 Advances in Bioscience and Biotechnology, 2013, 4, 900-907 ABB http://dx.doi.org/10.4236/abb.2013.49118 Published Online September 2013 (http://www.scirp.org/journal/abb/) Production of mono-, di-, and triglycerides from waste fatty acids through esterification with glycerol N. A. Mostafa1, Ashraf Maher2, Wael Abdelmoez2* 1Faculty of Applied Medical Science, Taif University, Taif, KSA 2Chemical Engineering Department, Faculty of Engineering, Minia University, Minia, Egypt Email: *drengwael2003@yahoo.com Received 10 July 2013; revised 10 August 2013; accepted 25 August 2013 Copyright © 2013 N. A. Mostafa et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Currently monoglyceride and diglyceride are repre- senting important products, as they have numerous applications such as modifying agents in food and pharmaceutical industries. In this work, the produc- tion of these economically value added compounds by estrifying the fatty acids with the glycerol is presented. Effects of various reaction parameters were optimized to obtain high yield of mono, di- and triglycerids. The effects of temperature (180˚C to 260˚C), ZnCl2 cata- lyst concentration (0.1%, 0.2%, 0.3%), glycerol to fatty acids molar ratio (1:1, 1:2, 1:3, 3:1), agitation speeds (200, 500, 1000 rpm), type of reaction system (opened and closed) and type of fatty acids including oleic and palmatic acids on esterification efficiency of fatty acids were investigated. The optimum conditions of esterification reaction were at temperature 195˚C, molar ratio 1:1, amount of catalyst 0.3% Zncl2, and agitation 500 rpm. Analysis of yield showed that at the optimum conditions mondi and triglycerids were produced in high purity, up to 99%. Infrared spec- troscopy IR and thin layer chromatograph TLC proved that the final product contains mono, di- and triglycerides. Keywords: Esterification; Fatty Acids; Glycerol; Monoglycerides; Diglycerides; Zinc chloride; Triglycerides 1. INTRODUCTION Soapstock is a by product of the caustic refining of oils and fats. Fatty acids are the main component in the soap- stock in the form of sodium salts. These fatty acids could be separated by soapstock acidulation to give waste oils. Distilled fatty acids could be obtained from waste oils by two processes namely splitting and distillation. These fr ee fatty acids could be used as a starting material for the production of very valuable products such as mono-, di- and triglycerides by esterification reaction with glycerol [1]. As consumers become more aware and concerned about the impact of the food they eat and the substances they commonly use on their health and general well- being, there is a growing interest in designer lipids. Among the lipid classes, surfactants, such as monoacylglycerols (monoglycerides, MAG), are desired by the food, cos- metic, pharmaceutical and chemical industries [2-5]. Pro- duction of tailor-made designer MAG with targeted fatty acids therefore offers promising industrial opportunities. The commercial synthesis of fatty acid esters of glycerol is carried out by two different routes: direct esterification of the fatty acid with the glycerol (Glycerolysis), cata- lyzed by a homogeneous acid, such as sulphuric or sul- fonic acids, or by transesterification of triglycerides and polyalcohol catalyzed by alkaline hydroxides like NaOH, KOH or Ca(OH)2 and sodium salts of low molecular weight a lco hols , su ch as meth an ol . The e steri fication me- thod is best suited for the production of designer MAG because the desired free fate acid (FFA) can easily be selected prior to MAG formation [6]. It could be esti- mated that approximately 80% of manufacturing proc- esses use acid catalysts [7]. The complete steps for es- terification reaction are demonstrated in Figure 1. The major factors affecting the conversion efficiency of the esterification process are molar ratio of alcohol/oil, amount of catalyst, reaction temperature, catalyst type and stirring speed according t o reaction duration [8,9]. Esterification of glycerol and fatty acids was studied under reduced pressure with and without the assistance of various metal chlorides and oxides as catalysts. Also super acids like sulphated zirconia and niobium acid wer e used as catalyst, for the reason that they could prevent *Corresponding a uthor. OPEN ACCESS 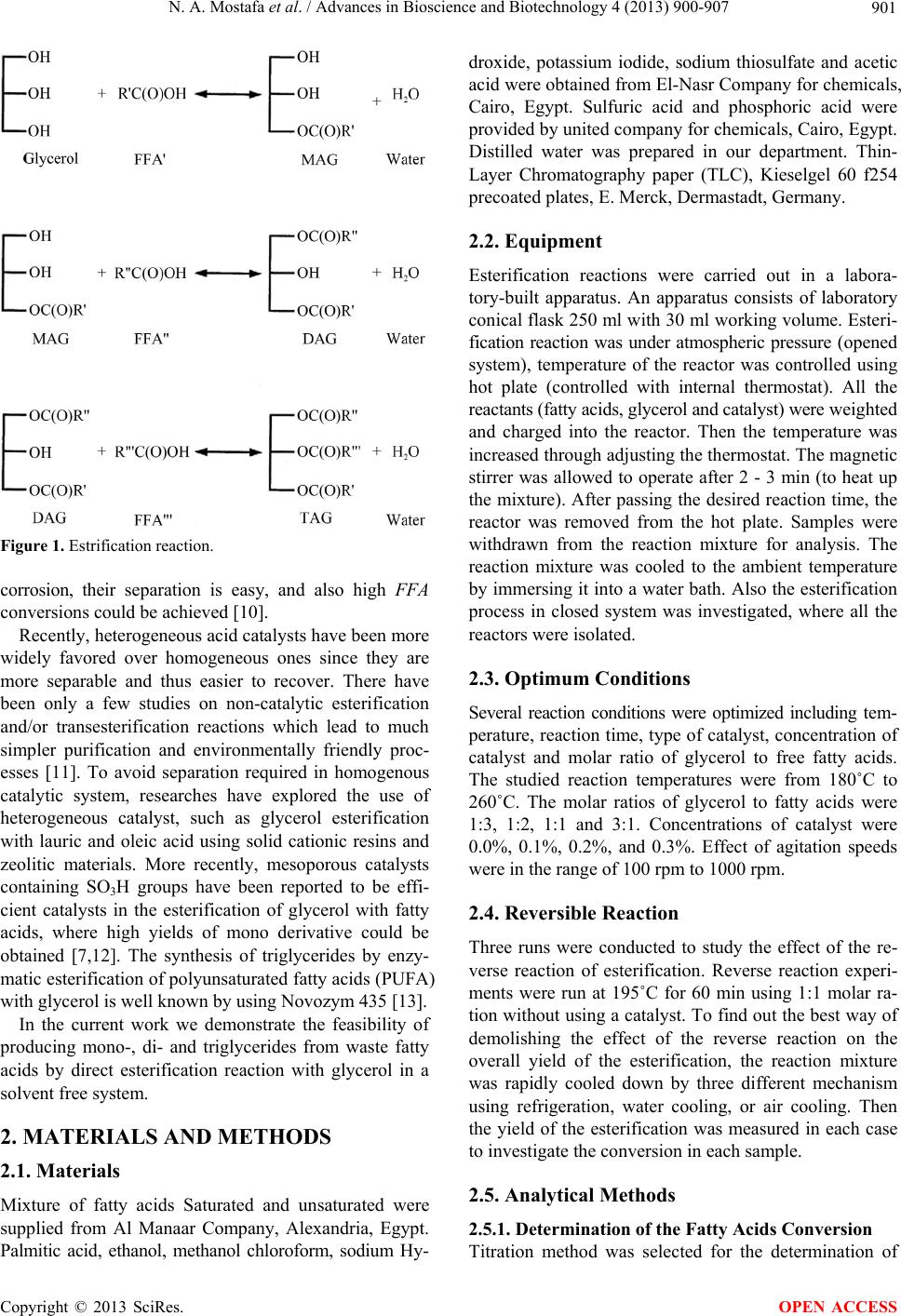 N. A. Mostafa et al. / Advances in Bioscience and Biotechnology 4 (2013) 900-907 901 Figure 1. Estrification reaction. corrosion, their separation is easy, and also high FFA conversions could be achieved [10]. Recently, heterogeneous acid catalysts have been more widely favored over homogeneous ones since they are more separable and thus easier to recover. There have been only a few studies on non-catalytic esterification and/or transesterification reactions which lead to much simpler purification and environmentally friendly proc- esses [11]. To avoid separation required in homogenous catalytic system, researches have explored the use of heterogeneous catalyst, such as glycerol esterification with lauric and oleic acid using solid cationic resins and zeolitic materials. More recently, mesoporous catalysts containing SO3H groups have been reported to be effi- cient catalysts in the esterification of glycerol with fatty acids, where high yields of mono derivative could be obtained [7,12]. The synthesis of triglycerides by enzy- matic esterification of polyunsaturated fatty acids (PUFA) with glycerol is well known by using Novozym 435 [13]. In the current work we demonstrate the feasibility of producing mono-, di- and triglycerides from waste fatty acids by direct esterification reaction with glycerol in a solvent free system. 2. MATERIALS AND METHODS 2.1. Materials Mixture of fatty acids Saturated and unsaturated were supplied from Al Manaar Company, Alexandria, Egypt. Palmitic acid, ethanol, methanol chloroform, sodium Hy- droxide, potassium iodide, sodium thiosulfate and acetic acid were obtained from El-Nasr Company for chemicals, Cairo, Egypt. Sulfuric acid and phosphoric acid were provided by united company for chemicals, Cairo, Egypt. Distilled water was prepared in our department. Thin- Layer Chromatography paper (TLC), Kieselgel 60 f254 precoated plates, E. Merck, Dermastadt, Germany. 2.2. Equipment Esterification reactions were carried out in a labora- tory-built apparatus. An apparatus consists of laboratory conical flask 250 ml with 30 ml work ing volume. Esteri- fication reaction was under atmospheric pressure (opened system), temperature of the reactor was controlled using hot plate (controlled with internal thermostat). All the reactants (fatty acids, glycerol and catalyst) were weighted and charged into the reactor. Then the temperature was increased through adjustin g the thermostat. Th e magnetic stirrer was allowed to operate after 2 - 3 min (to heat up the mixture). After passing the desired reaction time, the reactor was removed from the hot plate. Samples were withdrawn from the reaction mixture for analysis. The reaction mixture was cooled to the ambient temperature by immersing it into a water bath. Also the esterification process in closed system was investigated, where all the reactors were isolated. 2.3. Optimum Conditions Several reaction conditions were optimized including tem- perature, reaction time, type of catalyst, concentration of catalyst and molar ratio of glycerol to free fatty acids. The studied reaction temperatures were from 180˚C to 260˚C. The molar ratios of glycerol to fatty acids were 1:3, 1:2, 1:1 and 3:1. Concentrations of catalyst were 0.0%, 0.1%, 0.2%, and 0.3%. Effect of agitation speeds were in the range of 100 rpm to 1000 rpm. 2.4. Reversible Reaction Three runs were conducted to study the effect of the re- verse reaction of esterification. Reverse reaction experi- ments were run at 195˚C for 60 min using 1:1 molar ra- tion without using a catalyst. To find out the best way of demolishing the effect of the reverse reaction on the overall yield of the esterification, the reaction mixture was rapidly cooled down by three different mechanism using refrigeration, water cooling, or air cooling. Then the yield of the esterification was measured in each case to investigate the conversion in each sample. 2.5. Analytical Methods 2.5.1. Determination of the Fatty Acids Conversion Titration method was selected for the determination of Copyright © 2013 SciRes. OPEN ACCESS  N. A. Mostafa et al. / Advances in Bioscience and Biotechnology 4 (2013) 900-907 902 the fatty acids conversion during the esterification reac- tion because it was simple and efficient analytical me tho d that could be performed without the need for highly spe- cialized equipment [14]. Five grams of the sample at time intervals was dis- solved in ethanol and ph enolphthalein indicato r was used to determine the pH ch ange during esterification reaction . The titration was carried out against aqueous solution of 0.1 N KOH. Acid value (A.V) was determined according to the equation given below: 56.1 .mg KOHg oil NV AV where: N: normality of KOH solution V: the volume of solution employed for titration, ml M: weight of fatty acids sample, g The conversion of FFA was calculated by the follow- ing equation: ... conversion%100 it i AA FFA A Where Ai is the initial acidity of the mixture and At acidity at any time, t. 2.5.2. Thin-Layer Chromatography for the Produced Glycosides TLC was used to identify the types of glycrides produced during the esterification reaction (i.e mono, di or triglyc- erides). TLC experiments were carried out by dissolving one drop of the esterification products into 0.5 ml mix- ture of chloroform and methanol (9:1). Then, a drop from the final mixture was applied over the TLC paper im- mersed into a 100 ml-beaker containing 10 ml of a mix- ture of chloroform and methanol (9:1) which allows a contact between the edges of the TLC paper with the solvent mixture. The TLC paper was allowed to stand until the solvent reach a level which must be just below the end line. Then, the TLC paper was dried and the bands of the different produced glycerides were detected under UV lamp. In some cases, the bands were not cl ea r l y appearing under the UV lamp. Accordingly, the TLC paper was exposed to an iodine vapor which allows a direct visualizing of the band by naked eye. 3. RESULTS AND DISCUSSION Monoglyceride, diglyceride and triglyceride were the ma- jor products obtained in this study. First, it was necessary to optimize the esterification conditions. To find out the optimum esterification conditions, the esterification proc- ess was carried out under different operating conditions at any time only one parameter was changing while the other parameters were kept con st a n t . 3.1. Effect of Type of the System To investigate the effect of type of the system on the esterification yield, the same procedures described above were followed by applying both closed and opened sys- tems (isolated and atmospheric systems). The esterifica- tion reactions were carried out using molar ratio of 3:1 (glycerol to fatty acids) at 180˚C for 510 min. Figure 2 shows the effect of both systems on the obtained estrifi- cation yield. The results indicated that the closed system and the opened syste m were come to equilibriu m point at nearly the same time. However in the opened system the conversion (91%) was higher than that obtained by closed system (72%). Consequently it could be concluded that the opened system based process is more efficient than the closed one. This finding maybe due to the pres- ence of the side reactions products in the estrification reactor inhibit the reaction to be proceed, therefore when these by products allowed to get out from the reactor, favorabl e p roducts formation c ou ld be obtained. 3.2. Effect of Temperature and Time Both temperature and time are having major effects on the conversion of the esterification process. Accordingly, they were studied and optimized together. The obtained results showd that that by increasing the reaction tem- perature, the reaction conversion increases rapidly. Fig- ure 3 shows that after 20 min, the conversion reached 99%. However, the energy consumed was very high (260˚C) which is not practical from the economic point of view. Therefore another esterification reaction was carried out within a temperature range of 180 ˚C - 210˚C as sh own in Figure 4. The results revealed that by increasing the Figure 2. Effect of the type of esterification system (opened system & closed system) at 180˚C, 3:1 molar ratio and 510 min of the unsaturated fatty acid. Copyright © 2013 SciRes. OPEN ACCESS  N. A. Mostafa et al. / Advances in Bioscience and Biotechnology 4 (2013) 900-907 903 Figure 3. Effect of different temperatures on the esterification yiled of saturated fatty acid after 40 min and 3:1 molar raio (gly:fat). Figure 4. Effect of temperature at 3:1 gly:fat molar ratio on the esterification of unsaturated fatty acid. esterification time, the esterification yield increased up to a maximum conversation. To determine the optimum tem- perature of esterification process, not only the maximum yield of esterification should be considered but also the time required to reach the reaction temperature was taken into account. Heating time to reaction temperature was certainly longer and energy consumption was surely greater for higher reaction temperature. Consequently, a faster reaction at a lower temperature is desirable. From this economical point of view, the optimum temperature for the esterification was found to be 195˚C at which the conversion reached up to 99%. 3.3. Effect of Molar Ratio on the Esterification Yield The second studied parameter was the optimum esterifi- cation molar ratio (glycerol:fatty acids). In this part, all experiments were carried out at the optimum obtained temperature (195˚C). The esterification process was car- ried out at 1:1, 1:2, 1:3 and 3:1 (glycerol to fatty acids). As shown in the Figure 5, it could be easily concluded that 1:1 molar ratio gave the maximum conversion under the required lower amount of raw material which in turn reduces the running cost of the process. 3.4. Effect of the Concentration of Catalyst on the Esterification Yield After investigating the effect of temperature, time and molar ratio without catalyst, the effect of concentration of the catalyst (0.1%, 0.2%, and 0.3%) on the esterifica- tion yield under optimum temperature of 195˚C and 1:1. molar ratio was investigated. Figure 6 shows the effect of catalyst percentage on the esterification yield. As s ho wn from the figure, by increasing the catalyst concentration, the time required to reach the maximum conversion de- creases. So that 0.3% (by weight) ZnCl2 was considered to be the optimum dose, where the maximum yield of Figure 5. Effect of glycerol:fatty acid molar ratio on the esteri- fication of unsaturated fatty acid with glycerol at 195˚C. Figure 6. Effect of the concentration of catalyst on the esteri- fication yield at optimum temperature (195˚C). Copyright © 2013 SciRes. OPEN ACCESS  N. A. Mostafa et al. / Advances in Bioscience and Biotechnology 4 (2013) 900-907 904 99% could be obtained after 100 min. Further increasing in the catalyst concentration will make the downstream processes including catalyst separation more difficult. 3.5. Effect of Degree of Agitation (rpm) To investigate the effect of the degree of agitation (rpm), the esterification process was carried out at different agi- tating speeds (1 00 , 500 and 1000 rp m) at 195 ˚C, 1:1 fatty acids to glycerol and without catalyst. The results are presented in Figure 7. As shown from the figure the maximum conversion of 99% w as obtained at 500 rpm. 3.6. Effect of the Reverse Reaction Reverse reaction effect was stopped using three different cooling mechanisms as described in the method part. The results were presented in Figure 8. As shown in the fig- ure, the yield of esterification were 92%, 90%, and 88% for reactions cooled down by refrigerator, cooling water and air cooling, respectively. The rapid cooling of the reaction mixture prevents (through low temperature) the reversible reaction, which in turn keeping the conversion. Accordingly, by decreasing the cooling temperature the effect of reversible reaction is minimized. 3.7. Effect of the Type of Fatty Acids on the Esterification Yield In this part of the study, all experiments were carried out at the optimum obtained conditions including tempera- ture (195˚C), molar ratio (1:1) and different types of fatty Figutr 7. Effect of the degree of agitation at 195˚C, 1:1 (gly:fa t) molar ratio. Figure 8. Effect of reversible reaction at optimum temperature. acids (oleic acid, stearic acid and palmitic acid Figure 9 shows the effect of the type of fatty acids on th e esterifi- cation yield. The results showed that the yield of esteri- fication recorded a maximum conversion at time of 360, 240, 210 min in case of oleic acid, stearic acid and palmitic acid, respectively. From these results it co uld be concluded that the fatty acid chain length and the degree of unsaturation are having a considerable effect on the esterification reaction. Since long chain and unsaturated fatty acids need higher reaction time compared with the smaller chai n and saturat ed fatty acids. 3.8. Esterification Reaction Using the Optimized Conditions In this part, the esterification of free fatty acids was per- formed under the obtained optimum conditions. The time course of the esterification was measured and shown in Figure 10. The data showed that the maximum conver- sion (99%) was obtained after 100 min (instead of 180 min in the temperature effect experiments). Accordingly the esterification processes was achieved in a considera- bly short time and lower temperature. A sample was taken for IR and TLC analysis to prove the composition of the obtained esterification products. Figure 11 shows Figure 9. The esterification yield under the optimum condition (195˚C, 100 min, 0.3% ZnCl2, 500 rpm). Figure 10. Effect of degree of saturation and number of atoms of the fatty acids on the esterification reaction at optimum temperature. Copyright © 2013 SciRes. OPEN ACCESS  N. A. Mostafa et al. / Advances in Bioscience and Biotechnology 4 (2013) 900-907 Copyright © 2013 SciRes. 905 the IR analysis, the results proved the existance of the carbonyl group (at wavenumber 1738.96 of the ester for- mation [15]. plant with a capacity of 100 ton of fatty acids/day using ASPEN HYSYS 2006 as shown in Figure 13. As dem- onstrated in the figure the process starts with a feeding pump (P-100) which was used for transferring the free fatty acids to the esterification reactor (convertor type reactor, CRV-100) through a heat exchanger (E-100) equipped with control valve (VLV-102). Glycerol was transferred to mixing tank through control valve (VLV- 100) and zinc chloride was mixed with glycerol in the same mixing tank (V-100). Then the mixture was pumped to reactor (CRV-100) through a transfer pump (P-101) equipped with a flow control (VLV-101). The product w as cooled by the exchang er (E101). Th e resulting mixture was transferred to a washing tank (V-101). The washing was carried out using fresh water. Then, the washed mix- ture was transferred to a settling tank (V-102) using a control valve (VLV-103). The mixtu re inside the settling tank is divided into two phases. The first phase is com- posed mainly of esterified fatty acids and the second phase is composed mainly of catalyst, glycerol and water. The first phase was pumped through control (VLV-104) to a fractionation tower (T-100) equipped with ten stages fractionating the feed into glycerol from the top and es- terified fatty acids from the bottom. The results of the simulation were summarized in Table 1. As shown in Table 1 the yield of the final product was found to be Figure 12 shows the TLC paper plate of both estrfied and free fatty acid. The results showd the existence of the three main products of monoglyceride, diglyceride, and triglyceride. Now let us compare the results obtained in this study with others. Lilis Hermida [16] used a mesoporous cata- lyst in order to catalyzing the estrification reaction, the optimum conditions obtained were, 5% catalyst, 2:1 glyc - erol:fatty acid and 750 rpm which were higher than that obtained in this study. Furthermore the conversion ob- tained was 95% which was less than the 99% obtained in this study. Such a result indicated the effectiv eness of the proposed route for production of mono-, di- and triglyc- erides. Consequently, the esterification performed in the pre- sent work will make two major benefits; firstly, it will make the production of biodiesel more competitive with the existing diesel fuel market. Secondly, it will produce which is the most important emulsifier used in food in- dustry. 4. PROCESS SIMULATION The last step of this article was building an estrification Figure 11. IR analysis of esterification unsaturated fatty acids. OPEN ACCESS  N. A. Mostafa et al. / Advances in Bioscience and Biotechnology 4 (2013) 900-907 906 Figure 12. TLC analysis of esterified and non esterified fatty acids. Figure 13. Ester production plant from fatty acids and glycerol. Table 1. Results of the simulation. Feed M3/hr 4.9 Free fatty acid Mass% 99% m3/hr 1.05 Glycerol make up Mass% 99 kg/hr 16.5 ZnCl2 Mass% 99 m3/hr 1 Washing water Mass% 100 product m3/hr 5.09 Fatty acid ester Mass% 96 m3/hr 0.4267 glycerol Mass% 72.7 96% ester and 4% glycerol at the bottom of the distilla- tion tower. On the other hand, at the top of the tower it was recovered for about 73% glycerol. REFERENCES [1] Isabel, D., Federico, M., Joaquın, P. and Enrique, S. (2003) Synthesis of MCM-41 materials dialkyl silane groups and their catalytic activity in functionalised with the esterification of glycerol with fatty acids. Applied Catalysis A: General, 242, 161-169. doi:10.1016/S0926-860X(02)00501-X [2] Anonymous (2006) Lubricant compositions. Research Disclosure, 690. [3] Fernandez, A.M., Held, U., Willing, A. and Breuer, W.H. (2005) New green surfactants for emulsion polymeriza- tion. Progress in Organic Coatings, 53, 246-255. doi:10.1016/j.porgcoat.2004.12.011 [4] Eccleston, G.M. (1997) Functions of mixed emulsifiers and emulsifying waxes in dermatological lotions and creams. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 123-124, 169-182. doi:10.1016/S0927-7757(96)03846-0 [5] Garti, N. (1999) What can nature offer from an emulsifier point of view: Trends and progress? Colloids and Sur- faces A: Physicochemical and Engineering Aspects, 152, Copyright © 2013 SciRes. OPEN ACCESS  N. A. Mostafa et al. / Advances in Bioscience and Biotechnology 4 (2013) 900-907 907 125-146. doi:10.1016/S0927-7757(98)00621-9 [6] Guner, F.S., Sirkecioglu, A., Yilmaz, S., Erciyes, A.T. and Erdem-Senatalar, A. (1996) Esterification of oleic acid with glycerol in the presence of sulfated iron oxide catalyst. Journal of the American Oil Chemists’ Society, 73, 347-351. doi:10.1007/BF02523429 [7] Moquinp (2008) Hydrolysis, esterification and glyceroly- sis of lipids in supercritical carbon dioxide media. Pro- quest Dissertations and Theses, University of Alberta. [8] Farag, H., Azza, M. and Taha, N.A. (2011) Optimization of factors affecting esterification of mixed oil with high percentage of free fatty acid. Fuel Processing Technology, 92, 507-510. doi:10.1016/j.fuproc.2010.11.004 [9] Chetpattananondh, P., Rukprasoot, J., Bunyakan, C. and Tongurai, C. (2005) Glycerolysis of crude glycerol de- rived from biodiesel process. PSU-UNS International Conference on Engineering and Environment, Novi Sad, 19-21 May 2005. [10] Zbay, N.O., Oktar, N. and Alper Tapan, N. (2008) Esteri- fication of free fatty acids in waste cooking oils (WCO): Role of ion-exchange resins. Fuel, 87, 1789-1798. doi:10.1016/j.fuel.2007.12.010 [11] Hyun, J., Soo Hyun, K., Seok, W. and Yeong-Koo, Y. (2012) A single step non-catalytic esterification of palm fatty acid distillate (PFAD) for biodiesel production. Fuel, 93, 373-380. doi:10.1016/j.fuel.2011.08.063 [12] Sakthivel, A., Nakamura, R., Komura, K. and Komura, Y. (2007) Esterification of glycerol by lauric acid over alu- minium and zirconium containing mesoporous molecular sieves in supercritical carbon dioxide medium. Journal of Supercritical Fluids, 42, 219-225. doi:10.1016/j.supflu.2007.03.012 [13] Cramer, J., Dueholm, M., Nielsen, S., Pedersen, D. and Cramer, J.F. (2007) Controlling the degree of esterifica- tion in lipase catalysed synthesis of xylitol fatty acid es- ters. Enzyme and Microbial Technology, 41, 346-352. doi:10.1016/j.enzmictec.2007.03.001 [14] Egyptian Standard Specifications Codex 210/1999 m. k. m 49-6/2005. [15] Vlachos, N., Skopelitis, Y., Psaroudaki, M., Konstantini- dou, V., Chatzilazarou, A. and Tegou, E. (2006) Applica- tions of fourier transform-infrared spectroscopy to edible oils. Analytica Chimica Acta, 573-574, 459-465. doi:10.1016/j.aca.2006.05.034 [16] Hermida, L., Abdullah, A.Z. and Mohamed, A.R. (2011) Synthesis of monoglyceride through glycerol esterifica- tion with lauric acid over propyl sulfonic acid post-syn- thesis functionalized SBA-1 5 mesoporous catalyst. Chemi- cal Engineering Journal, 174, 668-676. Copyright © 2013 SciRes. OPEN ACCESS
|