 Open Journal of Nephrology, 2013, 3, 128-134 http://dx.doi.org/10.4236/ojneph.2013.33024 Published Online September 2013 (http://www.scirp.org/journal/ojneph) Antihypertensive Therapy in Non-Diabetic Chronic Kidney Disease Associated with Proteinuria in Adults Khawar Maqsood1, Adeel Siddiqui2, Geoffrey Teehan2* 1Department of Medicine, Baystate Medical Center, Tufts University School of Medicine, Springfield, USA 2Department of Nephrology, Lankenau Medical Center, Lankenau Institute for Medical Research, Wynnewood, USA Email: *gteehan@comcast.net Received July 3, 2013; revised August 4, 2013; accepted August 14, 2013 Copyright © 2013 Khawar Maqsood et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Controlling blood pressure and reducing proteinuria are common goals in Chronic Kidney Disease associated with hy- pertension and proteinuria and lead to fewer cardiovascular outcomes. This rev iew summarizes the available literature. Keywords: Hypertension; Proteinuria; Chronic Kidney Disease 1. Introduction Proteinuria is a risk factor for progression of chronic kid- ney disease (CKD) and is associated with adverse cardio- vascular outcomes. Controlling blood pressure particu- larly with strongly anti-proteinuric agents slows the pro- gression of chronic kidney disease (CKD) and delays/ prevents cardiovascular outcomes. Angiotensin convert- ing enzyme inhibitors (ACE-I) and angiotensin receptor antagonists (ARBs) have a blood pressure-independent antiproteinuric effect and are considered first line agents in this scenario [1]. This review will app rise th e literatu re to date on an tihyp erten s ive agents studied in non-d iabetic proteinuric kidney disease. Table 1 summarizes these trials. 2. ACE Inhibitors Captopril was the first ACE-I approved by Food and Drug Administration (FDA) in 1981 followed by enala- pril which was marketed two years later. Since then at least twelve other ACE-I have been marketed. Multiple trials have identified a preferential benefit of ACE-I in reducing proteinuria when compared with other anti- hypertensive drugs. The basis of all these studies was the observation that protein excretion varies directly with the intra-glomerular pressure in animals with structural glo- merular disease [2]. Apart from the reduction in intra-glomerular pressure, a variety of other mechanisms may contribute to ACE-I induced reduction in proteinuria. These include: The hemodynamic effects of ACE inhibition occur rapidly but then become stable, whereas protein ex- cretion progressively decreases over weeks to several months [3]. Acute administration of angiotensin II increases intra- glomerular pressure by causing renal and systemic vasoconstriction but does not reverse the antiprotein- uric effect of ACE-I [4]. Nephrin is a major component of the podocyte slit pore membrane and an important contributor to the glomerular filtration barrier. Angiotensin II reduces the expression of nephrin [5]. In contrast, ACE-I in- crease expression of nephrin [6]. ACE-I may contribute to slowing of renal disease by their possible anti fibrotic properties. ACE-I related reduction in proteinuria may be associ- ated with a reduction in serum lipid level which may have a beneficial effect on progression of renal dis- ease and systemic atherosclerosis. Multiple clinical trials have demonstrated a beneficial effect of antihypertensive therapy with renin-angiotensin system (RAS) inhibitors, mostly ACE-I in patients with proteinuric non-diabetic chronic kidney disease (CKD). Jafar et al studied 1860 patients with CKD in 11 ran- domized controlled trials (RCT) and the response to ACE-I versus other antihypertensives or placebo [7]. ACE-I use was associated with significant reductions in the rate of progr ession to end-stage renal disease (ESRD) (7.4% versus 11.6%, RR 0.69, 95% CI 0.51 - 0.94), while that for doubling of the baseline serum creatinine concentration or ESRD was 13.2 versus 20.5 percent (RR 0.70, 95% CI 0.55 - 0.88) independent of blood pressure *Corresponding author. C opyright © 2013 SciRes. OJNeph 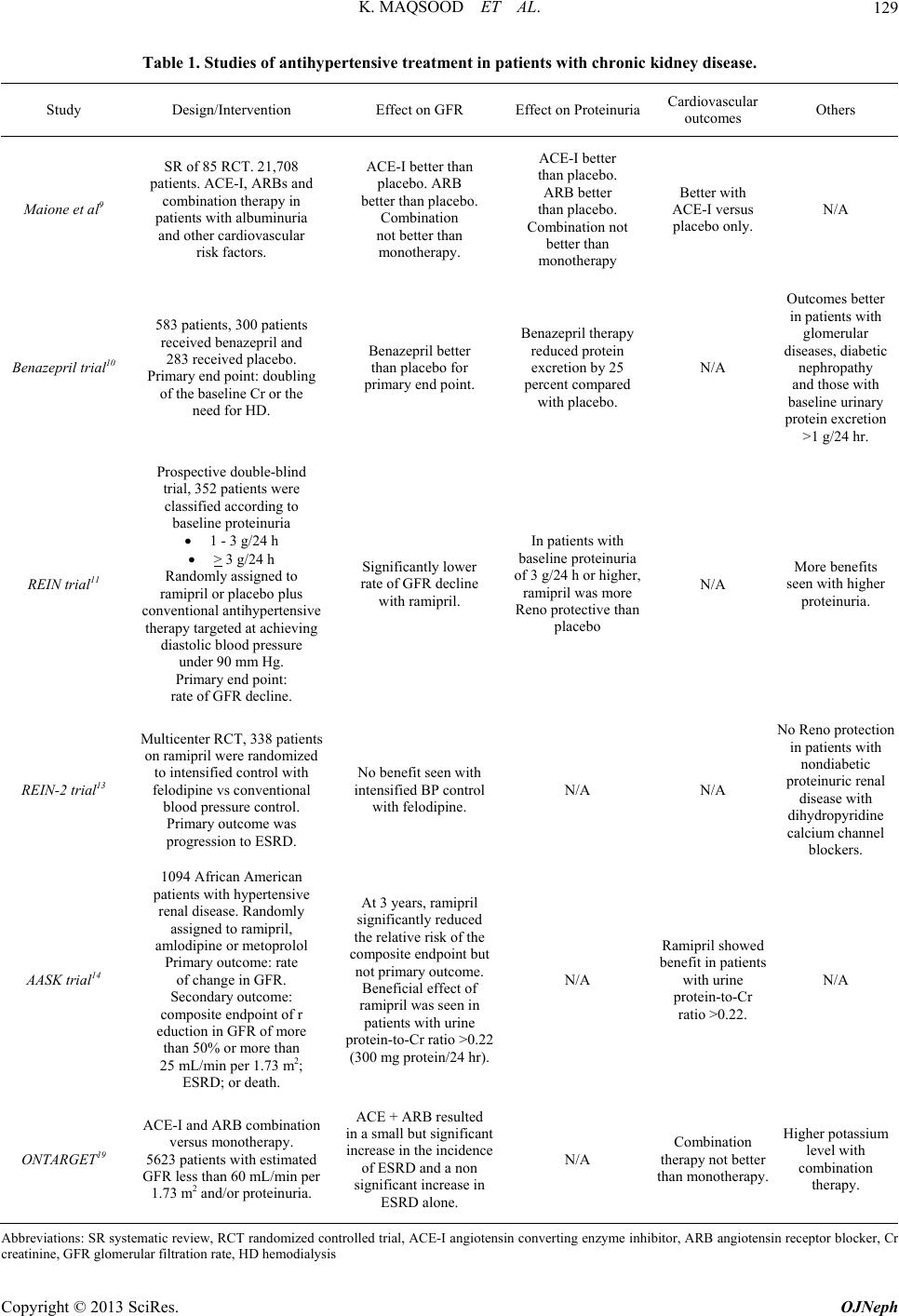 K. MAQSOOD ET AL. 129 Table 1. Studies of antihypertensive treatment in patients with chronic kidney disease. Study Design/Intervention Effect on GFR Effect on Protein uriaCardiovascular outcomes Others Maione et al9 SR of 85 RCT. 21,708 patients. ACE-I, ARBs and combination therapy in patients with albuminuria and other cardiovascular risk factors. ACE-I better than placebo. ARB better than placebo. Combination not better than monotherapy. ACE-I better than placebo. ARB better than placebo. Combination not better than monotherapy Better with ACE-I versus placebo only. N/A Benazepril trial10 583 patients, 300 patients received benazepril and 283 received placebo. Primary end po int: dou bling of the baseline Cr or the need for HD. Benazepril better than placebo for primary end point. Benazepril therapy reduced protein excretion by 25 percent compared with placebo. N/A Outcomes better in patients with glomerular diseases, diabetic nephropathy and those with baseline urinary protein excretion >1 g/24 hr. REIN trial11 Prospective double-blind trial, 352 patients were classified according to baseline proteinuria 1 - 3 g/24 h > 3 g/24 h Randomly assigned t o ramipril or placebo plus conventional antihypertensive therapy targeted at achieving diastolic blood pressure under 90 mm Hg. Primary end point: rate of GFR decline. Significantly lower rate of GFR decline with ramipril. In patients with baseline proteinuria of 3 g/24 h or higher, ramipril was more Reno protective than placebo N/A More benefits seen with higher proteinuria. REIN-2 trial13 Multicenter RCT, 338 patients on ramipri l were randomized to intensified control with felodipine vs conventional blood pressure control. Primary ou tc ome was progression to ESR D . No benefit seen with intensified BP control with felodipine. N/A N/A No Reno protection in patients with nondiabetic proteinuric renal disease with dihydropyridine calcium channel blockers. AASK trial14 1094 African American patients with hypertensive renal disease. Randomly assigned to ramipril, amlodipine or m etoprolol Primary outcome: rate of change in GFR. Secondary outcome: composite endpoint of r eduction in GFR of more than 50% or more than 25 mL/min per 1.73 m 2; ESRD; or death. At 3 years, ramipril significantly reduced the relative risk of the composite endpoint but not primary o utco me. Beneficial effect of ramipril was seen in patients with urine protein-to-Cr ratio >0.22 (300 mg prote i n/ 24 hr). N/A Ramipril sho w e d enefit in patients with urine protein-to-Cr ratio >0.22. N/A ONTARGET19 ACE-I and ARB combination versus monotherapy. 5623 patients with estimated GFR less than 60 mL/min per 1.73 m2 and/or proteinuria. ACE + ARB resulted in a small but significant increase in the incidence of ESRD and a non significant increase in ESRD alone. N/A Combination therapy not better than monotherapy. Higher potassium level with combination therapy. Abbreviations: SR systematic review, RCT randomized controlled trial, ACE-I angiotensin converting enzyme inhibitor, ARB angiotensin receptor bl ocker, Cr creatinine, GFR glomerular filtration rate, HD hemodialysis Copyright © 2013 SciRes. OJNeph  K. MAQSOOD ET AL. 130 control. These benefits of ACE-I correlated with increas- ing baseline proteinuria but did not apply to those with proteinuria below 500 to 1000 mg/day [8]. Maione et al. conducted a systematic review of 85 RCTs (21,708 subjects) to look at ACE-I, ARBs and combination therapy in patients with microalbuminuria, macroalbuminuria and other cardiovascular risk factors [9]. Neither ACE I, ARB, nor the combination of the two was associated with a significant reduction in the risk of all-cause morta lity or fatal card iac-cerebrovascular events. ACE I, and not ARB, did however reduce the risk of nonfatal cardiovascular events. Development of ESRD disease and progression of microalbuminuria to macro- albuminuria were reduced significantly with ACE-I ver- sus placebo, ARB versus placebo but not with combined therapy with ACE-I and ARB versus monotherapy. Au- thors concluded that ACE-I and ARB exert independent renal and nonfatal cardiovascular benefits while their effects on mortality and fatal cardiovascular disease are uncertain. Use of combination therap y was not supported by the evidence. Maschio et al. performed a placebo-controlled RCT involving 583 patients with renal insufficiency (crea- tinine clearance 30 - 60 ml per minute) in which 300 pa- tients received benazepril and 283 received placebo. The primary end point was a doubling of the baseline serum creatinine concentration or the n eed for dialysis. At three years the risk reduction attributed to benazepril was 53 percent overall (95% CI, 27% - 70%) and was particu- larly evident in milder k idney disease. The risk reduction was greatest among men; those with glomerular diseases, diabetic nephropathy, or miscellaneous or unknown cau- ses of renal disease; and those with baseline urinary pro- tein excretion above 1 g per 24 hours. Benazepril was not effective in patients with polycystic kidney disease [10]. The Ramipril Efficacy in Nephropathy (REIN) trial was a RCT in which patients (N = 352) with proteinuric non-diabetic CKD were randomized to ramipril or pla- cebo plus other antihypertensive therapy to attain a dia- stolic pressure below 90 mmHg. It showed similar bene- fits as the Maschio trial [11]. Stratified by level of daily proteinuria, the primary endpoint was the rate of glome- rular filtration rate (GFR) decline. The trial was termi- nated prematurely in patients excreting more than 3 grams of protein per day because of a significant benefit with ACE-I in ameliorating the rate of decline of renal function (0.53 versus 0.88 mL/min per month for pla- cebo). Subsequently all pa tients with pr oteinu r ia of 3 g or more per 24 h either continued on ramipril or were shift- ed to ramipril. A REIN follow-up study compared the rate of decline of renal function and the need for dialysis in patients who continued to receive ramipril (51 patients) and in those originally randomized to conventional antihypertensive therapy plus placebo who were switched to ramipril at the beginning of the observational follow-up (46 pa- tients). At 20 months (and at 44 months for the trial phase and observational follow-up combined), mean rate of GFR decline slowed significantly and end of trial GFRs were significantly higher in the ramipril group (36 vs 24 ml per minute, P = 0.01). Progression to ESRD was more common in those receiving placebo (35 versus 19, P = 0.027) [12]. The original and follow-up ramipril studies strongly suggest that patients who particularly benefit are those with prominent proteinuria, but signifi- cant benefit was also seen in patients with sub-nephrotic proteinuria (1.0 to 2.9 g/day). REIN-2 evaluated lowering blood pressure beyond usual targets in patients with ch ronic nephropath ies using ACE I. Patients receiving ACE I (Ramipril 2.5 - 5 mg/d) with non-diabetic proteinuric CKD (mean baseline GFR 35 mL/min and mean proteinuria 2.9 g/day, N = 338) were enrolled in this RCT [13]. Subjects were randomly assigned to either conventional (diastolic < 90 mm Hg; n = 169) or intensified (systolic/diastolic < 130 /80 mm Hg; n = 169) blood-pressure control with Felodipine (5 - 10 mg/day) added as needed to achieve the intensified blood-pressure level. The primary outcome measure, time to ESRD over 36 months did not differ between the two groups (20% vs 23%, P = 0.99). Further blood pres- sure reduction with Felodipine therefore is not warranted to slow progression to ESRD in this population. These findings are consistent with previous observations show- ing that dihydropyridine calcium channel blockers fail to provide renoprotection in patients with non-diabetic pro- teinuric renal disease. Hypertension and ESRD are more prevalent among African Americans than Caucasians. Monotherapy with a calcium channel blocker or a diuretic may be superior in this population than ACE-I. The African American Study of Kidney Disease and Hypertension (AASK trial) [14] included 1094 African American patients with hyperten- sive renal disease. Mean GFR was 46 ml/min (range 20- 65 mL/min per 1.73 m2) and mean protein excretion was about 600 mg/day in men and 400 mg/day in women. Patients were randomly assigned to three different anti- hypertensive drugs and to two different blood pressure goals. The blood pressure target was a mean arterial pres- sure of 92 mm Hg or less in the intensive-control group (corresponds to lower than the traditional blood-pressure target of 130/80 mm Hg for CKD) and 102 to 107 mm Hg in the standard-control group (corresponds to the tra- ditional blood-pressure target of 140/90 mm Hg). Pa- tients were randomly assigned to treatment with an ACE inhibitor (ramipril, 2.5 - 10 mg/day), a calcium channel blocker (amlodipine, 5 - 10 mg/day), or a beta blocker (metoprolol, 50 - 200 mg day); other antihypertensive drugs were added to initial monotherapy to achieve the Copyright © 2013 SciRes. OJNeph  K. MAQSOOD ET AL. 131 blood pressure goals. The primary outcome was the rate of change in GFR; the main secondary outcome was a composite endpoint of: reduction in GFR of more than 50 percent or more than 25 mL/min per 1.73 m2; ESRD; or death. Although the GFR decline with ramipril and amlodip- ine therapy were similar at 3 years, ramipril significantly reduced the relative risk of the composite endpoint by 38 percent. Additionally, ramipril was significantly better at slowing GFR decline among those with a urine protein-to creatinine ratio >0.22 (approximately equivalent to 300 mg protein in 24 hours). It also showed that sustained- release metoprolol has antiproteinuric effects nearly eq- ual to that of ramipril (P = 0.06 for the comparison of total change over 4 years) and better than that of am- lodipine. Proteinuria increased by 58% for the amlodip- ine group and declined by 14% in the sustained-release metoprolol group between baseline and 6 months (P < 0.001) [14]. After completion of the AASK trial, all of the partici- pants were invited to enroll in a cohort phase during which ramipril was prescribed to everyone. After an ad- ditional five years of follow-up progression of neph- ropathy was significan t ly slowed b ut n ot stoppe d [15]. 2.1. Use of ACEI/ARB in Advanced CKD The pitfalls of using an ACE-I or ARBs in advanced CKD include an initial GFR decline and hyperkalemia, prompting many practition ers to avoid their use. Fan Fan Hou et al. enrolled 422 patients with non-diabetic CKD and randomly assigned them to benazepril or placebo plus other antihypertensive therapy to attain a systolic and diastolic pressure below 130 and 80 mmHg, respec- tively [16]. Patients were divided into two groups based on renal function: Group 1 (N = 141, Serum Creatinine 1.5 - 3.0 mg/dL; mean GFR 37 ml/min/1.73 m2, mean proteinuria 1.6 g/d) and Group 2 (N = 281, Serum Creatinine 3.1 - 5.0 mg/dL, mean GFR 26 ml/min/1.73m2, mean proteinuria 1.6 g/d). After an eight-week run-in period in which they re- ceived benazepril at 10 mg/day for four weeks 104 pa- tients remained in Group 1 an d 224 r emained in Group 2. Group 1 subjects then received benazepril (at 10 mg twice daily, since it was deemed unethical to administer placebo), while Group 2 participants were randomly as- signed to benazepril (10 mg twice daily) or placebo. Ad- ditional antihypertensive therapy was administered to attain blood pressure goals. The primary endpoint was the composite of doubling of the serum creatinine level, ESRD, or death, while secondary endpoints were change in proteinuria and rate of progression of the renal disease. At a mean follow up of 3.4 years, significantly fewer Group 2 patients (mean GFR of 26 mL/min per 1.73 m2) treated with benazepril reached the primary endpoint (41 versus 60 percent with placebo), resulting in an overall risk reduction of 43 percent with active therapy. The primary endpoint was reached less often in group 1 pa- tients (22 percent), who had less severe disease and were all treated with benazepril. In group 2 doubling of the serum creatinine and rea- ching ESRD occurred in 51 and 40% fewer patients re- ceiving Benazepril. Fall in proteinuria (52 versus 20 per- cent) and a slower rate of decline in GFR (6.8 versus 8.8 mL/min per 1.73 m2 per year) were also noticed. The benefits with benazepril were independent of blood pressure. Benazepril conferred substantial renal benefits in highly selected patients without diabetes who had ad- vanced renal insufficiency. The REIN trial confirms the benefit from ACE-I in pa- tients with advanced CKD. As previously mentioned, patients with an initial GFR within the lowest group (11 to 33 mL/min/1.73 m2) had a 20 percent decrease in the rate of decline in GFR and a 33 percent reduction in the incidence of end - s t age renal disease. 2.2. Use of ACE I in Elderly Patients The benefits from RAS inhibition in proteinuric CKD patients older than 70 years are largely unknown. Most of the above trials did not include such individuals since older patients with CKD are less likely to have proteinu- ria. This is confirmed by an analysis of 1190 National Health and Nutrition Examination Survey (NHANES) participants who were over age 70 and had CKD, which was defined as an estimated GFR < 60 mL/min per 1.73 m2 or an albumin-to-creatinine ratio >200 mg/g of creat- inine (approximately 300 mg/day) [17]. This level of proteinuria was present in only 13 percent. There is no evidence of benefit from RAS inhibition in patients with protein excretion below 500 mg/day. It suggests that the great majority of patients over age 70 with CKD would not benefit from RAS inhibition for renal protection and may have harm from a higher rate of side effects. How- ever, this does not fully apply on patients excretin g more than 1 g/day. Treatment of proteinuric individuals over the age of 70 with CKD remains an area for investiga- tion. 2.3. Combination of ACE Inhibitors and ARBs A number of small trials have shown that combination ACE-I and ARB therapy has a greater antiproteinuric effect than either agent alone. A meta-analysis of 14 tri- als in non-diabetic renal disease found that combination therapy produ ced a significant 18% - 25% greater reduc- tion in proteinuria compared with monotherapy [18]. These trials did not attempt to identify a maximum anti- proteinuric dose, did not compare combination therapy to high dose monotherapy, and did not assess renal out- Copyright © 2013 SciRes. OJNeph  K. MAQSOOD ET AL. 132 comes. Combination therapy however may have adverse ef- fects in proteinuric CKD. The ONTARGET trial evalu- ated combination therapy versus monoth erapy in a sub set of 5623 patients who, at baseline, had reduced renal function (defined as an estimated GFR less than 60 mL/min per 1.73 m2) and/or proteinuria [19]. Combina- tion therapy resulted in a small but significant increase in the incidence of ESRD or doubling of the serum crea- tinine (0.79 versus 0.56 percent per year), but a nonsig- nificant increase in ESRD alone (0.34 versus 0.27 per- cent per year) and caused more hyperkalemia while fail- ing to reduce the risk of cardiovascular disease or death [20]. 3. Other Antihypertensive Drugs 3.1. Calcium Channel Blockers Numerous studies suggest that the dihydropyridine cal- cium antagonists (DCAs) and non-dihydropyridine cal- cium antagonists (NDCAs) have differential antiprotein- uric effects. The NDCAs, such as diltiazem and verapa- mil are generally considered to have significant antipro- teinuric effects while DCAs, such as amlodipine and nifedipine either have no effect or cause a small rise in protein excretion [21]. Bakris et al. systematically reviewed 23 studies of NDCA versus DCA and found NDCAs decreased mean proteinuria by 30 percent and DCAs increased proteinu- ria by 2 percent (95% CI 10% - 54% for the differences between the two drug classes). Similar observations were noted when these agents were used in combination with ACE-I or ARBs: despite similar reductions in blood pressure, the mean change in proteinuria was minus 39 and plus 2 percent for NDCAs and DCAs, respectively. They concluded that NDCA and DCAs lower blood pressure equivalently, but that NDCAs are superior to DCAs in reducing proteinuria in both diabetic and non- diabetic kidney disease [21]. 3.2. Aldosterone Antagonists Addition of aldosterone antagonists may provide renal benefits to proteinuric CKD patients over and above the inhibition of RAS blockers by virtue of partially elimi- nating secondary hyperaldosteronism. Navaneethan et al. performed a meta- analysis of 11 trials in which patients were treated with an ACE-I and/or ARB plus either spi- ronolactone (usually 25 mg/day) or placebo and found a significantly greater reduction in proteinuria in the spi- ronolactone group (weighted mean difference 800 mg/day, 95% CI 330 - 1270 mg/day). Short-term chang es in GFR (less than one year of follow-up) were similar with spi- ronolactone and placebo. However, most of these studies did not first maximize the dose of the ACE-I or ARB, and the aldosterone antagonist was associated with an increased risk of hyperkalemia (relative risk 3.1 in the meta-analysis) [22]. Longer-term data are lacking in this field. 3.3. Direct Renin Inhibitors Aliskiren, being the first oral direct renin inhibitor (DRI) became available in the United States in March 2007. A number of studies have evaluated the blood pressure lowering effect of aliskiren in combination with other antihypertensive drugs [23-26]. Its novel mechanism of action, inhibition of catalytic activity of renin, the most proximal and rate-limiting step in RAS activation, makes it of particular interest. In the AVOID trial, aliskiren plus losartan was associated with a 20 percent greater reduc- tion in proteinuria compared with losartan alone in pa- tients with type-2 diabetes and nephropathy in the ab- sence of a significantly greater effect on blood pressure [27]. However, this effect on proteinuria did not translate into a clinical benefit. In the ALTITUDE trial, 8600 patients with type-2 dia- betes and kidney disease already taking either an ACE-I or ARB were randomly assigned to additional therapy with aliskiren or placebo [28]. The trial was terminated early due to no benefit on the primary cardiovascular and renal outcomes and the preliminary analysis indicated that aliskiren therapy produced a higher rate of adverse events (i.e., non-fatal stroke, hypotension, hyperkalemia) [29]. Other studies also described an increased risk of hyperkalemia when aliskiren is combined with ACE-I or ARB [30]. Thus, aliskiren should not be combined with ACE-I or ARBs. 3.4. Drugs with Little or No Effect Other antihypertensive drugs have little or no effect on protein excretion. As an example, beta blockers, diuretics, and the alpha-1-blockers (such as prazosin) typically have a lesser antiproteinuric effect than RAS inhibitors [31-33]. A meta-analysis showed that ACE-I lowered protein excretion by 40 percent compared with 16 per- cent for beta blockers and 14 percent for other non-cal- cium channel blocker antihypertensive drugs [31]. Me- thyldopa and Guanfacine, have little effect on protein excretion. 4. Conclusion Proteinuria is associated with adverse renal and cardio- vascular outcomes. Retarding proteinuria in CKD slows progression of CKD and can limit some cardiovascular outcomes. ACE-I and ARBs are the first line agents in this setting because they have a blood pressure-inde- pendent antiproteinuric effect and have shown to im- prove renal and cardiovascular outcomes. If proteinuria Copyright © 2013 SciRes. OJNeph  K. MAQSOOD ET AL. 133 is not controlled with ACE-I or ARB, combination ther- apy is generally discouraged. We recommend the addi- tion of an NDCA such as diltiazem or verapamil, long acting metoprolol or a mineralocorticoid antagonist as the next line agent. Aggressive measures must be taken to improve blood pressure and reduce proteinuria for better renal and cardiovascular outcomes. REFERENCES [1] P. A. Sarafidis, N. Khosla and G. L. Bakris, “Antihyper- tensive Therapy in the Presence of Proteinuria,” American Journal of Kidney Diseases, Vol. 49, No. 1, 2007, pp. 12- 26. doi:10.1053/j.ajkd.2006.10.014 [2] T. Yoshioka, H. G. Rennke, D. J. Salant, W. M. Deen and I. Ichikawa, “Role of Abnormally High Transmural Pres- sure in the Permselectivity Defect of Glomerular Capil- lary Wall: A Study in Early Passive Heymann Nephritis,” Circulation Research, Vol. 61, No. 4, 1987, p. 531. doi:10.1161/01.RES.61.4.531 [3] R. T. Gansevoort, D. de Zeeuw and P. E. de Jong, “Disso- ciation between the Course of the Hemodynamic and An- tiproteinuric Effects of Angiotensin I Converting Enzyme Inhibition,” Kidney International, Vol. 44, No. 3, 1993, pp. 579-584. doi:10.1038/ki.1993.284 [4] J. E. Heeg, P. E. de Jong, G. K. van der Hem and D. de Zeeuw, “Angiotensin II Does Not Acutely Reverse the Reduction of Proteinuria by Long-Term ACE Inhibition,” Kidney International, Vol. 40, No. 4, 1991, pp. 734-741. doi:10.1038/ki.1991.268 [5] F. N. Ziyadeh and G. Wolf, “Pathogenesis of the Podo- cytopathy and Proteinuria in Diabetic Glomerulopathy,” Current Diabetes Reviews, Vol. 4, No. 1, 2008, pp. 39-45. doi:10.2174/157339908783502370 [6] R. G. Langham, D. J. Kelly, A. J. Cox, N. M. Thomson, H. Holthöfer, P. Zaoui, N. Pinel, D. J. Cordonnier and R. E. Gilbert, “Proteinuria and the Expression of the Podo- cyte Slit Diaphragm Protein, Nephrin, in Diabetic Neph- ropathy: Effects of Angiotensin Converting Enzyme Inhi- bition,” Diabetologia, Vol. 45, No. 11, 2002, pp. 1572- 1576. doi:10.1007/s00125-002-0946-y [7] T. H. Jafar, C. H. Schmid, M. Landa, I. Giatras, R. Toto, G. Remuzzi, G. Maschio, B. M. Brenner, A. Kamper, P. Zucchelli, G. Becker, A. Himmelmann, K. Bannister, P. Landais, S. Shahinfar, P. E. de Jong, D. de Zeeuw, J. Lau and A. S. Levey, “Angiotensin-Converting Enzyme In- hibitors and Progression of Nondiabetic Renal Disease. A Meta-Analysis of Patient-Level Data,” Annals of Internal Medicine, Vol. 135, No. 2, 2001, pp. 73-87. doi:10.7326/0003-4819-135-2-200107170-00007 [8] D. M Kent, T. H. Jafar, R. A. Hayward, H. Tighiouart, M. P. Landa , de Jong, D. de Zeeuw, G. Remuzzi, A. L. Kam- per and A. S. Levey, “Progression Risk, Urinary Protein Excretion, and Treatment Effects of Angiotensin-Con- verting Enzyme Inhibitors in Nondiabetic Kidney Dis- ease,” Journal of the American Society of Nephrology, Vol. 18, No. 6, 2007, pp. 1959-1965. doi:10.1681/ASN.2006101081 [9] A. Maione, S. D. Navaneethan, G. Graziano, R. Mitchell, D. Johnson, J. F. Mann, P. Gao, J. C. Craig, G. Tognoni, V. Perkovic, A. Nicolucci, S. De Cosmo, A. Sasso, O. La- macchia, M. Cignarelli, V. M. Manfreda, G. Gentile and G. F. Strippoli, “Angiotensin-Converting Enzyme Inhibi- tors, Angiotensin Receptor Blockers and Combined Ther- apy in Patients with Micro- and Macroalbuminuria and Other Cardiovascular Risk Factors: A Systematic Review of Randomized Controlled Trials,” Nephrology Dialysis Transplantation, Vol. 26, No. 9, 2011, pp. 2827-2847. doi:10.1093/ndt/gfq792 [10] G. Maschio, D. Alberti, G. Janin, F. Locatelli, J. F. Mann, M. Motolese, C. Ponticelli, E. Ritz and P. Zucchelli, “Ef- fect of the Angiotensin-Converting-Enzyme Inhibitor Be- nazepril on the Progression of Chronic Renal Insuffi- ciency,” The New England Journal of Medicine, Vol. 334, No. 15, 1996, pp. 939-945. doi:10.1056/NEJM199604113341502 [11] The GISEN Group “Randomised Placebo-Controlled Trial of Effect of Ramipril on Decline in glomerular Filtration Rate and Risk of Terminal Renal Failure in Proteinuric, Non-Diabetic Nephropathy,” Lancet, Vol. 349, No. 9069, 1997, pp. 1857-1863. doi:10.1016/S0140-6736(96)11445-8 [12] P. Ruggenenti, A. Perna, G. Gherardi, F. Gaspari, R. Be- nini and G. Remuzzi, “Renal Function and Requirement for Dialysis in Chronic Nephropathy Patients on Long- Term Ramipril: REIN Follow-Up Trial,” Gruppo Italiano di Studi Epidemiologici in Nefrologia (GISEN). Ramipril Efficacy in Nephropathy. Lancet, Vol. 352, No. 9136, 1998, pp. 1252-1256. doi:10.1016/S0140-6736(98)04433-X [13] P. Ruggenenti, A. Perna, G. Loriga, M. Ganeva, B. Ene- Iordache, M. Turturro, M. Lesti, E. Perticucci, I. N. Cha- karski, D. Leonardis, G. Garini, A. Sessa, C. Basile, M. Alpa, R. Scanziani, G. Sorba, C. Zoccali and G. Remuzzi, “REIN-2 Study Group Blood-Pressure Control for Reno Protection in Patients with Non-Diabetic Chronic Renal Disease (REIN-2): Multi Centre Randomized Controlled Trial,” Lancet, Vol. 365, No. 9463, 2005, pp. 939-946. doi:10.1016/S0140-6736(05)71082-5 [14] J. T. Wright Jr, G. Bakris, T. Greene, L. Y. Agodoa, L. J. Appel, J. Charleston, D. Cheek, J. G. Douglas-Baltimore, J. Gassman, R. Glassock, L. Hebert, K. Jamerson, J. Le- wis, R. A. Phillips, R. D. Toto, J. P. Middleton, S. G. Ro- stand and African American Study of Kidney Disease and Hypertension Study Group, “Effect of Blood Pressure Lowering and Antihypertensive Drug Class on Progres- sion of Hypertensive Kidney Disease: Results from the AASK Trial,” Journal of the American Medical Associa- tion, Vol. 288, No. 19, 2002, pp. 2421-2431. doi:10.1001/jama.288.19.2421 [15] L. J. Appel, J. T. Wright Jr., T. Greene, J. W. Kusek, J. B. Lewis, X. Wang, M. S. Lipkowitz, K. C. Norris, G. L. Bakris, M. Rahman, G. Contreras, S. G. Rostand, J. D. Kopple, F. B. Gabbai, G. I. Schulman, J. J. Gassman, J. Charleston and L. Y. Agodoa, “African American Study of Kidney Disease and Hypertension Collaborative Re- search Group Long-Term Effects of Renin-Angiotensin System-Blocking Therapy and a Low Blood Pressure Goal on Progression of hypertensive Chronic Kidney Dis- ease in African Americans,” JAMA Internal Medicine, Copyright © 2013 SciRes. OJNeph  K. MAQSOOD ET AL. Copyright © 2013 SciRes. OJNeph 134 Vol. 168, No. 8, 2008, pp. 832-839. doi:10.1001/archinte.168.8.832 [16] F. F. Hou, X. Zhang, G. H. Zhang, D. Xie, P Y. Chen, W. R. Zhang, J. P. Jiang, M. Liang, G. B. Wang, Z. R. Liu and R. W. Geng, “Efficacy and Safety of Benazepril for Advanced Chronic Renal Insufficiency,” The New Eng- land Journal of Medicine, Vol. 354, No. 2, 2006, pp. 131- 140. doi:10.1056/NEJMoa053107 [17] A. M. O'Hare, J. S. Kaufman, K. E. Covinsky, C. S. Lan- defeld, L. V. McFarland and E. B. Larson, “Current Guide- lines for Using Angiotensin-Converting Enzyme Inhibi- tors and Angiotensin II-Receptor Antagonists in Chronic Kidney Disease: Is the evidence Base Relevant to Older Adults?” Annals of Internal Medicine, Vol. 150, No. 10, 2009, pp. 717-724. doi:10.7326/0003-4819-150-10-200905190-00010 [18] R. Kunz, C. Friedrich, M. Wolbers and J. F. Mann, “Meta - Analysis: Effect of Monotherapy and Combination Ther- apy with Inhibitors of the Renin Angiotensin System on Proteinuria in Renal Disease,” Annals of Internal Medi- cine, Vol. 148, No. 1, 2008, pp. 30-48. doi:10.7326/0003-4819-148-1-200801010-00190 [19] S. W. Tobe, C. M. Clase, P. Gao, M. McQueen, A. Gros- shennig, X. Wang, K. K. Teo, S. Yusuf and J. F. Mann, “Cardiovascular and Renal Outcomes with Telmisartan, Ramipril, or Both in People at High Renal Risk: Results from the ONTARGET and TRANSCEND Studies,” Cir- culation, Vol. 123, No. 10, 2011, pp. 1098-1107. doi:10.1161/CIRCULATIONAHA.110.964171 [20] M. MacKinnon, S. Shurraw, A. Akbari, G. A. Knoll, J. Jaffey and H. D. Clark, “Combination Therapy with an Angiotensin Receptor Blocker and an ACE Inhibitor in Proteinuric Renal Disease: A Systematic Review of the Efficacy and Safety Data,” American Journal of Kidney Diseases, Vol. 48, No. 1, 2006, pp. 8-20. doi:10.1053/j.ajkd.2006.04.077 [21] G. L. Bakris, M. R. Weir, M. Secic, B. Campbell and A. Weis-McNulty, “Differential Effects of Calcium Antago- nist Subclasses on Markers of Nephropathy Progression,” Kidney International, Vol. 65, No. 6, 2004, pp. 1991- 2002. doi:10.1111/j.1523-1755.2004.00620.x [22] S. D. Navaneethan, S. U. Nigwekar, A. R. Sehgal and G. F. Strippoli, “Aldosterone Antagonists for Preventing the Progression of Chronic Kidney Disease: A Systematic Review and Meta-Analysis,” Clinical Journal of the Ame- rican Society of Nephrology, Vol. 4, No. 3, 2009, pp. 542- 551. doi:10.2215/CJN.04750908 [23] B. H. Oh, J. Mitchell, J. R. Herron, J. Chung, M. Khan and D. L. Keefe, “Aliskiren, an Oral Renin Inhibitor, Pro- vides Dose-Dependent Efficacy and Sustained 24-Hour Blood Pressure Control in Patients with Hypertension,” Journal of the American College of Cardiology, Vol. 49, No. 11, 2007, pp. 1157-1163. doi:10.1016/j.jacc.2006.11.032 [24] J. L. Pool, R. E. Schmieder, M. Azizi, J. C. Aldigier, A. Januszewicz, W. Zidek, Y. Chiang and A. Satlin, “Al- iskiren, an Orally Effective Renin Inhibitor, Provides An- tihypertensive Efficacy Alone and in Combination with Valsartan,” American Journal of Hypertension, Vol. 20, No. 1, 2007, pp. 11-20. doi:10.1016/j.amjhyper.2006.06.003 [25] A. Villamil, S. G. Chrysant, D. Calhoun, B. Schober, H. Hsu, L. Matrisciano-Dimichino and J. Zhang, “Renin In- hibition with Aliskiren Provides Additive Antihyperten- sive Efficacy When Used in Combination with Hydroch- lorothiazide,” Journal of Hypertension, Vol. 25, No. 1, 2007, pp. 217-226. doi:10.1097/HJH.0b013e3280103a6b [26] M. M. Shafiq, D. V. Menon and R. G. Victor, “Oral Di- rect Renin Inhibition: Premise, Promise, and Potential Limitations of a New Antihypertensive Drug,” The Ame- rican Journal of Medicine, Vol. 121, No. 4, 2008, pp. 265-271. doi:10.1016/j.amjmed.2007.11.016 [27] H. H. Parving, F. Persson, J. B. Lewis, E. J. Lewis and N. K. Hollenberg, “AVOID Study Investigators Aliskiren Combined with Losartan in Type 2 Diabetes and Neph- ropathy,” The New England Journal of Medicine, Vol. 358, No. 23, 2008, pp. 2433-2446. doi:10.1056/NEJMoa0708379 [28] H. H. Parving, B. M. Brenner, J. J. McMurray, D. de Ze- euw, S. M. Haffner, S. D. Solomon, N. Chaturvedi, M. Ghadanfar, N. Weissbach, Z. Xiang and J. Armbrecht, “Cardiorenal End Points in a Trial of Aliskiren for Type 2 Diabetes,” The New England Journal of Medicine, Vol. 367, 2012, pp. 2204-2213. doi:10.1056/NEJMoa1208799 [29] M. Azizi and J. Ménard, “Renin Inhibitors and Cardio- vascular and Renal Protection: An Endless Quest?” Car- diovascular Drugs and Therapy, 2012. [30] Z. Harel, C. Gilbert, R. Wald, C. Bell, J. Perl, D. Juurlink, J. Beyene and P. S. Shah, “The Effect of Combination Treatment with Aliskiren and Blockers of the Renin-An- giotensin System on Hyperkalemia and Acute Kidney In- jury: Systematic Review and Meta-Analysis,” British Medical Journal, Vol. 344, 2012, p. e42. doi:10.1136/bmj.e42 [31] R. T. Gansevoort, W. J. Sluiter, M. H. Hemmelder, D. de Zeeuw and P. E. de Jong, “Ant iproteinuric Effect of Bl ood- Pressure-Lowering Agents: A Meta-Analysis of Com- parative Trials,” Nephrology Dialysis Transplantation, Vol. 10, No. 11, 1995, p. 1963. [32] A. J. Apperloo, D. de Zeeuw, H. E. Sluiter and P. E. de Jong, “Differential Effects of Enalapril and Atenolol on Proteinuria and Renal Hemodynamics in Non-Diabetic Renal Disease,” British Medical Journal, Vol. 303, No. 6806, 1991, p. 821. doi:10.1136/bmj.303.6806.821 [33] M. E. Rosenberg and T. H. Hostetter, “Comparative Ef- fects of Antihypertensives on Proteinuria: Angiotensin- Converting Enzyme Inhibitor versus Alpha 1-Antagonist,” American Journal of Kidney Diseases, Vol. 18, No. 4, 1991, p. 472.
|