 Food and Nutrition Sciences, 2013, 4, 87-94 http://dx.doi.org/10.4236/fns.2013.49A2012 Published Online September 2013 (http://www.scirp.org/journal/fns) Polyphenol Oxidase Inactivation by Microwave Oven and Its Effect on Phenolic Profile of Loquat (Eriobotrya japonica) Fruit Yanet Chávez-Reyes1, Lidia Dorantes-Alvarez1, Daniel Arrieta-Baez2, Obed Osorio-Esquivel3, Alicia Ortiz-Moreno1* 1Departamento de Ingeniería Bioquímica, Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional, México D.F., México; 2Espectrometría de Masas y RMN Centro de Nanociencias y Micro y NanoTecnología, México D.F., México; 3Instituto de Salud Pública, Universidad de Chalcatongo, Chalcatongo de Hidalgo, Oaxaca, México. Email: *ortizalicia@hotmail.com Received June 15th, 2013; revised July 15th, 2013; accepted July 22nd, 2013 Copyright © 2013 Yanet Chávez-Reyes et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT The objective of this research was investigated the effect of polyphenol oxidase microwave treatment on phenolic com- position, antioxidant activity and microstructure of loquat fruit. Phenolic profile of methanolic extracts prepared from fresh, and microwave-treated samples were analyzed. Antioxidant activity was also evaluated by 2,2’-azinobis (3-ethyl- benzothiazoline-6-sulfonic acid) (ABTS•+) and 1,1-diphenyl-2-picrylhydrazyl (DPPH+) methods. In addition, polyphe- nol oxidase inactivation was carried out using a response surface methodology to establish the optimal conditions of treatment. The phenolic content of fresh mesocarp was 311 ± 0.60 mg gallic acid equivalents (GAE)/100g dry weight (DW) and that of microwave-treated mesocarp was 1230 ± 0.36 mg GAE/100g DW. Total phenolic content of water/ methanol extract significantly increases after microwave treatment rather than methanolic extract of fresh loquat. Five glycoside phenolics were identified by HPLC-DAD-MS as 3-caffeoylquinic acid, 3-p-coumaroylquinic acid, 5-caffeoyl- quinic acid and quercetin-3-O-sambubioside. Methanolic extract of microwave-treated mesocarp showed higher anti- oxidant activity than that of fresh mesocarp. Thus, polyphenol oxidase inactivation by microwave energy preserved the integrity of phenolic compounds as well as antioxidant activity in mesocarp extracts prepared from loquat fruit. It was also noted that phenolics were more abundant in the microwaved samples than in the fresh samples. Keywords: Loquat; Phenolic Compounds; Polyphenol Oxidase; Microwave; Eriobotrya japonica 1. Introduction The loquat (Eriobotrya japonica Lindl) plant is grown in subtropical areas of China, Japan, India, Israel and the Mediterranean [1]. However, its utilization is limited due to enzymatic browning that occurs immediately after removing the peel [2]. Enzymatic browning is an unde- sirable reaction because of its unattractive appearance and the resulting loss of quality [3] such as sensorial and nutritional modifications, softening, off-flavor develop- ment and darkening. Thus, enzymatic browning greatly depreciates the potential of loquat as a food product [4,5]. The extent of browning depends on phenol content and polyphenol oxidase (PPO) activity [5]. Polyphenol oxi- dase enzymes in loquat juices have been inhibited using sulfhydryl compounds [6] and the effectiveness of anti- browning agents may be due to the concentration of endo- genous phenolic compounds presenting in each fruit [3]. Recent research has reported the advantages of using microwaves for processing food products. Microwaves can penetrate material and deposit energy, and therefore heat can be generated throughout the volume of the ma- terial. Microwave treatment can reduce processing time and enhance product quality [7]. Microwave energy is a useful alternative in the processing of fruits and vege- tables because of its rapid heating rate and its non-ther- mal effect on enzyme inactivation. Moreover, microwave energy reduces the impact of elevated temperature and improves retention of thermolabile compounds such as polyphenols, vitamins, carotenoids and other secondary metabolites [8,9]. The main objective of this research *Corresponding author. Copyright © 2013 SciRes. FNS 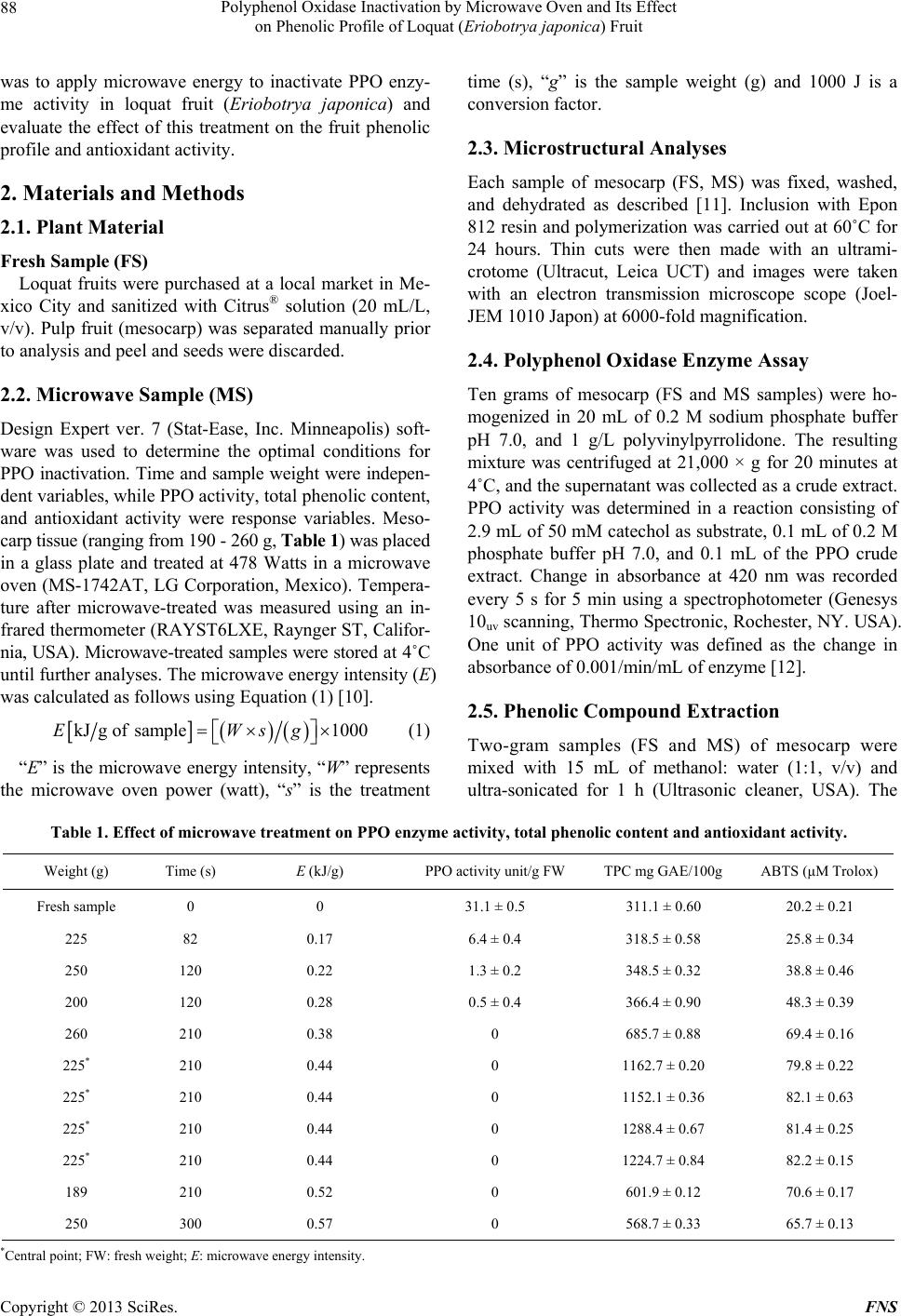 Polyphenol Oxidase Inactivation by Microwave Oven and Its Effect on Phenolic Profile of Loquat (Eriobotr ya jap oni ca) Fruit 88 was to apply microwave energy to inactivate PPO enzy- me activity in loquat fruit (Eriobotrya japonica) and evaluate the effect of this treatment on the fruit phenolic profile and antioxidant activity. 2. Materials and Methods 2.1. Plant Material Fresh Sample (FS) Loquat fruits were purchased at a local market in Me- xico City and sanitized with Citrus® solution (20 mL/L, v/v). Pulp fruit (mesocarp) was separated manually prior to analysis and peel and seeds were discarded. 2.2. Microwave Sample (MS) Design Expert ver. 7 (Stat-Ease, Inc. Minneapolis) soft- ware was used to determine the optimal conditions for PPO inactivation. Time and sample weight were indepen- dent variables, while PPO activity, total phenolic content, and antioxidant activity were response variables. Meso- carp tissue (ranging from 190 - 260 g, Table 1) was placed in a glass plate and treated at 478 Watts in a microwave oven (MS-1742AT, LG Corporation, Mexico). Tempera- ture after microwave-treated was measured using an in- frared thermometer (RAYST6LXE, Raynger ST, Califor- nia, USA). Microwave-treated samples were stored at 4˚C until further analyses. The microwave energy intensity (E) was calculated as follows using Equation (1) [10]. kJg ofsample1000EWsg (1) “E” is the microwave energy intensity, “W” represents the microwave oven power (watt), “s” is the treatment time (s), “g” is the sample weight (g) and 1000 J is a conversion factor. 2.3. Microstructural Analyses Each sample of mesocarp (FS, MS) was fixed, washed, and dehydrated as described [11]. Inclusion with Epon 812 resin and polymerization was carried out at 60˚C for 24 hours. Thin cuts were then made with an ultrami- crotome (Ultracut, Leica UCT) and images were taken with an electron transmission microscope scope (Joel- JEM 1010 Japon) at 6000-fold magnification. 2.4. Polyphenol Oxidase Enzyme Assay Ten grams of mesocarp (FS and MS samples) were ho- mogenized in 20 mL of 0.2 M sodium phosphate buffer pH 7.0, and 1 g/L polyvinylpyrrolidone. The resulting mixture was centrifuged at 21,000 × g for 20 minutes at 4˚C, and the supernatant was collected as a crude extract. PPO activity was determined in a reaction consisting of 2.9 mL of 50 mM catechol as substrate, 0.1 mL of 0.2 M phosphate buffer pH 7.0, and 0.1 mL of the PPO crude extract. Change in absorbance at 420 nm was recorded every 5 s for 5 min using a spectrophotometer (Genesys 10uv scanning, Thermo Spectronic, Rochester, NY. USA). One unit of PPO activity was defined as the change in absorbance of 0.001/min/mL of enzyme [12]. 2.5. Phenolic Compound Extraction Two-gram samples (FS and MS) of mesocarp were mixed with 15 mL of methanol: water (1:1, v/v) and ultra-sonicated for 1 h (Ultrasonic cleaner, USA). The Table 1. Effect of microwave treatment on PPO enzyme activity, total phenolic content and antioxidant activity. Weight (g) Time (s) E (kJ/g) PPO activity unit/g FW TPC mg GAE/100g ABTS (μM Trolox) Fresh sample 0 0 31.1 ± 0.5 311.1 ± 0.60 20.2 ± 0.21 225 82 0.17 6.4 ± 0.4 318.5 ± 0.58 25.8 ± 0.34 250 120 0.22 1.3 ± 0.2 348.5 ± 0.32 38.8 ± 0.46 200 120 0.28 0.5 ± 0.4 366.4 ± 0.90 48.3 ± 0.39 260 210 0.38 0 685.7 ± 0.88 69.4 ± 0.16 225* 210 0.44 0 1162.7 ± 0.20 79.8 ± 0.22 225* 210 0.44 0 1152.1 ± 0.36 82.1 ± 0.63 225* 210 0.44 0 1288.4 ± 0.67 81.4 ± 0.25 225* 210 0.44 0 1224.7 ± 0.84 82.2 ± 0.15 189 210 0.52 0 601.9 ± 0.12 70.6 ± 0.17 250 300 0.57 0 568.7 ± 0.33 65.7 ± 0.13 *Central point; FW: fresh weight; E: microwave energy intensity. Copyright © 2013 SciRes. FNS  Polyphenol Oxidase Inactivation by Microwave Oven and Its Effect on Phenolic Profile of Loquat (Eriobotr ya jap oni ca) Fruit 89 mixture was allowed to stand for 15 min at room tem- perature and the sonication was repeated for an addi- tional 1 h. The mixture was centrifuged (Beckman J2-H2, USA) at 13,000 × g for 5 min. The supernatant was collected and analyzed immediately. 2.6. Determination of Total Phenolic Content (TPC) TPC was determined according to the Folin-Ciocalteu method described by Sun et al. A 100 μL aliquot of me- thanolic extract was mixed with 750 μL of Folin-Cio- calteu phenol reagent (diluted 1:10 with water) and was allowed to stand for 5 min at room temperature; 0.75 mL of 6% sodium bicarbonate was added to the mixture. The mixture was then incubated for 90 min at room tempe- rature in the dark. Reactions were measured at 750 nm using a spectrophotometer (Genesys 10uv scanning, Ther- mo Spectronic, Rochester, NY, USA). A calibration curve using gallic acid at concentrations ranging from 0 to 0.25 mg/mL was prepared and reactions were tested under similar conditions. Results are expressed as mg GAE/g DW ± standard deviation (SD) for 3 replicates [13]. 2.7. HPLC-DAD-MS/MS Analysis of Phenolic Compounds Methanolic extracts were analyzed using a UHPLC-sys- tem (Ultimate 3000, Dionex, Sunnyvale, CA, USA) equipped with on-line degasser, binary pump, auto sam- pler, column heater, diode array detector and a 100 μL loop coupled to a MS detector (MicrOTOF-QII, Bruker Daltonics, Biellerica, MA, USA). Chromatographic ana- lysis was performed using a C18-reversed phase column (Kinetex C-18, 50 × 2.1 mm i.d. Phenomenex, Torrance, CA, USA), with a particle size of 2.6 μm. Mobile phase A was 100% methanol and mobile phase B consisted of 5% formic acid in water. Separation of phenolics was achieved using the following gradient: 0 min—5% B, 3 min—15% B, 13 min—25% B, 25 min— 30% B, 35 min—35% B, 39 min—45% B, 42 min—45% B, 44 min—50% B, 47 min—70% B, 50 min—70% B, 56 min—75% B, 61 min—80% B and a flow rate of 0.9 mL/min. The chromatograms were recorded at 280 nm for 65 min and the sample injection volume was 20 μL. Identification of phenolic compounds was performed on a HPLC-DAD-ESI-MS/MS system (Bruker micrO- TOF-Q II, Bruker Daltonics, Bremem, Germany) using Electrospray Ionization (ESI) analysis. Samples were dissolved in 100% methanol and were injected directly into the spectrometer. Polymer-related peaks were identi- fied in positive and negative ion mode. The capillary potential was −4.5 kV, the dry gas temperature was 200˚C and the drying gas flow was 4 L/min. Total ion chromatograms from m/z 500 to 3000 were obtained. 2.8. DPPH Radical Assay The activity of loquat methanolic extracts on the 1,1- diphenyl-2-picrylhydrazyl (DPPH) free radical was esti- mated according to the procedure described by Ferreres et al. (2009). A 100 µL aliquot of methanolic extract (FS and MS samples) was mixed with 2.9 mL of a metha- nolic solution of 150 µM DPPH. The reaction mixture was incubated for 30 min in darkness at room tem- perature. The absorbance of the resulting solution was measured at 515 nm in a spectrophotometer (Genesys 10uv scanning, Thermo Spectronic, Rochester, NY, USA). The control sample was composed of methanol instead of methanolic extracts. The antioxidant activity of the tested samples was expressed as % inhibition of DPPH ± SD, following Equation (2): Acontrol Asample Antioxidant activity%100 Acontr ol (2) 2.9. ABTS free Radical Scavenging Assay The antioxidant capacity of loquat extracts was also eva- luated by using 2,2´-azinobis (3-ethylbenzothiazoline-6- sulfonic acid) (ABTS) free radical scavenging following the modified method. Potassium persulfate was added to 7 mmol/L of ABTS•+ and the resulting solution was in- cubated for 16 h at room temperature in the dark. The ABTS•+ solution was diluted with ethanol and adjusted to an absorbance of 0.70 ± 0.02 at 734 nm before analysis. A 10 µL aliquot of loquat extract was added to 990 μL of the ABTS•+ cation solution and mixed thoroughly. After mixing, the absorbance was measured at 734 nm every minute for 7 min. Readings taken after 5 min of reaction were used to calculate the inhibition (%) of ABTS. The blank was made with ethanol in place of extracts. Ab- sorbance values were corrected for the solvent as follows [14] according to Equation (3): t=5sa t=0 sat=0 sot=5sot=0 so t=0 sa AA ΔAsa AAAA (3) In this case, “sa” refers to the sample and “so” refers to the solvent. A standard calibration curve was construc- ted by plotting absorbance values against varying con- centrations of Trolox. Antioxidant capacities of the sam- ples were calculated in Trolox equivalents and % ABTS inhibition using this curve. All measurements were made in triplicate and these experiments were repeated twice. Data are reported as means ± SD. 2.10. Statistical Analysis Statistical analyses were conducted using Sigma Stat ver- Copyright © 2013 SciRes. FNS  Polyphenol Oxidase Inactivation by Microwave Oven and Its Effect on Phenolic Profile of Loquat (Eriobotr ya jap oni ca) Fruit 90 sion 3.5 (Jandel Corp., San Raphael, CA). Data was analyzed by one-way ANOVA and differences among the means were compared using the Holm-Sidak method with a level of significance of P < 0.05. 3. Results and Discussion 3.1. Polyphenol Oxidase Inactivation by Microwave Treatment Results of microwave treatment indicated that PPO was inactivated in most combinations of microwave power and time (Table 1). However, conditions were selected in consideration of the E value, the amount of energy required to inactivate the enzyme, which is an important parameter in scaling up the process. Therefore, an opti- mal combination was considered to be the one where enzyme activity was inactivated and the largest fraction of phenolic compounds were extracted using the lowest microwave energy intensity. Matsui et al. reported that microwave treatment improves retention of thermolabile nutrients and sensorial characteristics [8]. The PPO en- zyme activity in loquat pulp was inactivated at 83˚C, which is consistent with published findings that purified PPO from loquat fruit (Mespilus germanica L., Rosacea) is heat-denatured at approximately 80˚C [4]. Compared to fresh mesocarp, significant reductions in PPO enzyme activity (approximately 79%, 96%, and 98%) were ob- served at E = 0.17 kJ/g, 0.22 kJ/g and 0.28 kJ/g respec- tively. Our study indicated that the optimal combination for PPO inactivation in loquat fruit was 478 W for 210 s, resulting in an E value of 0.44 kJ/g. Under these condi- tions, the largest fraction of phenolic compounds, aproxi- mately 311.1 ± 0.60 mg GAE/100g were retained com- pare to the other combinations. The E value required for enzyme inactivation depends on several factors such as the chemical composition, dielectric properties, pH, and other physical properties of the tissue [15]. Similar E values have been previously reported for different fruits and vegetables such as avocado puree (700 W/23 s, E = 0.80 kJ/g), potato cubes (600 W/300 s, E = 0.85 kJ/g), and mamey (937 W/165 s, E = 0.90 kJ/g) [15-17]. Mi- crowaves are useful because they transfer energy through- out the volume of the material, reducing processing time and enhancing overall quality by inactivating PPO activ- ity [18]. 3.2. Microstructural Analysis Microstructural analysis of fresh samples and micro- wave-treated samples (at 478 W/210 s) are shown in Figure 1. In fresh mesocarp sample (Figure 1(a)), the cell walls and vacuoles showed a defined structure. After microwave treatment, morphological alterations such as thinner cell walls and compromised vacuoles were ob- 500 nm wc v (a) 2 microns wc (b) Figure 1. Transmission electron micrographs of loquat tis- sue. (a) Fresh mesocarp (30 K), (b) Microwave-treated me- socarp (6000×). CW: cell wall, V: vacuole. The magnifica- tion used is indicated on each micrograph. served (Figure 1(b)). These morphological changes may favor the release of compounds from vacuoles and sig- nificantly increase the amount of extractable phenolics in microwaved tissue compared to fresh tissue. In agree- ment with our results, other labs have reported that anti- oxidant activity and phenolic extraction levels increased after microwave treatment [19]. The intense heat gener- ated from microwaves creates a high vapor pressure and temperature inside plant tissue, resulting in the disruption of plant cell wall polymers [20]. Consequently, cell wall phenolics or bond phenolics can be released, thus causing even more phenolics to be extracted [21]. However, more studies are needed to understand how structural modifi- cations caused by microwaves influence the extraction of phenolic compounds. 3.3. Effect of Microwave Treatment on Total Phenolic Content (TPC) A central composite design was used to study the influ- ence of microwave energy on TPC. Factors studied were the weight of the samples which varied from 200 g to Copyright © 2013 SciRes. FNS 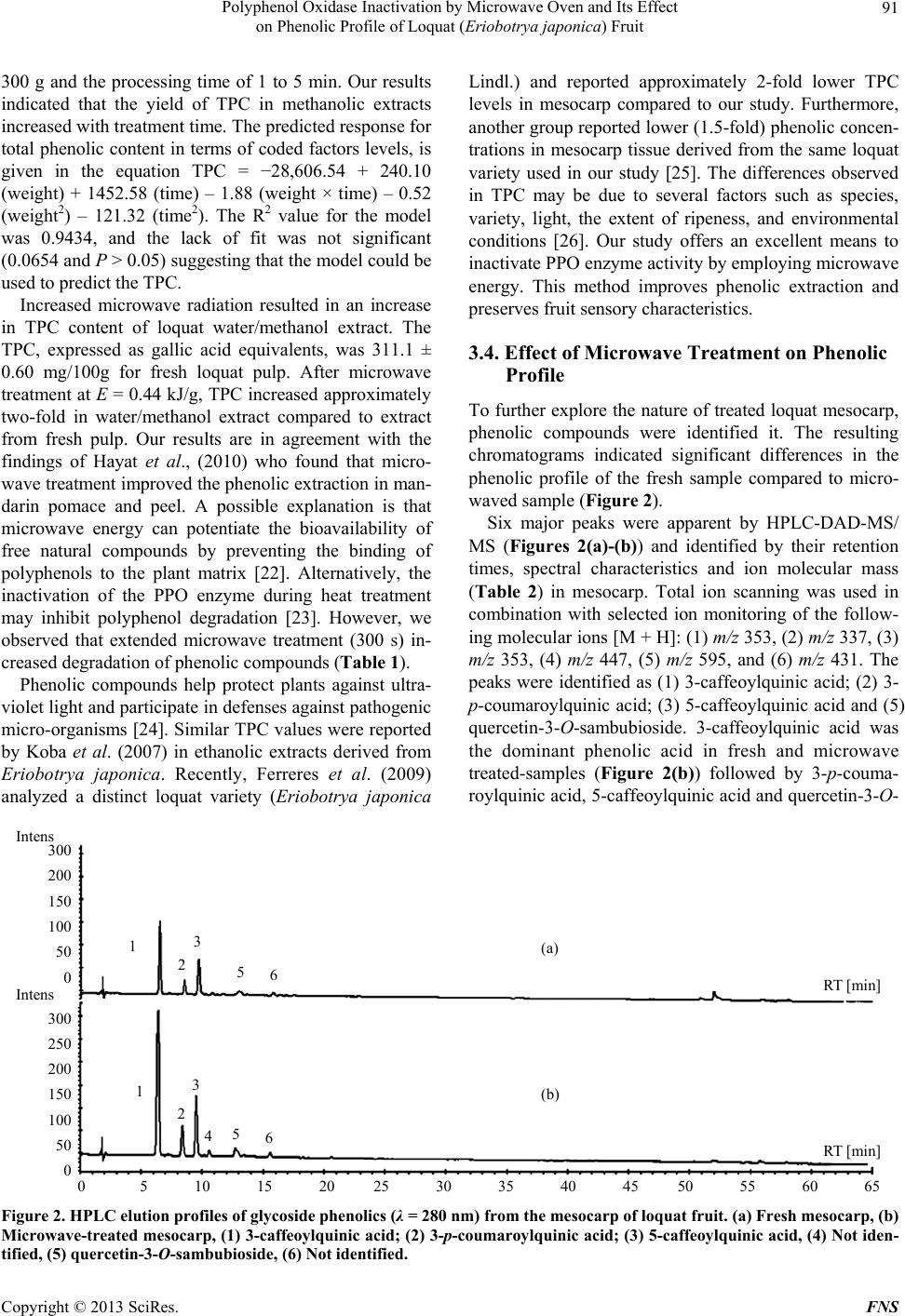 Polyphenol Oxidase Inactivation by Microwave Oven and Its Effect on Phenolic Profile of Loquat (Eriobotr ya jap oni ca) Fruit Copyright © 2013 SciRes. FNS 91 300 g and the processing time of 1 to 5 min. Our results indicated that the yield of TPC in methanolic extracts increased with treatment time. The predicted response for total phenolic content in terms of coded factors levels, is given in the equation TPC = −28,606.54 + 240.10 (weight) + 1452.58 (time) – 1.88 (weight × time) – 0.52 (weight2) – 121.32 (time2). The R2 value for the model was 0.9434, and the lack of fit was not significant (0.0654 and P > 0.05) suggesting that the model could be used to predict the TPC. Lindl.) and reported approximately 2-fold lower TPC levels in mesocarp compared to our study. Furthermore, another group reported lower (1.5-fold) phenolic concen- trations in mesocarp tissue derived from the same loquat variety used in our study [25]. The differences observed in TPC may be due to several factors such as species, variety, light, the extent of ripeness, and environmental conditions [26]. Our study offers an excellent means to inactivate PPO enzyme activity by employing microwave energy. This method improves phenolic extraction and preserves fruit sensory characteristics. Increased microwave radiation resulted in an increase in TPC content of loquat water/methanol extract. The TPC, expressed as gallic acid equivalents, was 311.1 ± 0.60 mg/100g for fresh loquat pulp. After microwave treatment at E = 0.44 kJ/g, TPC increased approximately two-fold in water/methanol extract compared to extract from fresh pulp. Our results are in agreement with the findings of Hayat et al., (2010) who found that micro- wave treatment improved the phenolic extraction in man- darin pomace and peel. A possible explanation is that microwave energy can potentiate the bioavailability of free natural compounds by preventing the binding of polyphenols to the plant matrix [22]. Alternatively, the inactivation of the PPO enzyme during heat treatment may inhibit polyphenol degradation [23]. However, we observed that extended microwave treatment (300 s) in- creased degradation of phenolic compounds (Table 1). 3.4. Effect of Microwave Treatment on Phenolic Profile To further explore the nature of treated loquat mesocarp, phenolic compounds were identified it. The resulting chromatograms indicated significant differences in the phenolic profile of the fresh sample compared to micro- waved sample (Figure 2). Six major peaks were apparent by HPLC-DAD-MS/ MS (Figures 2(a)-(b)) and identified by their retention times, spectral characteristics and ion molecular mass (Table 2) in mesocarp. Total ion scanning was used in combination with selected ion monitoring of the follow- ing molecular ions [M + H]: (1) m/z 353, (2) m/z 337, (3) m/z 353, (4) m/z 447, (5) m/z 595, and (6) m/z 431. The peaks were identified as (1) 3-caffeoylquinic acid; (2) 3- p-coumaroylquinic acid; (3) 5-caffeoylquinic acid and (5) quercetin-3-O-sambubioside. 3-caffeoylquinic acid was the dominant phenolic acid in fresh and microwave treated-samples (Figure 2(b)) followed by 3-p-couma- roylquinic acid, 5-caffeoylquinic acid and quercetin-3-O- Phenolic compounds help protect plants against ultra- violet light and participate in defenses against pathogenic micro-organisms [24]. Similar TPC values were reported by Koba et al. (2007) in ethanolic extracts derived from Eriobotrya japonica. Recently, Ferreres et al. (2009) analyzed a distinct loquat variety (Eriobotrya japonica 0 5 10 15 20 25 30 35 40 45 50 55 60 65 (a) (b) 300 200 150 100 50 0 300 250 200 150 100 50 0 Intens Intens 1 2 3 5 6 RT [min] RT [min] 1 2 3 4 5 6 Figure 2. HPLC elution pr ofile s of glycoside phenolics (λ = 280 nm) from the mesocarp of loquat fruit. (a) Fresh mesocarp, (b) Microwave-treated mesocarp, (1) 3-caffeoylquinic acid; (2) 3-p-coumaroylquinic acid; (3) 5-caffeoylquinic acid, (4) Not iden- tified, (5) quercetin-3-O-sambubioside, (6) Not identified. 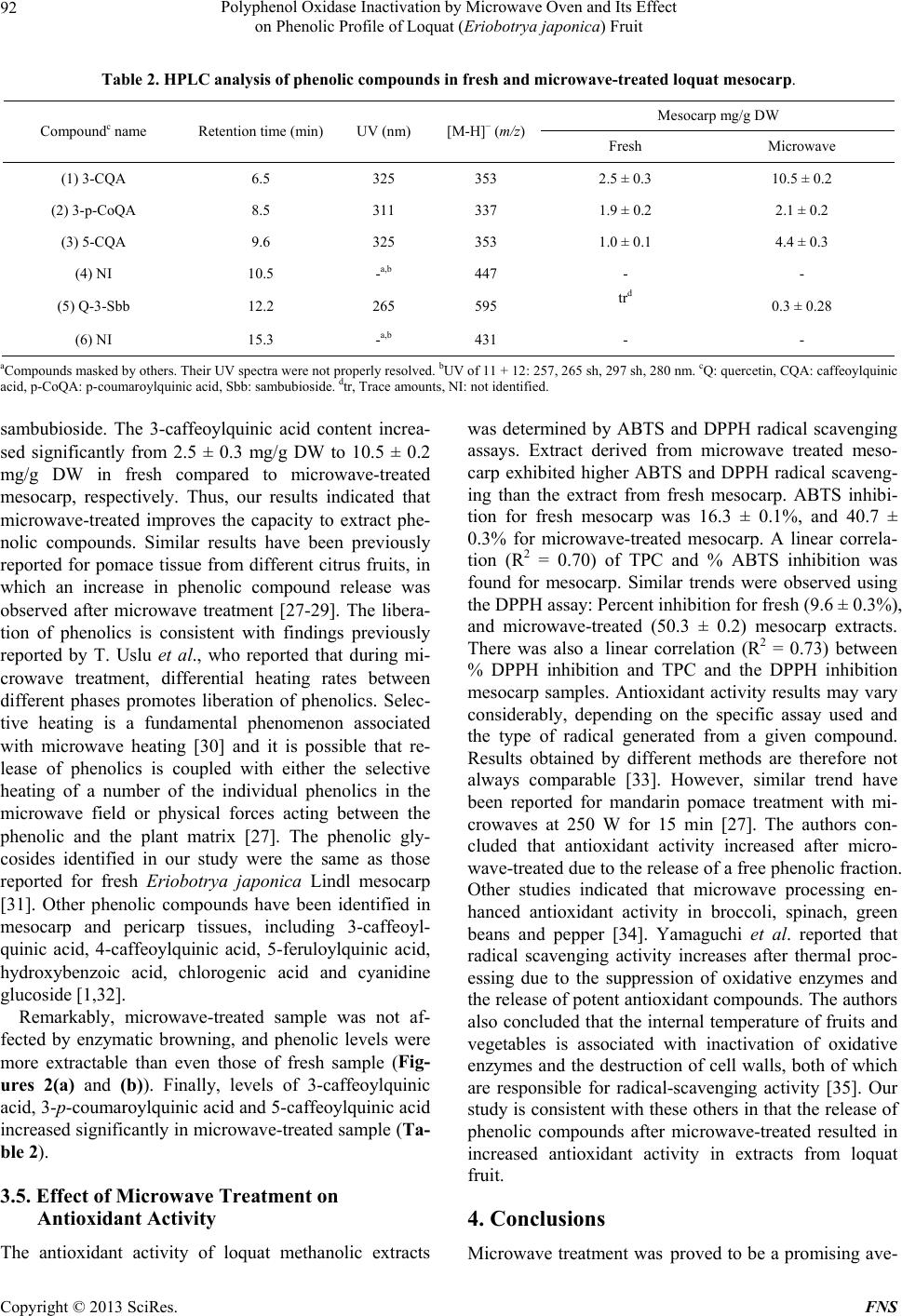 Polyphenol Oxidase Inactivation by Microwave Oven and Its Effect on Phenolic Profile of Loquat (Eriobotr ya jap oni ca) Fruit 92 Table 2. HPLC analysis of phenolic compounds in fresh and microwave-treated loquat mesocarp. Mesocarp mg/g DW Compoundc name Retention time (min) UV (nm) [M-H]− (m/z) Fresh Microwave (1) 3-CQA 6.5 325 353 2.5 ± 0.3 10.5 ± 0.2 (2) 3-p-CoQA 8.5 311 337 1.9 ± 0.2 2.1 ± 0.2 (3) 5-CQA 9.6 325 353 1.0 ± 0.1 4.4 ± 0.3 (4) NI 10.5 -a,b 447 - - (5) Q-3-Sbb 12.2 265 595 trd 0.3 ± 0.28 (6) NI 15.3 -a,b 431 - - aCompounds masked by others. Their UV spectra were not properly resolved. bUV of 11 + 12: 257, 265 sh, 297 sh, 280 nm. cQ: quercetin, CQA: caffeoylquinic acid, p-CoQA: p-coumaroylquinic acid, Sbb: sambubioside. dtr, Trace amounts, NI: not identified. sambubioside. The 3-caffeoylquinic acid content increa- sed significantly from 2.5 ± 0.3 mg/g DW to 10.5 ± 0.2 mg/g DW in fresh compared to microwave-treated mesocarp, respectively. Thus, our results indicated that microwave-treated improves the capacity to extract phe- nolic compounds. Similar results have been previously reported for pomace tissue from different citrus fruits, in which an increase in phenolic compound release was observed after microwave treatment [27-29]. The libera- tion of phenolics is consistent with findings previously reported by T. Uslu et al., who reported that during mi- crowave treatment, differential heating rates between different phases promotes liberation of phenolics. Selec- tive heating is a fundamental phenomenon associated with microwave heating [30] and it is possible that re- lease of phenolics is coupled with either the selective heating of a number of the individual phenolics in the microwave field or physical forces acting between the phenolic and the plant matrix [27]. The phenolic gly- cosides identified in our study were the same as those reported for fresh Eriobotrya japonica Lindl mesocarp [31]. Other phenolic compounds have been identified in mesocarp and pericarp tissues, including 3-caffeoyl- quinic acid, 4-caffeoylquinic acid, 5-feruloylquinic acid, hydroxybenzoic acid, chlorogenic acid and cyanidine glucoside [1,32]. Remarkably, microwave-treated sample was not af- fected by enzymatic browning, and phenolic levels were more extractable than even those of fresh sample (Fig- ures 2(a) and (b)). Finally, levels of 3-caffeoylquinic acid, 3-p-coumaroylquinic acid and 5-caffeoylquinic acid increased significantly in microwave-treated sample (Ta- ble 2). 3.5. Effect of Microwave Treatment on Antioxidant Activity The antioxidant activity of loquat methanolic extracts was determined by ABTS and DPPH radical scavenging assays. Extract derived from microwave treated meso- carp exhibited higher ABTS and DPPH radical scaveng- ing than the extract from fresh mesocarp. ABTS inhibi- tion for fresh mesocarp was 16.3 ± 0.1%, and 40.7 ± 0.3% for microwave-treated mesocarp. A linear correla- tion (R2 = 0.70) of TPC and % ABTS inhibition was found for mesocarp. Similar trends were observed using the DPPH assay: Percent inhibition for fresh (9.6 ± 0.3%), and microwave-treated (50.3 ± 0.2) mesocarp extracts. There was also a linear correlation (R2 = 0.73) between % DPPH inhibition and TPC and the DPPH inhibition mesocarp samples. Antioxidant activity results may vary considerably, depending on the specific assay used and the type of radical generated from a given compound. Results obtained by different methods are therefore not always comparable [33]. However, similar trend have been reported for mandarin pomace treatment with mi- crowaves at 250 W for 15 min [27]. The authors con- cluded that antioxidant activity increased after micro- wave-treated due to the release of a free phenolic fraction. Other studies indicated that microwave processing en- hanced antioxidant activity in broccoli, spinach, green beans and pepper [34]. Yamaguchi et al. reported that radical scavenging activity increases after thermal proc- essing due to the suppression of oxidative enzymes and the release of potent antioxidant compounds. The authors also concluded that the internal temperature of fruits and vegetables is associated with inactivation of oxidative enzymes and the destruction of cell walls, both of which are responsible for radical-scavenging activity [35]. Our study is consistent with these others in that the release of phenolic compounds after microwave-treated resulted in increased antioxidant activity in extracts from loquat fruit. 4. Conclusions Microwave treatment was proved to be a promising ave- Copyright © 2013 SciRes. FNS  Polyphenol Oxidase Inactivation by Microwave Oven and Its Effect on Phenolic Profile of Loquat (Eriobotr ya jap oni ca) Fruit 93 nue to inactivate PPO in loquat fruit. In addition, micro- wave treatment resulted in microstructural modifications that facilitated the release of phenolic compounds from the fruit matrix. The combination of PPO inactivation with increased TPC increased antioxidant activity in me- socarp. In the future, this technology may be scaled up to produce novel products derived from loquat fruit and made available throughout the year. However, further studies are needed to evaluate the effect of microwaves on biocompounds such as carotenes, vitamins, and other nutritional chemicals in loquat fruit. 5. Acknowledgements This research was partly funded by Consejo Nacional de Ciencia y Tecnología (CONACyT) scholarship 286143, Secretaria de Investigación y Posgrado-IPN Proyect num- ber 20090612 and 20100788, Comisión de Operación y Fomento de Actividades Académicas del IPN (COFAA- IPN). REFERENCES [1] C.-K. Ding, K. Chachin, Y. Ueda, Y. Imahori and C. Y. Wang, “Metabolism of Phenolic Compounds during Lo- quat Fruit Development,” Journal of Agricultural and Food Chemistry, Vol. 49, No. 6, 2001, pp. 2883-2888. http://dx.doi.org/10.1021/jf0101253 [2] C.-K. Ding, K. Chachin, Y. Ueda and Y. Imahori, “Puri- fication and Properties of Polyphenol Oxidase from Lo- quat Fruit,” Journal of Agricultural and Food Chemistry, Vol. 46, No. 10, 1998, pp. 4144-4149. http://dx.doi.org/10.1021/jf980093s [3] C.-K. Ding, K. Chachin, Y. Ueda and C. Y. Wang, “Inhi- bition of Loquat Enzymatic Browning by Sulfhydryl Compounds,” Food Chemistry, Vol. 76, No. 2, 2002, pp. 213-218. http://dx.doi.org/10.1016/S0308-8146(01)00270-9 [4] B. Dincer, A. Colak, N. Aydin, A. Kadioglu and S. Güner, “Characterization of Polyphenoloxidase from Medlar Fruits (Mespilus germanica L., Rosaceae),” Food Chemistry, Vol. 77, No. 1, 2002, pp. 1-7. http://dx.doi.org/10.1016/S0308-8146(01)00359-4 [5] A. Altunkaya and V. Gökmen, “Effect of Various Inhibi- tors on Enzymatic Browning, Antioxidant Activity and Total Phenol Content of Fresh Lettuce (Lactuca sativa),” Food Chemistry, Vol. 107, No. 3, 2008, pp. 1173-1179. http://dx.doi.org/10.1016/j.foodchem.2007.09.046 [6] S. Sellés-Marchart, J. Casado-Vela and R. Bru-Martínez, “Isolation of a Latent Polyphenol Oxidase from Loquat Fruit (Eriobotrya japonica Lindl.): Kinetic Characteriza- tion and Comparison with the Active Form,” Archives of Biochemistry and Biophysics, Vol. 446, No. 2, 2006, pp. 175-185. http://dx.doi.org/10.1016/j.abb.2005.12.004 [7] M. S. Venkatesh and G. S. V. Raghavan, “An Overview of Microwave Processing and Dielectric Properties of Agri-Food Materials,” Biosystems Engineering, Vol. 88, No. 1, 2004, pp. 1-18. http://dx.doi.org/10.1016/j.biosystemseng.2004.01.007 [8] K. N. Matsui, J. A. W. Gut, P. V. de Oliveira and C. C. Tadini, “Inactivation Kinetics of Polyphenol Oxidase and Peroxidase in Green Coconut Water by Microwave Proc- essing,” Journal of Food Engineering, Vol. 88, No. 2, 2008, pp. 169-176. http://dx.doi.org/10.1016/j.jfoodeng.2008.02.003 [9] K. Hayat, X. Zhang, U. Farooq, S. Abbas, S. Xia, C. Jia, F. Zhong and J. Zhang, “Effect of Microwave Treatment on Phenolic Content and Antioxidant Activity of Citrus Mandarin Pomace,” Food Chemistry, Vol. 123, No. 2, 2010, pp. 423-429. http://dx.doi.org/10.1016/j.foodchem.2010.04.060 [10] A. Ortiz-Moreno, L. Dorantes, J. Galindez and R. Guz- mán, “Effect of Different Extraction Methods on Fatty Acids, Volatile Compounds, and Physical and Chemical Properties of Avocado (Persea americana Mill) Oil,” Journal of Agricultural and Food Chemistry, Vol. 51, No. 8, 2003, pp. 2216-2221. http://dx.doi.org/10.1021/jf0207934 [11] Dykstra Biological Electron Microscopy, “Theory, Tech- niques and Troubleshooting,” Plenum Publishing Corp, New York, 1992. [12] M. Oktay, O. I. Kufrevioglu, I. Kocacaliskan and H. Saki- roglu, “Polyphenol Oxidase from Amasya Apple,” Jour- nal of Food Science, Vol. 60, No. 3, 1995, pp. 494-496. http://dx.doi.org/10.1111/j.1365-2621.1995.tb09810.x [13] T. Sun, Z. Xu, C.-T. Wu, M. Janes, W. Prinyawiwatkul and H. K. No, “Antioxidant Activities of Different Col- ored Sweet Bell Peppers (Capsicum annuum L.),” Journal of Food Science, Vol. 72, No. 2, 2007, pp. s98-s102. http://dx.doi.org/10.1111/j.1750-3841.2006.00245.x [14] R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang and C. Rice-Evans, “Antioxidant Activity Applying an Improved ABTS Radical Cation Decolourization As- say,” Free Radical Biology & Medicine, Vol. 26, No. 9-10, 1999, pp. 1231-1237. http://dx.doi.org/10.1016/S0891-5849(98)00315-3 [15] G. Palma-Orozco, J. G. Sampedro, A. Ortiz-Moreno and H. Nájera, “In Situ Inactivation of Polyphenol Oxidase in Mamey Fruit (Pouteria sapota) by Microwave Treat- ment,” Journal of Food Science, Vol. 77, No. 4, 2012, pp. c359-c365. http://dx.doi.org/10.1111/j.1750-3841.2012.02632.x [16] C. Severini, A. T. Baiano and T. Pili, “Microwave Blan- ching of Cubed Potatoes,” Journal of Food Processing and Preservation, Vol. 27, No. 6, 2003, pp. 475-491. http://dx.doi.org/10.1111/j.1745-4549.2003.tb00531.x [17] L. Dorantes-Álvarez, G. V. Barbosa-Cánovas and G. Gu- tiérrez-López, “Inovations in Food Processing, Food Pre- servation Technology Series. Blanching of Fruit and Ve- getables Using Microwaves,” Technomic Publishing Com- pany, 2000, pp. 149-161. [18] M. E. Latorre, P. R. Bonelli, A. M. Rojas and L. N. Ger- schenson, “Microwave Inactivation of Red Beet (Beta vulgaris L. var. conditiva) Peroxidase and Polyphenoloxi- Copyright © 2013 SciRes. FNS  Polyphenol Oxidase Inactivation by Microwave Oven and Its Effect on Phenolic Profile of Loquat (Eriobotr ya jap oni ca) Fruit Copyright © 2013 SciRes. FNS 94 dase and the Effect of Radiation on Vegetable Tissue Quality,” Journal of Food Engineering, Vol. 109, No. 4, 2012, pp. 676-684. http://dx.doi.org/10.1016/j.jfoodeng.2011.11.026 [19] T. Blessington, M. N. Nzaramba, D. Scheuring, A. Hale, L. Reddivari and J. C. Miller Jr., “Cooking Methods and Storage Treatments of Potato: Effects on Carotenoids, Antioxidant Activity, and Phenolics,” American Journal of Potato Research, Vol. 87, No. 6, 2010, pp. 479-491. http://dx.doi.org/10.1007/s12230-010-9150-7 [20] I. Hamrouni-Sellami, F. Rahali, I. Rebey, S. Bourgou, F. Limam and B. Marzouk, “Total Phenolics, Flavonoids, and Antioxidant Activity of Sage (Salvia officinalis L.) Plants as Affected by Different Drying Methods,” Food and Bioprocess Technology, Vol. 6, No. 3, 2013, pp. 806- 817. http://dx.doi.org/10.1007/s11947-012-0877-7 [21] S. Inchuen, W. Narkrugsa and P. Pornchaloempong, “Ef- fect of Drying Methods on Chemical Composition, Color and Antioxidant Properties of Thai Red Curry Powder,” Kasetsart Journal of Natural Science, Vol. 44, 2010, pp. 142-151. [22] A. Gulati, R. Rawat, B. Singh and S. D. Ravindranath, “Application of Microwave Energy in the Manufacture of Enhanced-Quality Green Tea,” Journal of Agricultural and Food Chemistry, Vol. 51, No. 16, 2003, pp. 4764- 4768. http://dx.doi.org/10.1021/jf026227q [23] A. M. Chuah, Y.-C. Lee, T. Yamaguchi, H. Takamura, L.-J. Yin and T. Matoba, “Effect of Cooking on the An- tioxidant Properties of Coloured Peppers,” Food Chemis- try, Vol. 111, No. 1, 2008, pp. 20-28. http://dx.doi.org/10.1016/j.foodchem.2008.03.022 [24] K. D. Croft, “The Chemistry and Biological Effects of Flavonoids and Phenolic Acidsa,” Annals of the New York Academy of Sciences, Vol. 854, No. 1, 1998, pp. 435-442. http://dx.doi.org/10.1111/j.1749-6632.1998.tb09922.x [25] J.-Y. Lin and C.-Y. Tang, “Determination of Total Phe- nolic and Flavonoid Contents in Selected Fruits and Ve- getables, as Well as Their Stimulatory Effects on Mouse Splenocyte Proliferation,” Food Chemistry, Vol. 101, No. 1, 2007, pp. 140-147. http://dx.doi.org/10.1016/j.foodchem.2006.01.014 [26] G. G. Duthie, P. T. Gardner and J. A. Kyle, “Plant Poly- phenols: Are They the New Magic Bullet?” Proceedings of The Nutrition Society, Vol. 62, No. 3, 2003, pp. 599- 603. http://dx.doi.org/10.1079/PNS2003275 [27] K. Hayat, X. Zhang, H. Chen, S. Xia, C. Jia and F. Zhong, “Liberation and Separation of Phenolic Compounds from Citrus Mandarin Peels by Microwave Heating and Its Ef- fect on Antioxidant Activity,” Separation and Purifica- tion Technology, Vol. 73, No. 3, 2010, pp. 371-376. http://dx.doi.org/10.1016/j.seppur.2010.04.026 [28] G. Xu, X. Ye, J. C. Chen and L. D. Liu, “Effect of Heat Treatment on the Phenolic Compounds and Antioxidant Capacity of Citrus Peel Extract,” Journal of Agricultural and Food Chemistry, Vol. 55, No. 2, 2007, pp. 330-335. http://dx.doi.org/10.1021/jf062517l [29] S.-M. Jeong, S.-Y. Kim, D.-R. Kim, S.-C. Jo, K. C. Nam, D. U. Anh and S.-C. Lee, “Effect of Heat Treatment on the Antioxidant Activity of Extracts from Citrus Peels,” Journal of Agricultural and Food Chemistry, Vol. 52, No. 11, 2004, pp. 3389-3393. http://dx.doi.org/10.1021/jf049899k [30] T. Uslu, Ü. Atalay and A. I. Arol, “Effect of Microwave Heating on Magnetic Separation of Pyrite,” Colloids and Surfaces A: Physicochemical and Engineering Aspects, Vol. 225, No. 1-3, 2003, pp. 161-167. http://dx.doi.org/10.1016/S0927-7757(03)00362-5 [31] F. Ferreres, D. Gomes, P. Valentão, R. Gonçalves, R. Pio, E. A. Chagas, R. M. Seabra and P. B. Andrade, “Im- proved Loquat (Eriobotrya japonica Lindl.) Cultivars: Variation of Phenolics and Antioxidative Potential,” Food Chemistry, Vol. 114, No. 3, 2009, pp. 1019-1027. http://dx.doi.org/10.1016/j.foodchem.2008.10.065 [32] K. Koba, A. Matsuoka, K. Osada and Y.-S. Huang, “Ef- fect of Loquat (Eriobotrya japonica) Extracts on LDL Oxidation,” Food Chemistry, Vol. 104, No. 1, 2007, pp. 308-316. http://dx.doi.org/10.1016/j.foodchem.2006.11.043 [33] K. Krishnaswamy, V. Orsat, Y. Gariépy and K. Than- gavel, “Optimization of Microwave-Assisted Extraction of Phenolic Antioxidants from Grape Seeds (Vitis vinif- era),” Food and Bioprocess Technology, Vol. 6, No. 2, 2013, pp. 441-455. http://dx.doi.org/10.1007/s11947-012-0800-2 [34] N. Turkmen, F. Sari and Y. S. Velioglu, “The Effect of Cooking Methods on Total Phenolics and Antioxidant Activity of Selected Green Vegetables,” Food Chemistry, Vol. 93, No. 4, 2005, pp. 713-718. http://dx.doi.org/10.1016/j.foodchem.2004.12.038 [35] T. Yamaguchi, M. Taeko, K. Rie, K. Hiroko, M. Fumiko, T. Junji, T. Hitoshi and M. Teruyoshi, “Radical-Scaveng- ing Activity of Vegetables and the Effect of Cooking on Their Activity,” Food Science and Technology Research, Vol. 7, No. 3, 2001, pp. 250-257. http://dx.doi.org/10.3136/fstr.7.250
|