 Materials Sciences and Applicatio ns, 2011, 2, 14-19 doi:10.4236/msa.2011.21003 Published Online January 2011 (http://www.SciRP.org/journal/msa) Copyright © 2011 SciRes. MSA Synthesis of TiO2/Al-MCM-41 Composites with Coal-Measure Kaolin and Performance in Its Photocatalysis Qisheng Wu, Shuiping Li School of Materials Engineering, Yancheng Institute of Technology, Yancheng, China Email: qishengwu@ycit.cn, lishuiping2002@126.com Received October 17th, 2010; revised December 13th, 2010; accepted December 21st, 2010. ABSTRACT TiO2/Al-MCM-41 composites with various titania content were prepared by loading titania into the mesopores of Al-MCM-41 mesoporous molecular sieve from coal-measure kaolin as silicon and aluminum source via general sol-gel method and incipient wetness impregnation method. TiO2/Al-MCM-41 composites were characterized by XRD, FT-IR and SEM, and the photocatalytic degradation of methyl orange solution under visible light irradiation. The results showed that the titania crystalline phase was anatase, and the particles size of TiO2 increase with TiO2 content. The photocatalytic degradation rates of all samples prepared by sol-gel method and incipient wetness impregnation have been become stable by visible light irradiation for 150 min, and the highest degradation rate was 83.7% and 76% re- spectively. Keywords: Coal-Measure Kaolin, Al-MCM-41, TiO2, Photocatalytic Degradation, Methyl Orange 1. Introduction Kaolin, relatively pure clay, has a wide variety of appli- cations in industry, particularly as paper mill wastewater, rubber filler, ceramic, organic matter intercalation mate- rial, adsorbent, catalyst, and cement [1-6]. Kaolin is rock comprised largely of the kaolin group mineral including kaolinite, nacrite, dickite and halloysite. The particle size of kaolin group mineral is less than 2 μm, and particle shape is flake, layer, tubular or folded sheet. The most common kaolin mineral is kaolinite (Al2[Si2O5](OH)4), which is a layer structure consisting of silicon oxygen tetrahedron layer and aluminum-oxide octahedral layer. Coal-measure kaolin, as one kind of kaolin, has been investigated by researchers in many fields, such as zeo- lites, mesoporous material, and intercalated material [7-12]. Nanoparticles of titania have a large specific surface area, which is responsible for the better performance [13,14]. Because of its wide band gap of 3.0-3.2 eV, the effective industries application of titanias gets bot- tle-necks. Since the discovery of mesoporous materials [15,16], MCM-41 has attracted particular attention and widely used as catalyst and hosts for nanomaterials syn- thesis because of their high specific surface area, uni- formly pore size and uniform and ordered mesoporous channels [17-22]. In order to overcome the bottle-necks of commercials application of TiO2 nanoparticles, there are many work focused on the loading onto MCM-41 mesoporous materials of TiO2 nonpareils [23-25]. Long-term water shortages driven by population growth, industries pollution and climate change are forc- ing people to learn to live with less water and treat wastewater for circulation [26]. Wastewaters from vari- ous industries, factories, laboratories, etc. are serious problems to the environment [27]. Actually, in many cases, much industrial wastewater streams are only slightly contaminated and contain low levels of dissolved organic compounds, such as dyes. In recent years, photocatalytic degradation of dyes using MCM-41 mesororous materials loaded/doped/coated/grafted/sub- stituted by TiO2 nanoparticles or titanium has become very important [28-34]. However, there is no study on the photocatalytic degradation of dyes by TiO2 loading on Al-MCM-41 mesoporous materials under visible light irradiation. It was proved that there were more solid acid sites on the surface of MCM-41 mesoporous materials if aluminum was inducted into its framework. Our research group have devoted to the application of  Synthesis of TiO/Al-MCM-41 Composites with Coal-Measure Kaolin and Performance in Its Photocatalysis15 2 nature mineral, especially coal-measure kaolin. We syn- thesized basic molecular sieve [9], 4A zeolites [7], 5A zeolites [10] and mesoporous material [8].Here we report the formation of TiO2/Al-MCM-41 composites using coal-measure kalin as starting materials and the photo- catalytic degradation of methyl orange by these compos- ites under visible light irradiation. We have used tetrabutyl titanate as Ti source and ethanol medium for general sol-gel method and incipient wetness impregnation method. It has been observed that the titania particles are loaded onto Al-MCM-41 mesoporous materials, which controls the particles size and narrows the band gap of titania. 2. Experimental 2.1. Raw Materials The coal-measure kaolin was supplied from the Xuzhou COAL MINING GROUP CORPORATION (China). The chemical composite of coal-measure kaolin was deter- mined by XRF and given as follows: SiO2 39.24%; Al2O3 36.12%; CaO 0.39%; TiO2 0.65%; K2O 0.12%; Na2O 1.21%; MgO 0.18%; Fe2O3 0.89%; ignition loss is 20.78%. Cetyltrimethylammonium Bromide (CTAB) used as template was obtained from SHANGHAI SHANPU CHEMICAL CO., LTD and NaOH was bought from Merck Company with 99.9% analytical grade. Sulfuric acid was obtained from SHANGHAI SHENXIANG CHEMICAL REAGENT CO, .LTD. 2.2. Synthesis of Al-MCM-41 Mesoporous Materials Al-MCM-41 was synthesized through hydrothermal treat- ment as follows: CTAB was dissolved in deionized water. NaOH was added into CTAB solution, stirred for 30 min. The precursor prepared by selectively acid leaching method from coal-measure kaolin was then added into the mixed solution, magnetic stirring for 1 h. The mixture was then transferred into a Teflon-lined steel autoclave and kept under hydrothermal condition at 110˚C with continuous stirring (200 rpm) for 12 h. The resultant white product was filtered, washed for 3 times with de- ionized water, dried at 110˚C for 10 h, and calcined at 550˚C in air for 6 h to produce Al-MCM-41 mesoporous materials. 2.3. Synthesis of TiO2/Al-MCM-41 Composites TiO2/Al-MCM-41 composites can be obtained with tetrabutyl titanate as Ti source. TiO2 was introduced onto Al-MCM-41with loading of 10%, 20%, 40%, 60% and 80% by general sol-gel method. In a typical synthesis, 1.0 g Al-MCM-41 was added into ethanol and ultrasonic for 10 min. Different amount of tetrabutyl titanate was then added to ethanol and stirred for 45 min to form a solution. A certain amount of distilled water was dropped to above solution, stirred for 2 h. The white resultant product was washed and centrifuged with distilled water and ethanol for several times, dried at 80˚C for 8 h, and then calcined at 500˚C for 4h to produce TiO2/Al- MCM-41 composites. The products are named as GS10, GS20, GS40, GS60 and GS80, where GS means as pre- pared by general sol-gel method, and 10, 20, 40, 60 and 80 are the Ti/Si mass ratio, respectively. Another kind of TiO2/Al-MCM-41 composites for comparison was prepared by an incipient wetness im- pregnation method. Typically the loaded process was as follows: the as-prepared Al-MCM-41 mesoporous mate- rials were dried in a vacuum oven at 60˚C overnight, then 1.0 g dried sample was dispersed in a mixed solution containing certain amount of tetrabutyl titanate and dis- tilled water, magnetic stirred for 2 h. The white resultant product was dried at 80˚C for 8 h, calcined at 500˚C for 4 h to produce TiO2/Al-MCM-41 composites. The products are named as IS10, IS20, IS40, IS60 and IS80, where IS mean as prepared by an incipient wetness impregnation method, and 10, 20, 40, 60 and 80 are the Ti/Si mass ratio, respectively. 2.4. Characterization The XRD data were collected by the Y500 full automatic diffractometer (Dandong, China) with Cu radiation at 30 kV and 20 mA (step size: 0.09˚ per step). The chemical composition of coal-measure kaolin was determined by X-ray fluorescence (ADVANT´XP, ARL, Switzerland). A Fourier transform infrared spectrophotometer, NEXUS 670 (Nicolet, USA, spectral range from 4000 cm–1 to 400 cm–1), was used to confirm the composition of raw mate- rial, metakaolinite, precursor and the resultant material. The microstructures of the samples were observed using a QANTA200 (FEI, USA) at an accelerating voltage of 20 kV. The photocatalytic activity of TiO2/Al-MCM-41 com- posites was evaluated from an analysis of the photo- degradation of methyl orange (MO) under visible light irradiation. The visible light source was a commercial 300 W halogen lamp (a cut-off filter was used to remove the light with a wavelength bellow 400 nm). 0.03 g TiO2/Al-MCM-41 composites were ultrasonically dis- persed in 20 ml methyl orange solution (50 mg L–1, the pH value of MO solution was adjusted to 6 with distilled hydrochloric acid). After stirred in dark for 1 h, the ad- sorption/desorption of MO on TiO2/Al-MCM-41 com- posites reached equilibrium. The solution was then irra- diated with a visible light. The distance between MO solution and the visible light source was 20 mm. The concentration of solution was detected every 30 min with Copyright © 2011 SciRes. MSA 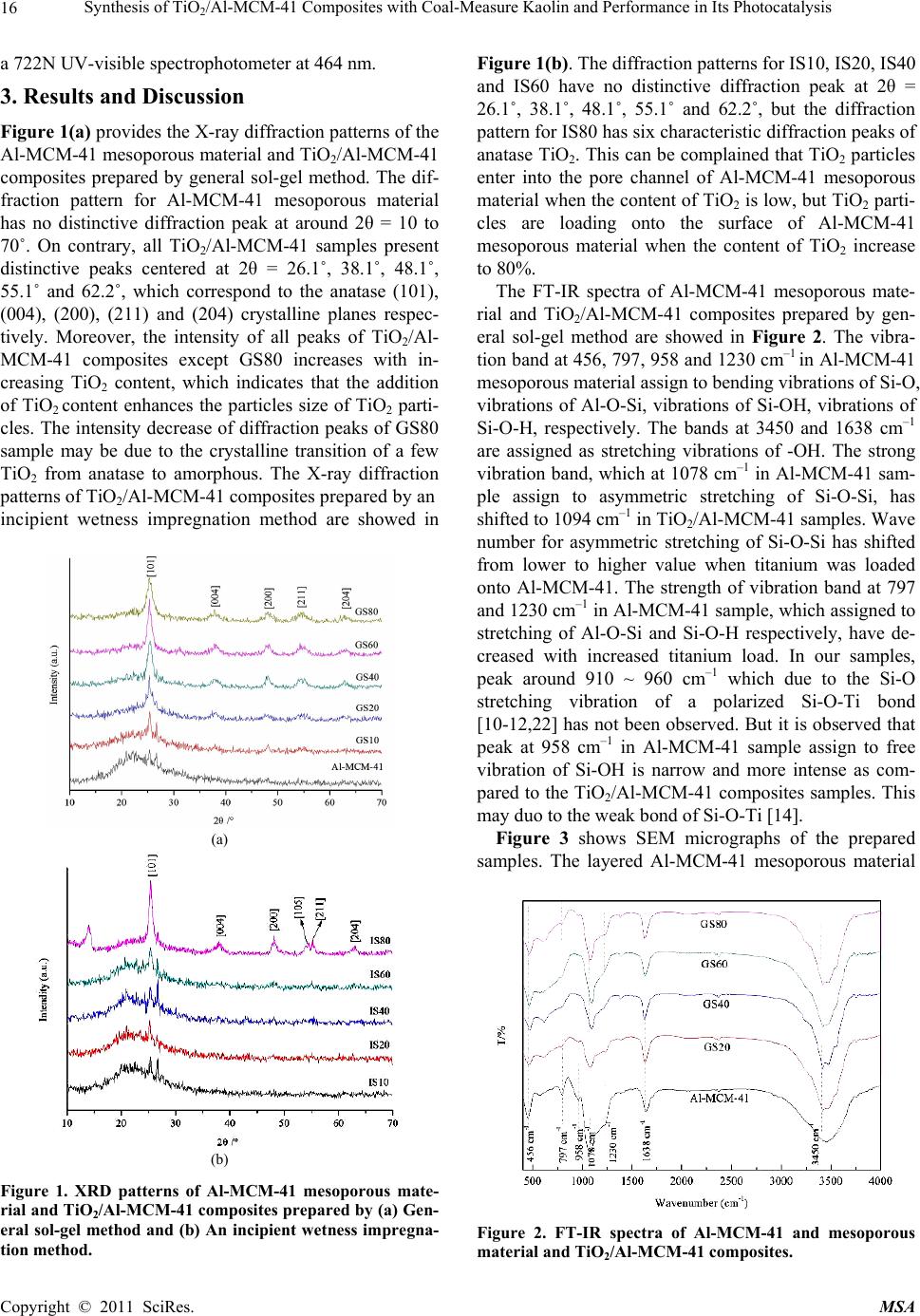 Synthesis of TiO/Al-MCM-41 Composites with Coal-Measure Kaolin and Performance in Its Photocatalysis 16 2 a 722N UV-visible spectrophotometer at 464 nm. 3. Results and Discussion Figure 1(a) provides the X-ray diffraction patterns of the Al-MCM-41 mesoporous material and TiO2/Al-MCM-41 composites prepared by general sol-gel method. The dif- fraction pattern for Al-MCM-41 mesoporous material has no distinctive diffraction peak at around 2θ = 10 to 70˚. On contrary, all TiO2/Al-MCM-41 samples present distinctive peaks centered at 2θ = 26.1˚, 38.1˚, 48.1˚, 55.1˚ and 62.2˚, which correspond to the anatase (101), (004), (200), (211) and (204) crystalline planes respec- tively. Moreover, the intensity of all peaks of TiO2/Al- MCM-41 composites except GS80 increases with in- creasing TiO2 content, which indicates that the addition of TiO2 content enhances the particles size of TiO2 parti- cles. The intensity decrease of diffraction peaks of GS80 sample may be due to the crystalline transition of a few TiO2 from anatase to amorphous. The X-ray diffraction patterns of TiO2/Al-MCM-41 composites prepared by an incipient wetness impregnation method are showed in (a) (b) Figure 1. XRD patterns of Al-MCM-41 mesoporous mate- rial and TiO2/Al-MCM-41 composites prepared by (a) Gen- eral sol-gel method and (b) An incipient wetness impregna- tion method. Figure 1(b). The diffraction patterns for IS10, IS20, IS40 and IS60 have no distinctive diffraction peak at 2θ = 26.1˚, 38.1˚, 48.1˚, 55.1˚ and 62.2˚, but the diffraction pattern for IS80 has six characteristic diffraction peaks of anatase TiO2. This can be complained that TiO2 particles enter into the pore channel of Al-MCM-41 mesoporous material when the content of TiO2 is low, but TiO2 parti- cles are loading onto the surface of Al-MCM-41 mesoporous material when the content of TiO2 increase to 80%. The FT-IR spectra of Al-MCM-41 mesoporous mate- rial and TiO2/Al-MCM-41 composites prepared by gen- eral sol-gel method are showed in Figure 2. The vibra- tion band at 456, 797, 958 and 1230 cm–1 in Al-MCM-41 mesoporous material assign to bending vibrations of Si-O, vibrations of Al-O-Si, vibrations of Si-OH, vibrations of Si-O-H, respectively. The bands at 3450 and 1638 cm–1 are assigned as stretching vibrations of -OH. The strong vibration band, which at 1078 cm–1 in Al-MCM-41 sam- ple assign to asymmetric stretching of Si-O-Si, has shifted to 1094 cm–1 in TiO2/Al-MCM-41 samples. Wave number for asymmetric stretching of Si-O-Si has shifted from lower to higher value when titanium was loaded onto Al-MCM-41. The strength of vibration band at 797 and 1230 cm–1 in Al-MCM-41 sample, which assigned to stretching of Al-O-Si and Si-O-H respectively, have de- creased with increased titanium load. In our samples, peak around 910 ~ 960 cm–1 which due to the Si-O stretching vibration of a polarized Si-O-Ti bond [10-12,22] has not been observed. But it is observed that peak at 958 cm–1 in Al-MCM-41 sample assign to free vibration of Si-OH is narrow and more intense as com- pared to the TiO2/Al-MCM-41 composites samples. This may duo to the weak bond of Si-O-Ti [14]. Figure 3 shows SEM micrographs of the prepared samples. The layered Al-MCM-41 mesoporous material Figure 2. FT-IR spectra of Al-MCM-41 and mesoporous material and TiO2/Al-MCM-41 composites. Copyright © 2011 SciRes. MSA 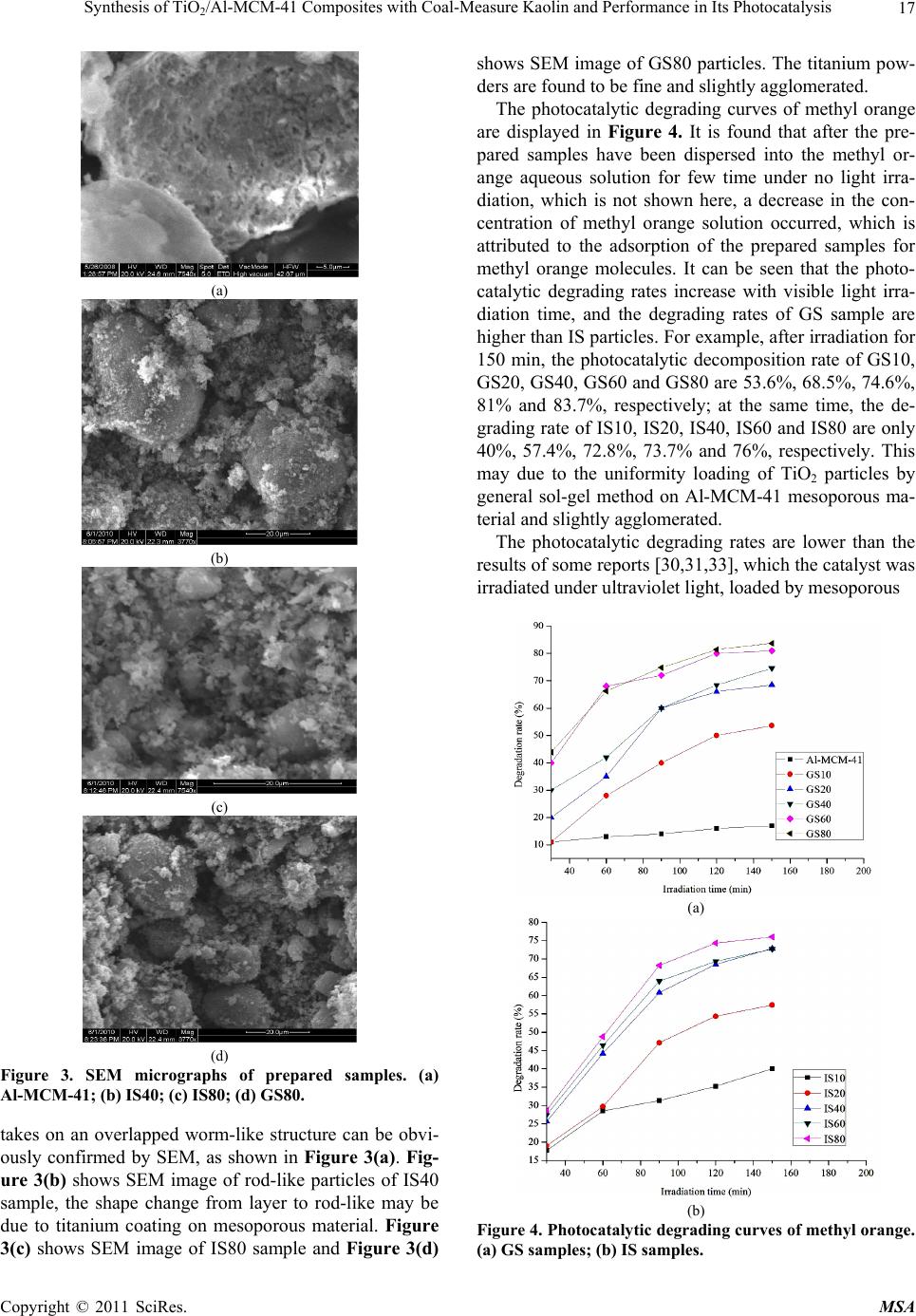 Synthesis of TiO/Al-MCM-41 Composites with Coal-Measure Kaolin and Performance in Its Photocatalysis17 2 (a) (b) (c) (d) Figure 3. SEM micrographs of prepared samples. (a) Al-MCM-41; (b) IS40; (c) IS80; (d) GS80. takes on an overlapped worm-like structure can be obvi- ously confirmed by SEM, as shown in Figure 3(a). Fig- ure 3(b) shows SEM image of rod-like particles of IS40 sample, the shape change from layer to rod-like may be due to titanium coating on mesoporous material. Figure 3(c) shows SEM image of IS80 sample and Figure 3(d) shows SEM image of GS80 particles. The titanium pow- ders are found to be fine and slightly agglomerated. The photocatalytic degrading curves of methyl orange are displayed in Figure 4. It is found that after the pre- pared samples have been dispersed into the methyl or- ange aqueous solution for few time under no light irra- diation, which is not shown here, a decrease in the con- centration of methyl orange solution occurred, which is attributed to the adsorption of the prepared samples for methyl orange molecules. It can be seen that the photo- catalytic degrading rates increase with visible light irra- diation time, and the degrading rates of GS sample are higher than IS particles. For example, after irradiation for 150 min, the photocatalytic decomposition rate of GS10, GS20, GS40, GS60 and GS80 are 53.6%, 68.5%, 74.6%, 81% and 83.7%, respectively; at the same time, the de- grading rate of IS10, IS20, IS40, IS60 and IS80 are only 40%, 57.4%, 72.8%, 73.7% and 76%, respectively. This may due to the uniformity loading of TiO2 particles by general sol-gel method on Al-MCM-41 mesoporous ma- terial and slightly agglomerated. The photocatalytic degrading rates are lower than the results of some reports [30,31,33], which the catalyst was irradiated under ultraviolet light, loaded by mesoporous (a) (b) Figure 4. Photocatalytic degrading curves of methyl orange. (a) GS samples; (b) IS samples. Copyright © 2011 SciRes. MSA  Synthesis of TiO/Al-MCM-41 Composites with Coal-Measure Kaolin and Performance in Its Photocatalysis 18 2 TiO2 or modified TiO2 with other ions doped respectively. 4. Conclusions TiO2/Al-MCM-41 composites with different ratios of TiO2 using coal-measure kaolin as starting materials were prepared at room temperature in ethanol solution by general sol-gel method and incipient wetness impregna- tion method. The photocatalysis properties of these composites were also studied by photocatalytic degrada- tion of methyl orange visible light irradiation. The results showed demonstrate that TiO2 nanoparticles enter into the pore channel of Al-MCM-41 mesoporous material and enhance the photocatalysis property of this material. TiO2/Al-MCM-41 composites can degrade methyl or- ange under visible light irradiation. The degradation rate of methyl orange under visible light irradiation of TiO2/Al-MCM-41 composites prepared by general sol- gel method is higher than the rates of IS sample at the same time. 5. Acknowledgements This work was supported by the Research fund for Social Development Plan of the Science and Technology De- partment of Jiangsu provincial government (BS2007 038). REFERENCES [1] H. Z. Ma, B. Wang and Y. Wang, “Application of Mo- lybdenum and Phosphate Modified Kaolin in Electro- chemical Treatment of Paper mill Wastewater,” Journal of Hazard Materials, Vol. 145, No. 3, July 2007, pp. 417-423. doi:10.1016/j.jhazmat.2006.11.038 [2] T. Toya, Y. Kameshima, A. Nakajima and K. Okada, “Preparation and Properties of Glass-Ceramics from Kao- lin Clay Refining Waste (Kira) and Paper Sludge Ash,” Ceramics International, Vol. 32, No. 7, 2006, pp. 789- 796. doi:10.1016/j.ceramint.2005.06.008 [3] B. M. Steenari and K. K. Fedje, “Addition of Kaolin as Potassium Sorbent in the Combustion of Wood Fuel —Effects on Fly Ash Properties,” Fuel, Vol. 89, No. 8, August 2010, pp. 2026-2032. [4] H. H. Liu, H. J. Zhao, X. H. Gao and J. T. Ma. “A Novel FCC Catalyst Synthesized via in Situ Overgrowth of NaY Zeolite on Kaolin Microspheres for Maximizing Propyl- ene Yield,” Catalysis Today, Vol. 125, No. 3-4, July 2007, pp. 163-168. doi:10.1016/j.cattod.2007.05.005 [5] H. S. Wong and H. A. Razak, “Efficiency of Calcined Kaolin and Silica Fume as Cement Replacement Material for Strength Performance,” Cement and Concrete Re- search, Vol. 35, No. 4, April 2005, pp. 696-702. doi:10.1016/j.cemconres.2004.05.051 [6] H. F. Cheng, Q. F. Liu, J. Yang, Q. Zhang and R. L. Frost, “Thermal Behavior and Decomposition of Kaolin- ite-Potassium Acetate Intercalation Composite,” Ther- mochimica Acta, Vol. 503-504, No. 1-2, May 2010, pp. 16-20. doi:10.1016/j.tca.2010.02.014 [7] Q. S. Wu, S. P. Li and S. S. Su, “Preparation of 4A Zeo- lite Molecular Sieve from Xuzhou Coal-Measure Kaolin,” Chemical Industry Engineering and Progress, Vol. 28, No. 1, January 2009, pp. 130-134. [8] Q. S, Wu, S. P. Li and S. S. Su, “Hydrothermal Synthesis of Mesoporous Materials from Coal-Measure Kaolin,” Chemical Industry Engineering and Progress, Vol. 28, No. 3, March 2009, pp. 458-461. [9] S. P. Li and Q. S. Wu, “Preparation of Basic Molecular Sieve Materials from Coal-Measure Kaolin,” Journal of Materials Science and Engineering, Vol. 27, No. 2, April 2009, pp. 233-237. [10] S. P. Li and Q. S. Wu, “Preparation on 5A Zeolite from Coal-Measure Kaolin and Adsorption Properties,” Envi- ronmental Engineering, Vol. 28, No. S1, September 2010, pp. 403-406. [11] Q. S. Wu and S. P. Li, “The Effect of Surfactant/Silica and Hydrothermal Time on the Specific Surface Area of Mesoporous Materials from Coal-Measure Kaolin,” Journal of WuHan University of Technology (Materials Science Edition), Vol. 26, No, 1, February 2011, pp. 1-6. [12] H. L. Xu, M. Wang, Q. F. Liu, D. L. Chen, H. L. Wang, K. J. Yang, H. D. Lu, R. Zhang and S. K. Guan. “Stability of the Compounds Obtained by Intercalating Potassium Acetate Molecules into Kaolinite from Coal Measures,” Journal of Physics and Chemistry of Solids, Vol. 72, No. 1, January 2010, pp. 24-28. doi:10.1016/j.jpcs.2010.10.019 [13] A. Tuel and L. G. Hubert-Ptalzgrat, “Nanometric Mono- dispersed Titanium Oxide Particles on Mesoporous Silica: Synthesis, Characterization, and Catalytic Activity in Oxidation Reactions in the Liquid Phase,” Journal of Catalysis, Vol. 217, No. 2, July 2003, pp. 343-353. [14] N. B. Lihitkar, M. K. Abyaneh, V. Samuel, R. Pasricha, S. W. Gosavi and S. K. Kulkarni, “Titania Nanoparticles Synthesis in Mesoporous Molecular Sieve MCM-41,” Journal of Colloid Interface Science, Vol. 314, No. 1, October 2007, pp. 310-316. doi:10.1016/j.jcis.2007.05.069 [15] C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, “Ordered Mesoporous Molecular Sieves Synthesized by a Liquid-Crystal Template Mechanism,” Nature, Vol. 359, No. 710-712, October 1992, pp. 710- 712. doi:10.1038/359710a0 [16] J. S. Beck, J. C. Vartuli, W. J. Roth, M. E. Leonowicz, C. T. Kresge, K. D. Schmitt, C. T. W. Chu, D. H. Olson and E. W. Sheppard, “A New Family of Mesoporous Mo- lecular Sieves Prepared with Liquid Crystal Templates,” Journal of American Chemistry Society, Vol. 114, No. 27, December 1992, pp. 10834-10843. [17] X. Zhang, F. Zhang and K. Y. Chan, “Synthesis of Tita- nia-Silica Mixed Oxide Mesoporous Materials, Charac- Copyright © 2011 SciRes. MSA  Synthesis of TiO2/Al-MCM-41 Composites with Coal-Measure Kaolin and Performance in Its Photocatalysis Copyright © 2011 SciRes. MSA 19 terization and Photocatalytic Properties,” Applied Cataly- sis A: General, Vol. 284, No. 1-2, April 2005, pp. 193-198. doi:10.1016/j.apcata.2005.01.037 [18] X. X. Wang, W. H. Lian, X. Z. Fu, J. M. Basset and F. Lefebvre, “Structure, Preparation and Photocatalytic Ac- tivity of Titanium Oxides on MCM-41 Surface,” Journal of Catalysis, Vol. 238, No. 1, February 2006, pp. 13-20. doi:10.1016/j.jcat.2005.11.027 [19] L. J. Wang, D. Li, R. Wang, Yuan He, Q. Qi, Y. Wang and T. Zhang, “Study on Humidity Sensing Property Based on Li-Doped Mesoporous Silica MCM-41,” Sen- sors Actuators, B: Chemical, Vol. 133, No. 2, August 2008, pp. 622-627. doi:10.1016/j.snb.2008.03.028 [20] V. Mavrodinova, M. Popova, V. Valchev, R. Nickolov and Ch. Minchev, “Beta Zeolite Colloidal Nanocrystals Supported on Mesoporous MCM-41,” Journal of Colloid Interface Science, Vol. 286, No. 1, June 2005, pp. 268- 273. doi:10.1016/j.jcis.2005.01.006 [21] H. Y. Chen, H. X. Xi, X. Y. Cai and Y. Qian, “Experi- mental and Molecular Simulation Studies of A ZSM-5-MCM-41 Micro-Mesoporous Molecular Sieve,” Microporous and Mesoporous Materials, Vol. 118, No. 1-3, February 2009, pp. 396-402. doi:10.1016/j.micromeso.2008.09.020 [22] Z. J. Li, B. Hou, Y. Xu, D. Wu and Y. H. Sun, “Hydro- thermal Synthesis, Characterization, and Photocatalytic Performance of Silica-Modified Titanium Dioxide Nanoparticles,” Journal of Colloid and Interface Science, Vol. 288, No. 1, August 2005, pp. 149-154. doi:10.1016/j.jcis.2005.02.082 [23] H. M. Yang, Y. H. Deng and C. F. Du, “Synthesis and Optical Properties of Mesoporous MCM-41 Containing Doped TiO2 Nanoparticles,” Colloids and Surfaces A: Physicochemical and Engineering Aspects, Vol. 339, No. 1-3, May 2009, pp. 111-117. doi:10.1016/j.colsurfa.2009.02.005 [24] M. S. Sadjadi, N. Farhadyar and K. Zare, “Improvement of the Alkaline Protease Properties via Immobilization on the TiO2 Nanoparticles Supported by Mesoporous MCM- 41,” Superlattices and Microstructures, Vol. 46, No. 1-2, July-August 2009, pp. 77-83. doi:10.1016/j.spmi.2008.10.022 [25] T. Kawahara, Y. Konishi, H. Tada, N. Tohge, J. J. Nishii and S. Ito, “A Patterned TiO2 (Anatase)/TiO2 (Rutile) Bi- layer-Type Photocatalyst: Effect of the Anatase/Rutile Junction on the Photocatalytic Activity,” Angewandte Chemie (International Edition), Vol. 41, No. 15, August 2002, pp. 2811-2813. doi:10.1002/1521-3773(20020802)41:15<2811::AID-AN IE2811>3.0.CO;2-# [26] W. Zhang, L. D. Zou and L. Z. Wang, “Photocatalytic TiO2/Adsorbent Nanocomposites Prepared via Wet Che- mical Impregnation for Wastewater Treatment: A Review”. Appllied Catalysis, A: General, Vol. 371, No. 1-2, De- cember 2009, pp. 1-9. [27] U. G. Akpan and B. H. Hameed, “Parameters Affecting the Photocatalytic Degradation of Dyes Using TiO2- Based Photocatalysts: A Review,” Journal of Hazardous Materials, Vol. 170, No. 2-3, October 2009, pp. 520-529. doi:10.1016/j.jhazmat.2009.05.039 [28] Y. Xie, Y. Z. Li and X. J. Zhao, “Low-Temperature Preparation and Visible-Light-Induced Catalytic Activity of Anatase F–N-Codoped TiO2,” Journal of Molecular Catalysis A: Chemical, Vol. 277, No. 1-2, November 2007, pp. 119-126. doi:10.1016/j.molcata.2007.07.031 [29] S. Yuan, Q. R. Sheng, J. L. Zhang, F. Chen, M. Anpo and Q. H. Zhang, “Synthesis of La3+ Doped Mesoporous Ti- tania with Highly Crystallized Walls,” Microporous and Mesoporous Materials, Vol. 79, No. 1-3, April 2005, pp. 93-99. doi:10.1016/j.micromeso.2004.10.028 [30] S. Yuan, Q. R. Sheng, J. L. Zhang, H. Yamashita and D. N. He, “Synthesis of Thermally Stable Mesoporous TiO2 and Investigation of Its Photocatalytic Activity,” Micro - porous and Mesoporous Materials, Vol. 110, No. 2-3, April 2008, pp. 501-507. doi:10.1016/j.micromeso.2007.06.039 [31] X. X. Fan, X. Y. Chen, S. P. Zhu, Z. S. Li, T. Yu, J. H. Ye and Z. G. Zou, “The Structural, Physical and Photo- catalytic Properties of the Mesoporous Cr-Doped TiO2,” Journal of Molecular Catalysis A: Chemical, Vol. 284, No. 1-2, April 2008, pp. 155-160. doi:10.1016/j.molcata.2008.01.005 [32] Y. Xie, X. J. Zhao, Y. Z. Li, Q. N. Zhao, X. D. Zhou and Q. H. Yuan, “CTAB-Assisted Synthesis of Mesoporous F–N-Codoped TiO2 Powders with High Visible-Light- Driven Catalytic Activity and Adsorption Capacity”. Journal of Solid State Chemistry, Vol. 181, No. 1, August 2008, pp. 1936-1942. doi:10.1016/j.jssc.2008.04.021 [33] J. H. Huang, X. C. Wang, Y. D. Hou, X. F. Chen, L. Wu, X. X. Wang and X. Z. Fu, “Synthesis of Functionalized Mesoporous TiO2 Molecular Sieves and Their Applica- tion in Photocatalysis,” Microporous and Mesoporous Materials, Vol. 110, No. 8, April 2008, pp. 543-552. doi:10.1016/j.micromeso.2007.06.055 [34] Y. Xiao, L. Q. Dang, L. Z. An, S. Y. Bai and Z. B. Lei, “Photocatalytic Degradation of Rhodamine B and Phenol by TiO2 Loaded on Mesoporous Graphitic Carbon,” Chi- nese Journal of Catalysis, Vol. 29, No. 2-3, January 2008, pp. 31-36. doi:10.1016/S1872-2067(08)60014-5
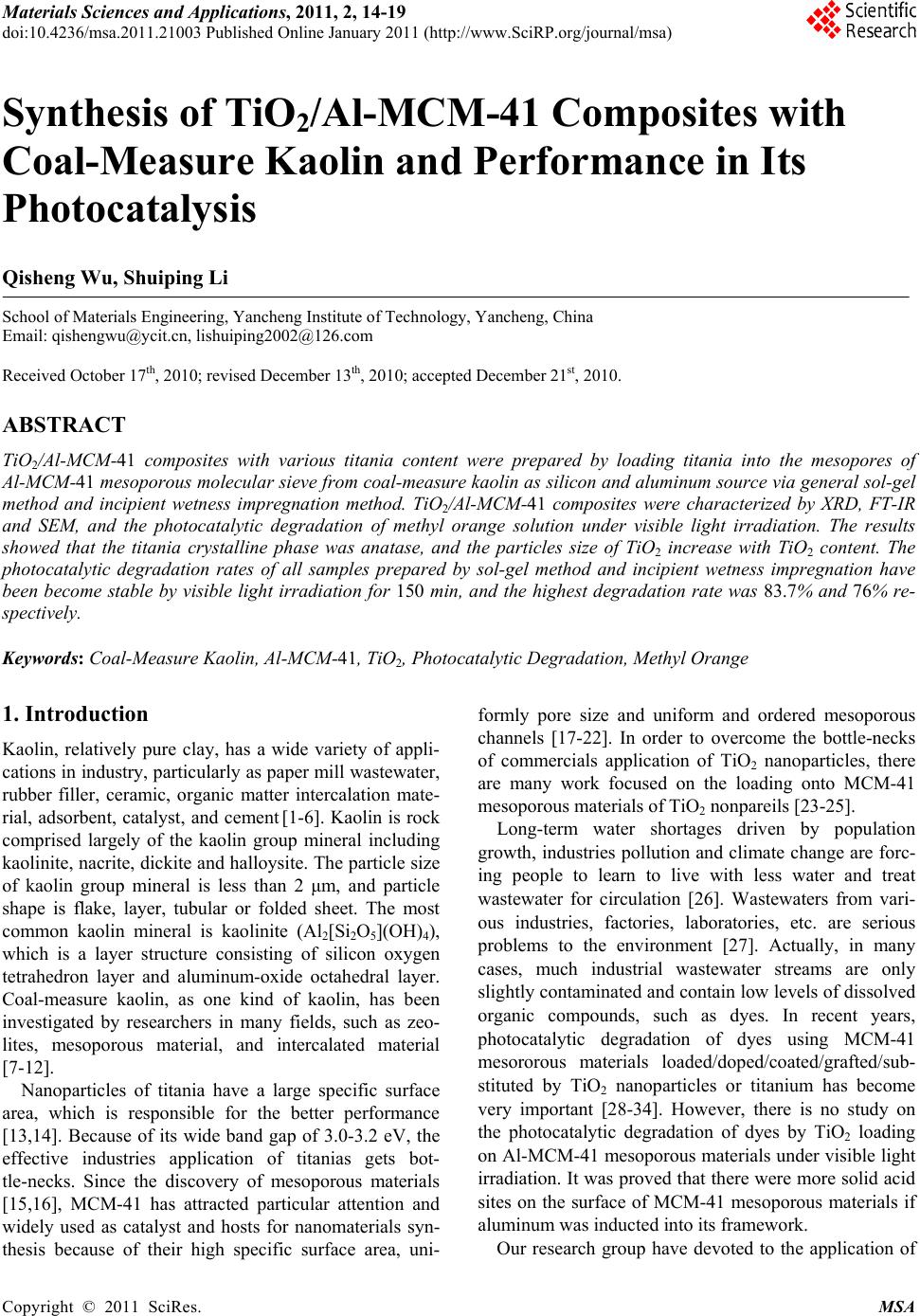
|