Maintenance Chemotherapy in Ovarian Cancer: A Trial-Sequential Analysis 1243
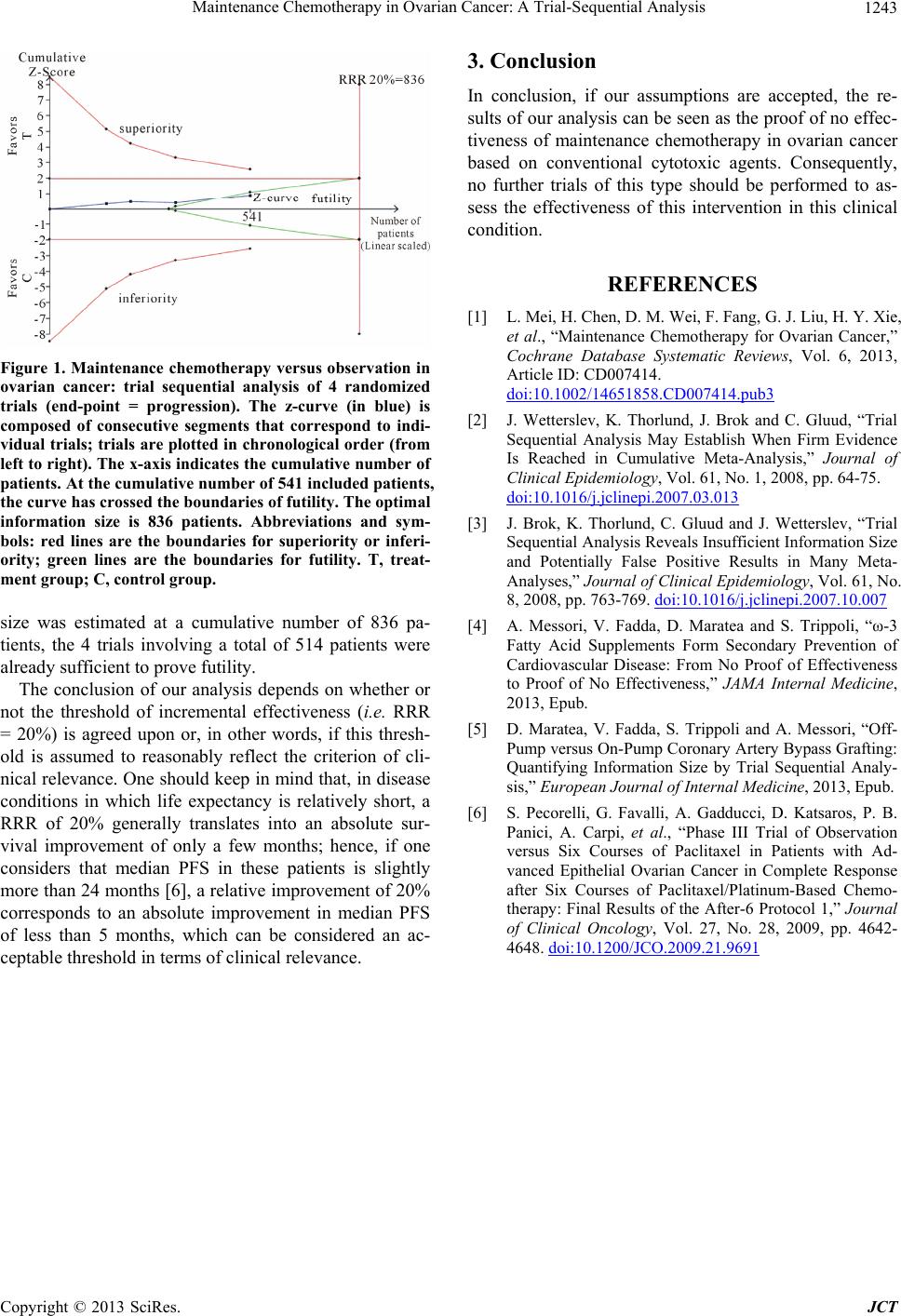
Figure 1. Maintenance chemotherapy versus observation in
ovarian cancer: trial sequential analysis of 4 randomized
trials (end-point = progression). The z-curve (in blue) is
composed of consecutive segments that correspond to indi-
vidual trials; trials are plotted in chronological order (from
left to right). The x-axis indicates the cumulative number of
patients. At the cumulative number of 541 included patients,
the curve has crossed the boundaries of futility. The optimal
information size is 836 patients. Abbreviations and sym-
bols: red lines are the boundaries for superiority or inferi-
ority; green lines are the boundaries for futility. T, treat-
ment group; C, control group.
size was estimated at a cumulative number of 836 pa-
tients, the 4 trials involving a total of 514 patients were
already sufficient to prove futility.
The conclusion of our analysis depends on whether or
not the threshold of incremental effectiveness (i.e. RRR
= 20%) is agreed upon or, in other words, if this thresh-
old is assumed to reasonably reflect the criterion of cli-
nical relevance. One should keep in mind that, in disease
conditions in which life expectancy is relatively short, a
RRR of 20% generally translates into an absolute sur-
vival improvement of only a few months; hence, if one
considers that median PFS in these patients is slightly
more than 24 months [6], a relative improvement of 20%
corresponds to an absolute improvement in median PFS
of less than 5 months, which can be considered an ac-
ceptable threshold in terms of clinical relevance.
3. Conclusion
In conclusion, if our assumptions are accepted, the re-
sults of our analysis can be seen as the proof of no effec-
tiveness of maintenance chemotherapy in ovarian cancer
based on conventional cytotoxic agents. Consequently,
no further trials of this type should be performed to as-
sess the effectiveness of this intervention in this clinical
condition.
REFERENCES
[1] L. Mei, H. Chen, D. M. Wei, F. Fang, G. J. Liu, H. Y. Xie,
et al., “Maintenance Chemotherapy for Ovarian Cancer,”
Cochrane Database Systematic Reviews, Vol. 6, 2013,
Article ID: CD007414.
doi:10.1002/14651858.CD007414.pub3
[2] J. Wetterslev, K. Thorlund, J. Brok and C. Gluud, “Trial
Sequential Analysis May Establish When Firm Evidence
Is Reached in Cumulative Meta-Analysis,” Journal of
Clinical Epidemiolo gy, Vol. 61, No. 1, 2008, pp. 64-75.
doi:10.1016/j.jclinepi.2007.03.013
[3] J. Brok, K. Thorlund, C. Gluud and J. Wetterslev, “Trial
Sequential Analysis Reveals Insufficient Information Size
and Potentially False Positive Results in Many Meta-
Analyses,” Journal of Clinical Epidemiology, Vol. 61, No.
8, 2008, pp. 763-769. doi:10.1016/j.jclinepi.2007.10.007
[4] A. Messori, V. Fadda, D. Maratea and S. Trippoli, “ω-3
Fatty Acid Supplements Form Secondary Prevention of
Cardiovascular Disease: From No Proof of Effectiveness
to Proof of No Effectiveness,” JAMA Internal Medicine,
2013, Epub.
[5] D. Maratea, V. Fadda, S. Trippoli and A. Messori, “Off-
Pump versus On-Pump Coronary Artery Bypass Grafting:
Quantifying Information Size by Trial Sequential Analy-
sis,” European Journal of Internal Medicine, 2013, Epub.
[6] S. Pecorelli, G. Favalli, A. Gadducci, D. Katsaros, P. B.
Panici, A. Carpi, et al., “Phase III Trial of Observation
versus Six Courses of Paclitaxel in Patients with Ad-
vanced Epithelial Ovarian Cancer in Complete Response
after Six Courses of Paclitaxel/Platinum-Based Chemo-
therapy: Final Results of the After-6 Protocol 1,” Journal
of Clinical Oncology, Vol. 27, No. 28, 2009, pp. 4642-
4648. doi:10.1200/JCO.2009.21.9691
Copyright © 2013 SciRes. JCT