsorptive loading method [15]. Briefly, two types of drug loading methods were carried out to produce samples with the extensions “RT” (room temperature) and “PHT” (pressure and high tem- perature) to their sample names, respectively. All sam- ples were made in triplicate for both loading methods being studied. During RT loading, the HA coated pins were placed for 5 min in tubes containing 5 ml of To- bramycin stock solution with a concentration of 20 mg/ml. PHT samples were prepared by placing the HA- coated pins and 30 ml of stock solution containing 20 mg/ml Tobramycin in a stainless steel tube under an ap- plied pressure of 6 bar and a temperature of 90˚C. For the preparation of Co-4 samples, TiO2 coated pins were in a first step coated with a thin HA layer through immer- sion for 3 days in 50 ml PBS at 60˚C followed by im- mersion in PBS with an antibiotic concentration of 4 mg/ml for 6 days at 37˚C. After the loading procedure, the samples were placed for drying in an oven at 37˚C for 24 hours. 2.3. Insertion into Polyethylene Foam Biomechanical properties were evaluated using polyure- thane (25 PU) foam blocks (20 mm × 20 mm × 4 mm) mimicking spongy bone quality. 25 PU was chosen, since preliminary tests demonstrated that this particular grade induced more severe coating wear-effects after insertion compared to higher density synthetic bone qua- lities reflecting cancellous bone structures [32]. Three fixation pins of each sample type were loaded with To- bramycin via adsorption at room temperature, denoted as HA-B_RT_BML, or under elevated temperature and pressure, denoted as HA-B_PHT_BML followed by biomechanical insertion. Co-precipitated samples de- noted as Co-4_BML were also tested in triplicate. Inser- tion was performed vertically without predrilling at a rotation speed of 50 rotations per minute over the full thread length of the coated pin. The pin was removed from the bone model materials by cutting two notches from both sides into the discs and plates with a distance of approximate 3 mm towards the center of the pin re- sulting in two separate pieces of bone model material. The bone model pieces were subsequently broken into two halves by applying an abrupt mechanical force, thus freeing the pin. 2.4. Gamma Sterilization Gamma Sterizlization of HA-B_PHT samples was car- ried out by Beta-Gamma-Service GmbH & Co KG, Wiehl, Germany with a minimum dose of 25.4 kGy and the sterilized samples were denoted as HA-B_PHT gam- ma sterilized. 2.5. Antibiotic Release High performance liquid chromatography (HPLC) was used to quantify the released drug content as well as the release kinetics of the three different sample types after insertions. The measurements were performed and modi- fied according to the British Pharmacopoeia [33] and Fabre et al. [34] using pre-column derivatization of the aminoglycoside antibiotic. Following the insertion into Copyright © 2013 SciRes. JBNB 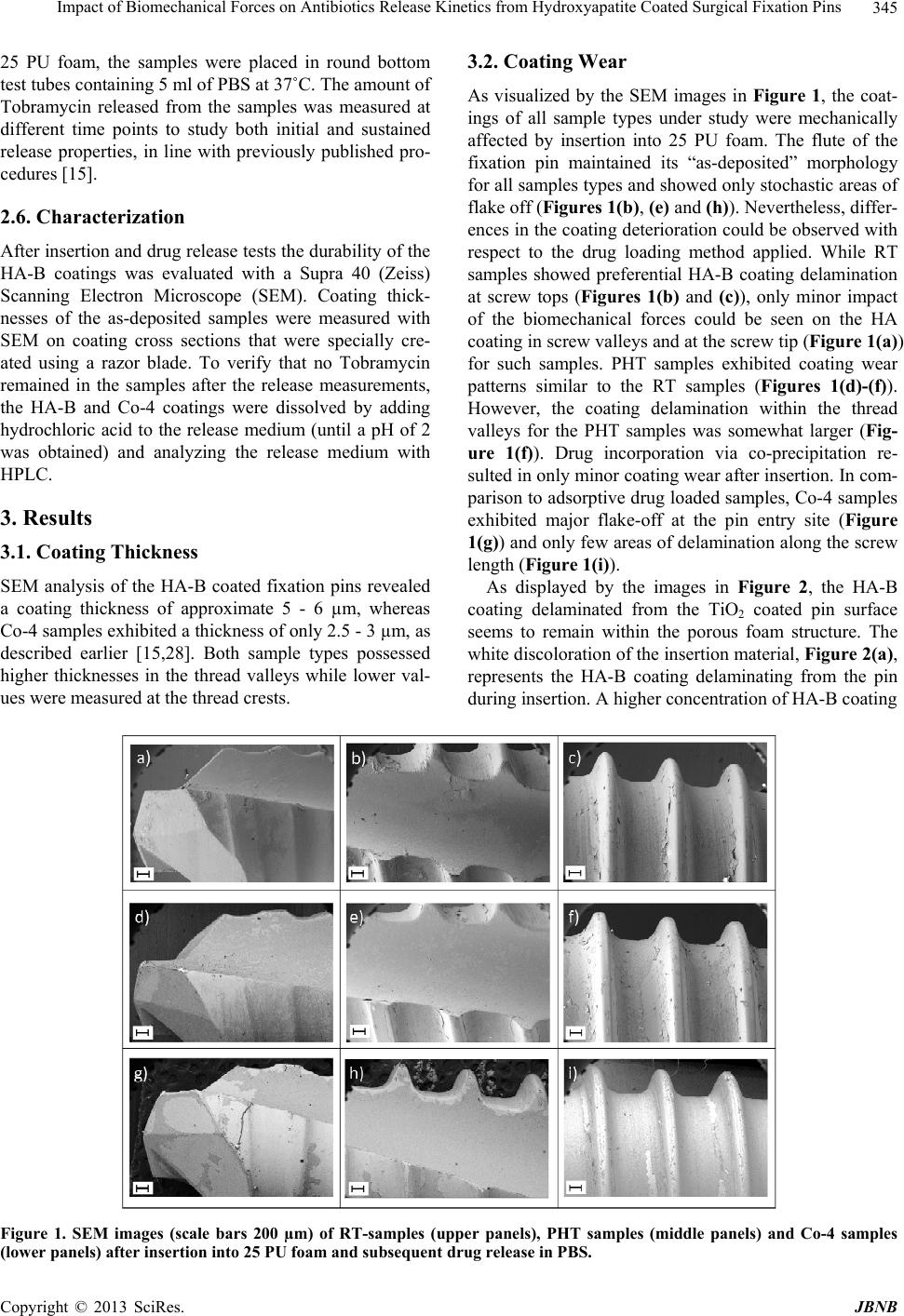 Impact of Biomechanical Forces on Antibiotics Release Kinetics from Hydroxyapatite Coated Surgical Fixation Pins Copyright © 2013 SciRes. JBNB 345 3.2. Coating Wear 25 PU foam, the samples were placed in round bottom test tubes containing 5 ml of PBS at 37˚C. The amount of Tobramycin released from the samples was measured at different time points to study both initial and sustained release properties, in line with previously published pro- cedures [15]. As visualized by the SEM images in Figure 1, the coat- ings of all sample types under study were mechanically affected by insertion into 25 PU foam. The flute of the fixation pin maintained its “as-deposited” morphology for all samples types and showed only stochastic areas of flake off (Figures 1(b), (e) and (h)). Nevertheless, differ- ences in the coating deterior ation could be observed with respect to the drug loading method applied. While RT samples showed preferential HA-B coating delamination at screw tops (Figures 1(b) and (c)), only minor impact of the biomechanical forces could be seen on the HA coating in screw valleys and at the screw tip (Figure 1(a)) for such samples. PHT samples exhibited coating wear patterns similar to the RT samples (Figures 1(d)-(f)). However, the coating delamination within the thread valleys for the PHT samples was somewhat larger (Fig- ure 1(f)). Drug incorporation via co-precipitation re- sulted in only minor coating wear after insertion. In com- parison to adsorptive drug loaded samples, Co-4 samples exhibited major flake-off at the pin entry site (Figure 1(g)) and only few areas of delamination along the screw length (Figure 1(i)). 2.6. Characterization After insertion and drug release tests the durability of th e HA-B coatings was evaluated with a Supra 40 (Zeiss) Scanning Electron Microscope (SEM). Coating thick- nesses of the as-deposited samples were measured with SEM on coating cross sections that were specially cre- ated using a razor blade. To verify that no Tobramycin remained in the samples after the release measurements, the HA-B and Co-4 coatings were dissolved by adding hydrochloric acid to the release medium (until a pH of 2 was obtained) and analyzing the release medium with HPLC. 3. Results 3.1. Coating Thickness SEM analysis of the HA-B coated fixation pins revealed a coating thickness of approximate 5 - 6 µm, whereas Co-4 samples exhibited a thickness of only 2.5 - 3 µm, as described earlier [15,28]. Both sample types possessed higher thicknesses in the thread valleys while lower val- ues were measured at the thread crests. As displayed by the images in Figure 2, the HA-B coating delaminated from the TiO2 coated pin surface seems to remain within the porous foam structure. The white discoloration of the insertion material, Figure 2(a), represents the HA-B coating delaminating from the pin during insertion. A higher concentration of HA-B coating Figure 1. SEM images (scale bars 200 µm) of RT-samples (upper panels), PHT samples (middle panels) and Co-4 samples (lower panels) after insert ion into 25 PU foam and subsequent drug release in PBS.  Impact of Biomechanical Forces on Antibiotics Release Kinetics from Hydroxyapatite Coated Surgical Fixation Pins 346 Figure 2. Photo (a) and SEM images (b), (c) of 25 PU foam after insertion. The insertion direction is indicated by an arrow (a). SEM images were taken at an insertion depth of approximate 2 mm from the pin entry point. The scale bars in panels (a), (b) and (c) are 50 mm, 20 µm and 10 µm, respectively. could be observed at the pin entry side, whereas less staining of the material could be seen with increasing insertion depth. HA-B coating flakes could be identified in the foam structure, as shown by the SEM image taken at a depth of 2 mm from the pin entry poin t (Figure 2(b)). Within the foam structure, the delaminated HA-B frag- ments showed a flake like morphology, which reflects the “as-deposited” coating morphology [15]. The size of delaminated HA-B fragments, Figure 2(c), is compara- ble to those observed during convention al scratch testing of the TiO2/HA-B coating system [32]. 3.3. Drug Release Tobramycin release profiles were evaluated by HPLC. For all samples under study, the Tobramycin release can be described by an initial burst release followed by a sustained release. The inserted samples (BML) released significantly lower amounts of drug during the initial 15 minute phase as compared to samples that had not been inserted into the bone model, Figure 3. The sustained release, however, seemed to be less effected by the inser- tion into 25 PU foam. The longest release period under the prevailing conditions was detected for the PHT coated samples and lasted for 8 days, followed by PHT_ BML inserted samples showing a release up to 5 days. The samples loaded at room temperature demonstrated maximum release duration of 2 days. The amount of Tobramycin for all sample types was above the minimum inhibition concentration (MIC) for Staphylococcus aureus for all time points measured, which corresponds to a Tobramycin concentration of 1 mg/ml in the release medium [35]. Figure 4 displays the effect of insertion of Co-4 coated pins into 25 PU on the drug release. The biome- chanical forces significantly impacted the initial burst release and only slightly decreased the amounts of drug released at later time points. The total amount of Tobra- mycin released from the inserted samples was ~35% lower that the released amount from the Co-4 reference samples. These reference samples released a detectable amount of antibiotics for as long as 12 days [28], while the Co-4_BML samples demonstrated a release period of 8 days. For all time points under study the amounts of Tobramycin released was above the MIC for S. aureus for all time points measured. 3.4. Impact of Gamma Sterilization Figure 5 describes the impact of gamma sterilization on the amount of Tobramycin released from HA-B_PHT coated fixation pins. No significant difference between Figure 3. Non-cumulative amount of Tobramycin released in 37˚C PBS from HA-B coated pins after RT and PHT loading in a solution containing 20 mg/ml of the antibiotics and following insertion into 25 PU foam (BML samples). Release results from mechanically untreated RT and PHT samples are incorporated as reference. Error bars denote the standard deviation of 3 measurements. The average total amounts of Tobramycin released from each sample type are also displayed. Copyright © 2013 SciRes. JBNB  Impact of Biomechanical Forces on Antibiotics Release Kinetics from Hydroxyapatite Coated Surgical Fixation Pins 347 Figure 4. Non-cumulative amount of Tobramycin released in 37˚C PBS from Co-4 coated pins after insertion into 25 PU foam (BML samples). Release results from mechanically untreated Co-4 samples are incorporated as reference. Error bars denote the standard deviation of 3 measure- ments. The average total amounts of Tobramycin released from each sample type ar e also display ed. Figure 5. Non-cumulative amount of Tobramycin released in 37˚C PBS from PHT loaded pins after gamma sterili- zation. Release results from PHT samples are incorporated as reference. Error bars denote the standard deviation of 3 measurements. The average total amounts of Tobramycin released from each sample type are also displayed. the release kinetic from HA-B_PHT and its sterilized counterpart could be observed. The total release period lasted for 8 days and as for the samples tested above, the released amounts were found to be above the MIC of S. aureus for each time period under study. Furthermore, no additional peaks were identified in the HPLC analysis indicating that there is no detectable impact or inactiva- tion of the drug molecule due to irradiation. 4. Discussion Functionalizing implant surfaces with bioactive HA coat- ings has been shown to play an essential role in forming an intimate contact between the implant and bone [36]. However, for successful clinical implementation of such coatings the chemical and mechan ical stability of the HA layer on the implant surface are considered to be impor- tant criteria [37,38]. Related to mechanical stability of HA-B coatings and the local release of drugs from such, the question arises of what would happen at the im- plant-bone interface in case of coating wear and loss. Th e data presented in this study confirm that insertion into bone mimicking foam material induces mechanical wear of the HA-B coatings. The biomechanical forces during insertion not only lead to compression and smearing of the coating but also to partial fragmentation and coating loosening from the TiO2 covered implant surface at high stress areas along the thread profile of the pin. While the total amount of coating wear is known to be dependent on the insertion torque and the total distance that the im- plant is moved when in contact with bone [29], also the bone properties have been shown to influence the forces required in screw insertion and pullout [29-41]. In agree- ment with literature [42], SEM images of the investigated sample types confirmed a high value of stresses at the pin entry site and at the thread tops giving rise to HA-B coating deterioration. The observed coating wear led to a decrease of the amount of Tobramycin released for all sample types in- vestigated, with the most significant reduction, ~50%, stemming from the initial burst period of the release. This shows that a substantial amount of antibiotics is su- perficially incorporated into the HA-B coating during loading by adsorption in agreement with earlier findings [15]. During the sustained release period following the initial burst, insertion-induced wear had only minor ef- fects on the drug release process governed by released from deeper sections of the coating. Co-precipitated samples suffered less coating wear-off than HA samples that were drug loaded after coating fabrication. This difference in coating wear may be re- lated to the coating growth condition s, which could result in different mechanical coating properties or be con- nected to the lower coating thickness of the co-precipi- tated Co-4 samples [28]. The delaminated HA coating was found to remain within the synthetic bone material- for all sample types. During in vivo bone insertion, the screw design, the friction at the coating interface as well as the bone density will be factors influencing the inser- tion torque and coating wear properties [43]. In fact, blood and fatty substances in vivo may act as lubricants and, hence, contribute to reducing the dry conditions prevailing at HA coating wear during insertion into syn- thetic bone [44]. Also, the delaminated HA fragments are still in place within the bone and will contribute posi- tively to the antibiotic effect. Furthermore, the rather smooth topography of biomimetic HA coatings [15] could present an additional advantage to minimize inser- tion torques as well as to minimize heat evolution during insertion. Copyright © 2013 SciRes. JBNB  Impact of Biomechanical Forces on Antibiotics Release Kinetics from Hydroxyapatite Coated Surgical Fixation Pins 348 5. Conclusion The impact on Tobramycin release and coating wear of insertion into synthetic bone of fixation pins functional- ized with antibiotics containing biomimetic HA coatings has been investigated. The investigated coatings were either prepared by co-precipitated of antibiotics during HA formation or by adsorption loading with antibiotics post HA deposition. All coatings under study were af- fected by insertion into the 25 PU synthetic bone material; at high stress areas of the pin surface coating delamina- tion was observed leading to a significant reduction in the amount of antibiotics released in vitro during the first 15 minutes of release. However, the insertion had only minor effects on the sustained drug release period lasting for 5 and 8 days, respectively, for the adsorption-loaded and co-precipitation-deposited coatings. The delaminated coating fragments were found to remain within the syn- thetic bone material structure. Hence, the in vitro ob- served reduction of the initial burst effect will most likely not prevail in vivo and the sustained release is expected to not only eliminate bacteria directly at the pin surface but also at some distance from the implant. With respect to the higher concentration of delaminated HA coating found at the pin entry site of the bone model materials, a high concentration of antibiotic can be expected to be delivered at the insertion point of the implant. The pin entry site is a likely pathway for bacteria to infiltrate during and post-surgery, hence, a high local concentra- tion at this site is potentially be neficial. The drug release profile of all HA coated implants under study was further shown to be unaffected by gamma sterilization. This de- monstrates that the incorporation of drugs into nanopor- ous HA coatings represents a feasible approach even when producing functional, therapeutic coatings, where the drugs are industrially-incorporated into the device (drug/device combination product). REFERENCES [1] J. Schalamon, T. Petnehazy, H. Ainoedhofer, E. B. Zwick, G. Singer and M. E. Hoellwarth, “Pin Tract Infection with External Fixation of Pediatric Fractures,” Journal of Pe- diatric Surgery, Vol. 42, No. 9, 2007, pp. 1584-1587. http://dx.doi.org/10.1016/j.jpedsurg.2007.04.022 [2] T. Miclau, A. Remiger, S. Tepic and R. Lindsey, “Me- chanical Comparison of the Dynamic Compression Plate, Limited Contact-Dynamic Compression Plate, and Point Contact Fixator,” Journal of Orthopaedic Trauma, Vol. 9, No. 1, 1995, pp. 17-22. http://dx.doi.org/10.1097/00005131-199502000-00003 [3] J. Mahan, D. Seligson, S. L. Henry, P. Hynes and J. Dob- bins, “Factors in Pin Tract Infections,” Orthopedics, Vol. 14, No. 3, 1991, pp. 305-308. [4] G. Pizá, V. L. Caja, M. A. Gonzalez-Viejo and A. Navarro, “Hydroxyapatite-Coated External-Fixation Pins: The Effect on Pin Loosening and Pin-Track Infection in Leg Lengthening for Short Stature,” Journal of Bone & Joint Surgery, Vol. 86B, No. 6, 2004, pp. 892-897. http://dx.doi.org/10.1302/0301-620X.86B6.13875 [5] H. G. Ahlborg and P. O. Josefsson, “Pin-Tract Complica- tions in External Fixation of Fractures of Distal Radius,” Acta Orthopaedica Scandinavica, Vol. 70, No. 2, 1999, pp. 116-118. http://dx.doi.org/10.3109/17453679909011246 [6] A. Moroni, L. Orienti, S. Stea and M. Visentin, “Im- provement of the Bone Pin Interface with Hydroxyapatite Coating: An in Vivo Long Term Experimental Study,” Journal of Orthopaedic Trauma, Vol. 10, No. 2, 1996, pp. 236-242. http://dx.doi.org/10.1097/00005131-199605000-00003 [7] Y. C. Tsui, C. Doyle and T. W. Clyne, “Plasma Sprayed Hydroxyapatite Coatings on Titanium Substrates. Part 1: Mechanical Properties and Residual Stress Levels,” Bio- materials, Vol. 19, No. 22, 1998, pp. 2015-2029. http://dx.doi.org/10.1016/S0142-9612(98)00103-3 [8] C. C. Berndt, G. N. Haddad, A. J. D. Farmer and K. A. Gross, “Thermal Spraying for Bioceramic Applications,” Materials Science Forum, Vol. 14, No. 3, 1990, pp. 161- 173. [9] A. Moroni, F. Vannini, M. Mosca and S. Giannini, “State of the Art Review: Techniques to Avoid Pin Loosening and Infection in External Fixation,” Journal of Orthopae- dic Trauma, Vol. 16, No. 3, 2002, pp. 189-195. http://dx.doi.org/10.1097/00005131-200203000-00009 [10] R. Placzek, M. Ruffer, G. Deuretzbacher, E. Heijens and A. L. Meiss, “The Fixation Strength of Hydroxyapatite- Coated Schanz Screws and Standard Stainless Steel Schanz Screws in Lower Extremity Lengthening: A Com- parison Based on a New Torque Value Index: The Fixa- tion Index,” Archives of Orthopaedic and Trauma Sur- gery, Vol. 126, No. 6, 2006, pp. 369-373. http://dx.doi.org/10.1007/s00402-006-0142-5 [11] A. Pommer, G. Muhr and A. David, “Hydroxyapatite- Coated Schanz Pins in External Fixators Used for Dis- traction Osteogenesis: A Randomized, Controlled Trial,” Journal of Bone and Joint Surgery, Vol. 84A, No. 7, 2002, pp. 1162-1166. [12] M. J. Raschke and G. Schmidmaier, “Biological Coating of Implants in Trauma and Orthopedic Surgery,” Unfall- chirurg, Vol. 107, No. 8, 2004, pp. 653-663. [13] S. Piskounova, J. Forsgren, U. Brohede, H. Engqvist and M. Strømme, “In Vitro Characterization of Bioactive Ti- tanium Dioxide/Hydroxyapatite Surfaces Functionalized with BMP-2,” Journal of Biomedical Materials Research Part B, Vol. 91B, No. 2, 2009, pp. 780-787. http://dx.doi.org/10.1002/jbm.b.31456 [14] J. Forsgren, U. Brohede, H. Engqvist and M. Strømme, “Co-Loading of Bisphosphonates and Antibiotics to a Biomimetic Hydroxyapatite Coating,” Biotechnology Let- ters, Vol. 33, No. 6, 2011, pp. 1265-1268. http://dx.doi.org/10.1007/s10529-011-0542-7 [15] M. Lilja, J. Sörensen, U. Brohede, M. Åstrand, J. Arnoldi, P. Procter, H. Steckel and M. Strømme, “Drug Loading and Release of Tobramycin from Hydroxyapatite Coated Copyright © 2013 SciRes. JBNB  Impact of Biomechanical Forces on Antibiotics Release Kinetics from Hydroxyapatite Coated Surgical Fixation Pins 349 Fixation Pins,” Journal of Materials Science: Materials in Medicine, Vol. 24, No. 9, 2013, pp. 2265-2274. http://dx.doi.org/10.1007/s10856-013-4979-1 [16] U. Brohede, J. Forsgren, S. Roos, A. Mihranyan, H. Eng- qvist and M. Strømme, “Multifunctional Implant Coat- ings Providing Possibilities for Fast Antibiotics Loading with Subsequent Slow Release,” Journal of Materials Science: Materials in Medicine, Vol. 20, No. 9, 2009, pp. 1859-1867. http://dx.doi.org/10.1007/s10856-009-3749-6 [17] J. Forsgren, U. Brohede, S. Piskounova, A. Mihranyan, S. Larsson, M. Strømme and H. Engqvist, “In Vivo Evalua- tion of Functionalized Biomimetic Hydroxyapatite for Local Delivery of Active Agents,” Journal of Biomate- rials and Nanobiotechnology, Vol. 2, No. 2, 2011, pp. 149-154. http://dx.doi.org/10.4236/jbnb.2011.22019 [18] M. Lilja, J. Forsgren, K. Welch, M. Åstrand, H. Engqvist and M. Strømme, “Photocatalytic and Antimicrobial Pro- perties of Surgical Implant Coatings of Titanium Dioxide Deposited though Cathodic Arc Evaporation,” Biotech- nology Letters, Vol. 34, No. 12, 2012, pp. 2299-2305. http://dx.doi.org/10.1007/s10529-012-1040-2 [19] M. Lilja, C. Lindahl, W. Xia, H. Engqvist and M. Strøm- me, “The Effect of Si-Doping on the Release of Antibi- otic from Hydroxyapatite Coatings,” Journal of Biomate- rials and Nanobiotechnology, Vol. 4, No. 3, 2013, pp. 237-241. http://dx.doi.org/10.4236/jbnb.2013.43029 [20] J. Åberg, U. Brohede, A. Mihranyan, M. Strømme and H. Engqvist, “Bisphosphonate Incorporation in Surgical Im- plant Coatings by Fast Loading and Co-Precipitation at Low Drug Concentrations,” Journal of Materials Science: Materials in Medicine, Vol. 20, No. 10, 2009, pp. 2053- 2061. http://dx.doi.org/10.1007/s10856-009-3771-8 [21] L. Sun, C. C. Berndt, K. A. Gross and A. Kucuk, “Mate- rial Fundamentals and Clinical Performance of Plasma- Sprayed Hydroxyapatite Coatings: A Review,” Journal of Biomedical Materials Research, Vol. 58, No. 5, 2001, pp. 570-592. http://dx.doi.org/10.1002/jbm.1056 [22] H. W. Debissen, W. Kalk, H. M. de Nieuport, J. C. Maltha and A van deHooff, “Mandibular Bone Response to Plasma Sprayed Coatings of Hydroxyapatite,” Interna- tional Journal of Prosthodontics, Vol. 3, No. 1, 1990, pp. 53-58. [23] A. David, J. Eitenmueller, G. Muhr, A. Pommer, H. F. Baer, P. A. W. Ostermann and T. A. Schildhauer, “Me- chanical and Histological Evaluation of Hydroxyapatite- Coated, Titanium-Coated and Grit Blasted Surfaces under Weight-Bearing Conditions,” Archives of Orthopaedic and Trauma Surgery, Vol. 114, No. 2, 1995, pp. 112-118. http://dx.doi.org/10.1007/BF00422838 [24] R. J. Friedman, J. Black, K. A. Gustke, W. M. Braunohler, W.D. Guyer and C. Savory, “Four to Six Year Results of Hydroxyapatite Total Hip Arthroplasty,” 20th Annual Meeting of The Society of Biomaterials, 1994, p. 37. [25] H. Kurzweg, R. B. Heimann, T. Troczynski and M. L. Wayman, “Development of Plasma-Sprayed Bioceramics Coatings with Bond Coats Based on Titania and Zirco- nia,” Biomaterials, Vol. 19, No. 16, 1998, pp. 1507-1511. http://dx.doi.org/10.1016/S0142-9612(98)00067-2 [26] W. N Capello and T. W. Bauer, “Hydroxyapatite in Or- thopedic Surgery,” In: H. U. Cameron, Ed., Bone Implant Interface, C.V. Mosby, St. Louis, 1994, pp. 191-202. [27] T. W. Bauer, “Hydroxyapatite: Coating Controversies,” Orthopedics, Vol. 18, No. 9, 1995, pp. 885-888. [28] J. Sörensen, M. Lilja, T. Sörensen, M. Åstrand, P. Procter, M. Strømme and H. Steckel, “Co-Precipitation of To- bramycin into Hydroxyapatite Coatings,” unpublished. [29] K. A. Gross and M. Babovic, “Influence of Abrasion on the Surface Characteristics of Thermally Sprayed Hy- droxyapatite Coatings,” Biomaterials, Vol. 23, No. 24, 2002, pp. 4731-4737. http://dx.doi.org/10.1016/S0142-9612(02)00222-3 [30] M. Lilja, K. Welch, M. Åstrand, H. Engqvist and M. Strømme, “Effect of Deposition Parameters on the Photo- catalytic Activity and Bioactivity of TiO2 Thin Films Deposited by Vacuum Arc on Ti-6Al-4V Substrates,” Journal of Biomedical Materials Research Part B, Vol. 100, No. 4, 2012, pp. 1078-1085. http://dx.doi.org/10.1002/jbm.b.32674 [31] A. Mihranyan, J. Forsgren, M. Strømme and H. Engqvist, “Assessing Surface Area Evolution during Biomimetic Growth of Hydroxyapatite Coatings,” Langmuir, Vol. 25, No. 3, 2009, pp. 1292-1295. http://dx.doi.org/10.1021/la803520k [32] J. Sörensen, M. Lilja, T. Sörensen, M. Åstrand, P. Procter, M. Strømme and H. Steckel, “Biomechanical and Ant- bacterial Properties of Hydroxyapatite Coated Fixation Pins,” unpublished. [33] “HPLC Detection of Gentamicin Sulphate,” In: British Pharmacopoeia, London, England, 1999, pp. 695-697. [34] H. Fabre, M. Sekkat, M. D. Blanchin and B. Mandrou, “Determination of Aminoglycosides in Pharmaceutical Formulations-II. High-Performance Liquid Chromatog- raphy,” Journal of Pharmaceutical and Biomedical Ana- lysis, Vol. 7, No. 12, 1989, pp. 1711-1718. http://dx.doi.org/10.1016/0731-7085(89)80185-2 [35] M. D’Arrigo, G. Ginestra, G. Mandalari and P. M. Fur- neri, “Synergism and Postantibiotic Effect of Tobramycin and Melaleuca alternifolia (teatree) Oil against Staphylo- coccus aureus and Escherichia coli,” Phytomedicine, Vol. 17, No. 5, 2010, pp. 317-322. http://dx.doi.org/10.1016/j.phymed.2009.07.008 [36] S. Ban, S. Maruno, N. Arimoto, A. Harada and J. Hase- gawa, “Effect of Electrochemically Deposited Apatite Coating on Bonding of Bone to the HA-G-Ti Composite and Titanium,” Journal of Biomedical Materials Re- search, Vol. 36, No. 1, 1997, pp. 9-15. http://dx.doi.org/10.1002/(SICI)1097-4636(199707)36:1< 9::AID-JBM2>3.0.CO;2-P [37] J. H. Lee, H. S. Ryu, K. S. Hong, D. S. Lee, K. B. S. Chang and C. K. Lee, “Biomechanical and Histomor- phometric Study on the Bone-Screw Interface of Bioac- tive Ceramic-Coated Titanium Screws,” Biomaterials, Vol. 26, No. 16, 2005, pp. 3249-3257. http://dx.doi.org/10.1016/j.biomaterials.2004.08.033 [38] U. Brohede, S. Zhao, F. Lindberg A. Mihranyan, J. Fors- gren, M. Strømme and H. Engqvist, “A Novel Graded Bi- oactive High Adhesion Implant Coating,” Applied Sur- face Science, Vol. 225, No. 17, 2009, pp. 7723-7728. Copyright © 2013 SciRes. JBNB  Impact of Biomechanical Forces on Antibiotics Release Kinetics from Hydroxyapatite Coated Surgical Fixation Pins Copyright © 2013 SciRes. JBNB 350 http://dx.doi.org/10.1016/j.apsusc.2009.04.149 [39] A. Kuhn, T. McIff, J. Cordey, F. W. Baumgart and B. A. Rahn, “Bone Deformation by Thread-Cutting and Thread- Forming Cortex Screws,” Injury, Vol. 26, No. 1, 1995, pp. 12-20. http://dx.doi.org/10.1016/0020-1383(95)90117-5 [40] A. W. Kwok, J. A. Finkelstein, T. Woodside, T. C. Hearn and R. W. Hu, “Insertional Torque and Pull-Out Strengths of Conical and Cylindrical Pedicle Screws in Cadaveric Bone,” Spine, Vol. 21, No. 1, 1996, pp. 2429-2434. http://dx.doi.org/10.1097/00007632-199611010-00004 [41] A. F. Tencer and K. D. Johnson, “Biomechanics in Or- thopedic Trauma,” In: Bone Fracture and Fixation, M. Dunitz, Ltd, London, 1994, pp. 118-157. [42] M. Capper, C. Soutis and O. O. A. Onit, “Comparison of the Stresses Generated at the Pin-Bone Interface by Stan- dard and Conical External Fixator Pins,” Biomaterials, Vol. 15, No. 6, pp. 471-473. http://dx.doi.org/10.1016/0142-9612(94)90227-5 [43] A. Koistinen, S. S. Santavirta, H. Kröger and R. Lap- palainen, “Effect of Bone Mineral Density and Amor- phous Diamond Coatings on Insertion Torque of Bone Screws,” Biomaterials, Vol. 26, No. 28, 2005, pp. 5687- 5694. http://dx.doi.org/10.1016/j.biomaterials.2005.02.003 [44] S. M. T. Chan, C. P. Neu, K. Komvopoulos and A. H. Redd, “The Role of Lubricant Entrapment at Biological Interfaces: Reduction of Friction and Adhesion in Articu- lar Cartilage,” Journal of Biomechanics, Vol. 44, No. 11, 2011, pp. 2015-2020. http://dx.doi.org/10.1016/j.jbiomech.2011.04.015
|