 American Journal of Plant Sciences, 2013, 4, 1821-1833 http://dx.doi.org/10.4236/ajps.2013.49224 Published Online September 2013 (http://www.scirp.org/journal/ajps) Phenotypic and Molecular Characterization of Wheat Leaf Rust Resistance Gene Lr34 in Iranian Wheat Cultivars and Advanced Lines Seyed Taha Dadrezaei1,2*, Kumarse Nazari3, Farzad Afshari2, Ebrahim Mohammadi Goltapeh1 1College of Agriculture, TarbiatModares University, Tehran, Iran; 2Seed and Plant Improvement Institute (SPII), Karaj, Iran; 3Inter- national Center for Agricultural Research in the Dry Areas (ICARDA), Aleppo, Syria. Email: *tahareza2000@yahoo.com Received April 20th, 2013; revised May 20th, 2013; accepted June 15th, 2013 Copyright © 2013 Seyed Taha Dadrezaei et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Lr34 is a vital gene in developing resistance to leaf rust, stripe rust, and powdery mildew of wheat. Providing simulta- neous resistance to various pathogens has made this gene valuable in breeding for wheat resistance to many diseases. The present study investigates the csLV34 marker’s capability in diagnosing this locus in130 wheat commercial cultivars and advanced wheat lines from Iran, and assesses the impact of this gene on disease severity in field conditions. To as- sess the reactions of cultivars and lines which contained Lr34 under epidemic conditions of leaf rust, these cultivars were cultivated during the 2009 and 2010 cropping season. Of the 130 studied cultivars, 43 contained Lr34. Cultivars that were selected and studied in stress conditions had the most frequent presence of Lr34. It can be concluded that this gene plays a vital role in increasing the tolerance of cultivars under stress conditions. Lr34 seems to cause active transition of materials out of the cell. In addition to being resistant to several important diseases of wheat, Lr34 can increase tolerance to stresses such as salinity. Considering the calculated value for AUDPC (3% - 440%/d) in cultivars containing Lr34, it seems that some cultivars contained additional resistance genes. The rate of infection in all cultivars, when presence of Lr34 was detected through the molecular marker, was lower than in other cultivars. Field results confirmed the results of the analysis using the csLV34b molecular marker. Keywords: Lr34 Gene; AUDPC; Salinity Stress; Leaf Rust; Puccinia triticina 1. Introduction Leaf rust caused by Puccinia triticina (Pt) is the most common and widely distributed of the three wheat rusts. Although losses from leaf rust are usually less damaging than those from stem rust and stripe rust, because of its regular and widespread occurrence, the global leaf rust damages are greater than the other two rust [1,2]. Wheat leaf rust is present in all wheat growing areas in Iran. In general, leaf rust is the second most important disease of wheat in Iran but in southern areas leaf rust is the most important disease of wheat [3]. The Pt population in Iran is extremely dynamic and a large number of races were found in a recent study of pathogenic variability of Pt population in Iran [4]. Improving the resistance of wheat cultivars to this disease is a preventive strategy with the greatest effect on reducing its damage. Rust resistance genes in wheat can be categorized into two groups: seed- ling resistance genes and adult plant resistance (APR) genes. Seedling resistance genes appear in both the seed- ling and adult plant stages and can be recognized; as a result, they show resistance in all phenotypic stages. Seedling resistance genes often lead to a hyper sensitive reaction (cell death-HR) or to lignifications of the cell membrane [5]. Adult plant gene resistance acts non-spe- cifically on race pathogens in the adult plant stage, and cultivars containing these genes are susceptible at seed- ling stage and have various levels of comparative resis- tance to disease at the adult plant stage [6]. This type of resistance is called race non-specific gene resistance since there is no relationship between host genes and pathogen genes. Additionally, it provides resistance to all pathogen isolates. Compared with susceptible plants, Lr34 resistance has a longer period of infection with fewer and smaller uredinial pustules at two weeks after infection [6,7]. *Corresponding author. Copyright © 2013 SciRes. AJPS  Phenotypic and Molecular Characterization of Wheat Leaf Rust Resistance Gene Lr34 in Iranian Wheat Cultivars and Advanced Lines 1822 More than 70 leaf rust resistance genes have been iden- tified, most of which are involved in race-specific cate- gory and follow the boom and bust cycle due to the high pathogenic variability of Pt population. Among the race non-specific genes, the Lr34/Yr18 complex [7], Lr46 [8] and Lr67 [9] are the most commonly used genes in global wheat. The Lr34 gene was initially introduced as an APR gene in cultivar “Frontana” [10], this gene encoding ATP Binding Cassette (ABC) transporter[7], with the locus of the gene on the short arm of wheat chromosome 7D [11]. With few exceptions, the race specific genes are asso- ciated with a very short durability. There is increasing interests in identification and development of race-non- specific slow rusting genes which have been shown to be more durable than race-specific genes [12,13]. So far leaf rust pathogens have not been reported as virulent on Lr34 [7,14]. Lr34 is genetically linked with the stripe rust adult-plant resistance gene Yr18, morphological marker leaf tip necrosis (Ltn1) [15], and an adult-plant powdery mildew resistance gene (Pm38) [16,17]. Tolerance to Barley yellow dwarf virus (Bdv1) in Lr34 carrying culti- vars is also reported [18]. The incorporation of leaf rust resistance gene Lr34 into “Thatcher” background is known to enhance stem rust resistance [10,11]. These si- multaneous resistances to several pathogens have made the Lr34/Yr18 locus one of the most valuable gene regions for disease resistance breeding in wheat. Leaf tip necrosis (Ltn1) has been used as a phenotypic marker for field selection of slow-rusting resistance conferred by Lr34/ Yr18 [15] by national and international breeding programs, but because of variable expression of Ltn1 under differ- ent environmental conditions and in different genetic background [18], the leaf tip necrosis could not be used as a reliable and diagnostic marker. Development and application of molecular markers for Lr34/Yr18 have been an important objective in marker-as- sisted selection in breeding for durable leaf rust resis- tance. Application of previously developed markers, such as gwm295 and gwm1220 [19,20], has shown their lim- ited use in breeding application due to their low diagnos- tic capability in precise detection of Lr34 in different ge- netic backgrounds. During the last few years significant progress has been made in the development of more closely linked markers for Lr34/Yr18 complex trait such as SWM10 and csL V 34 [21]. More recently, Kolmer et al. (2008) [18] confirmed robustness of a tightly linked csLV34 marker with Lr34/Yr18 across a wide range of global wheat germplasm and its utility in wheat breeding. In present study seedling and adult-plant assessment of resistance to leaf rust coupled with application of the tightly linked marker csLV34 with Lr34/Yr18 were used in the characterization of adult-plant resistance of some of the Iranian bread and durum wheat genotypes to leaf rust. 2. Materials and Methods 2.1. Plant Materials There were 130 commercial wheat cultivars and ad- vanced lines of hexaploid and tetraploid wheat from Iran (Table 1). Seeds of test genotypes were obtained from department of cereal research at Seed and Plant Improve- ment Institute Research (SPII), Karaj, Iran. A set of That- cher near isogenic lines (TcNILs) were used both at seed- ling and adult-plant assessments. Seeds of TcNILs were kindly provided by International Maize and Wheat Im- provement Center (CIMMYT). The universal leaf rust susceptible cultivar “Thatcher” and a local susceptible cultivar “Bolani” were used in seedling and adult plant tests. 2.2. Seedling Test Assessment of seedling resistance was carried out at ce- real rust pathology laboratory at SPII. The 130 test ge- notypes and leaf rust Thatcher near isogenic lines were used in seedling assessment against a local Pt isolate. Eight to 10 seeds of each genotype were planted in a 9 cm diameter pot filled with potting mix at two replica- tions. The seedlings were grown in a rust-free green- house at 20˚C and 16 hours light. At two leaf stage, seed- lings were inoculated with the local leaf rust isolate col- lected from the field trial site at Khuzestan Agricultural Research Station, in south of Iran. This isolate used in seedling tests and field inoculations. Urediniospores stored at −80˚C were first heat shocked at 42˚C for 5 minutes and then mixed with Talcum powder (1:4). Seedlings were inoculated with the talc-spore mixture using a small duster. Inoculated plants were placed overnight in a hu- mid chamber at 17˚C ± 2˚C and dark condition. After the incubation period, plants were placed in a greenhouse with 20˚C ± 2˚C and 16 hrs supplementary light. Seed- ling infection types were recorded 12 days post-inocu- lation using; (fleck) and 0 to 4 scale [22]. Infection types; and 0 to 3 were considered resistant reactions, while in- fection types higher than 3 were considered as suscepti- ble. 2.3. Field Experiments In order to evaluate adult-plant resistance of test geno- types and TcNILs, a field trial was carried out under mist irrigation system at Khuzestan Agricultural Research Center in 2009. Each genotype was planted as two 1-m row plot and 30 cm space. To facilitate inoculum build- up and uniform dissemination of infection, the suscepti- ble cultivar Bolani was planted perpendicular to the rows of entries. Bolani was also planted at each 10 plot inter- vals. Disease epidemic was created by artificial inocula- Copyright © 2013 SciRes. AJPS  Phenotypic and Molecular Characterization of Wheat Leaf Rust Resistance Gene Lr34 in Iranian Wheat Cultivars and Advanced Lines Copyright © 2013 SciRes. AJPS 1823 ger UV (Gel DocTM XR Bio rad Universal Hood II) was used for visualization and documentation of banding pat- terns. tion of the local leaf rust isolate collected from the same site in 2008. Preserved urediniospores were first multi- plied on susceptible cultivar Bolani under greenhouse conditions following the above described procedure. Fre- shly collected urediniospores were mixed with talcum powder and inoculation was carried over entries after misting irrigation at late afternoons by atomizer back- pack duster. First inoculation was started at 20 January 2010 when plants were at tillering stage and it was re- peated four times at fortnight intervals. Disease severities (0% - 100%) were recorded according to the Modified Cobb’s scale [23] and the adult-plant reactions were re- corded for the major infection types R (resistant), MR (moderately resistant), MS (moderately susceptible) and S (susceptible) according to Roelfs et al. (1992) [24]. Field scoring started from early onset of uniform infec- tions in Bloani with 10 days intervals. Data on the dis- ease severities and infection types were used in calcula- tion of coefficient of infection (CI) for each individual score [24].The area under the disease progress curve (AUDPC) [25] was then calculated as follows using the CIs for three disease scores: 3. Results 3.1. Molecular Marker Screening The presence of a 150-bp band is diagnostic of the Lr34 gene, indicating the presence of Lr34 in cultivars and lines carrying the gene. This band belongs to the csLV34b al- lele, which is associated with Lr34. Another longer band (229-bp) is produced and belongs to the csLV34a allele, which is associated with the absence of Lr34; i.e. when the cultivar does not contain Lr34, and then the longer band is produced. However, in cultivars that are het- erozygous both alleles are present and so both bands are generated simultaneously. Of the130 investigated genotypes, 87 lacked Lr34; shown by the presence of the 229-bpband of allele csLV34a. There were 43 genotypes with Lr34, shown by the presence of the 150-bp band. These 43genotypes were divided into two groups: 26 homozygous cultivars with the 150-bp band and17 genotypes with both the 229- and 150-bp bands. Of the 17 hetero zygous geno- types, 15 had unique phenotype markers. In these 15 ge- notypes, there produced PCR product possessed a three- band pattern consisting csLV34 a and csLV34b alleles, both of which were accompanied by an additional band with a higher molecular weight (280 bp). In the study of global wheat cultivars Kolmer et al. (2008) observed the three-band pattern in heterozygous cultivars; however, most heterozygous cultivars had a two-band pattern and cultivars with a three-band pattern were less frequent. Moreover, the positive control sample in the present study was the 150-bp band; and “Thatcher”, susceptible “Bo- lani”, and all susceptible cultivars only produced the 229- bp band and the negative control sample (water) had no bands (Figure 1). i1 N ii1 i1 i i1 yy AUDPCt t 2 with: i—index for scoring date; yi—Coefficient of leaf rust infection at scoring date i; ti—scoring date i expressed in days after scoring date 1; n—total number of scoring dates in the trial. 2.4. DNA Extraction and PCR Analysis For extraction of genomic DNA, 100 mg of harvested leaves from 14 days old seedlings of each tested geno- type was ground to fine powder on liquid nitrogen. The fine powder was immediately transferred into a 2 ml tubes and the small scale DNA extraction protocol was followed as described in CIMMYT applied molecular protocol [26]. PCR reaction was performed in 20 μl for the CsLV34 marker following published protocol [18,21] in a PTC 100 Thermocycler (MJ Research, Waltham, MA). The PCR product was separated on 1.5% agarose gel containing TBE 0.5× buffer. Digital molecular ima- There was a high frequency of csLV34 allele (150 bp), which is associated with Lr34 in the assessed Iranian lines and cultivars. Of the 130 studied genotypes, 43 contained the diagnostic Lr34 allele, representing a frequency of 33% (Table 1) as follows: csLV34b (229 bp) csL 34b (150 bp) 1 2 3 4 5 6 7 8 Figure 1. Polymerase chain reaction amplification products from wheat cultivars using csLV34 marker. 1. Thatcher +Lr34 (Lr34); 2. Falat (Lr34); 3. Ghods (Lr34); 4. Star (heterozygote); 5. Bam (Lr34); 6. Dez (Lr34); 7. Aflak (Lr34); 8. Cham ran (Lr34). 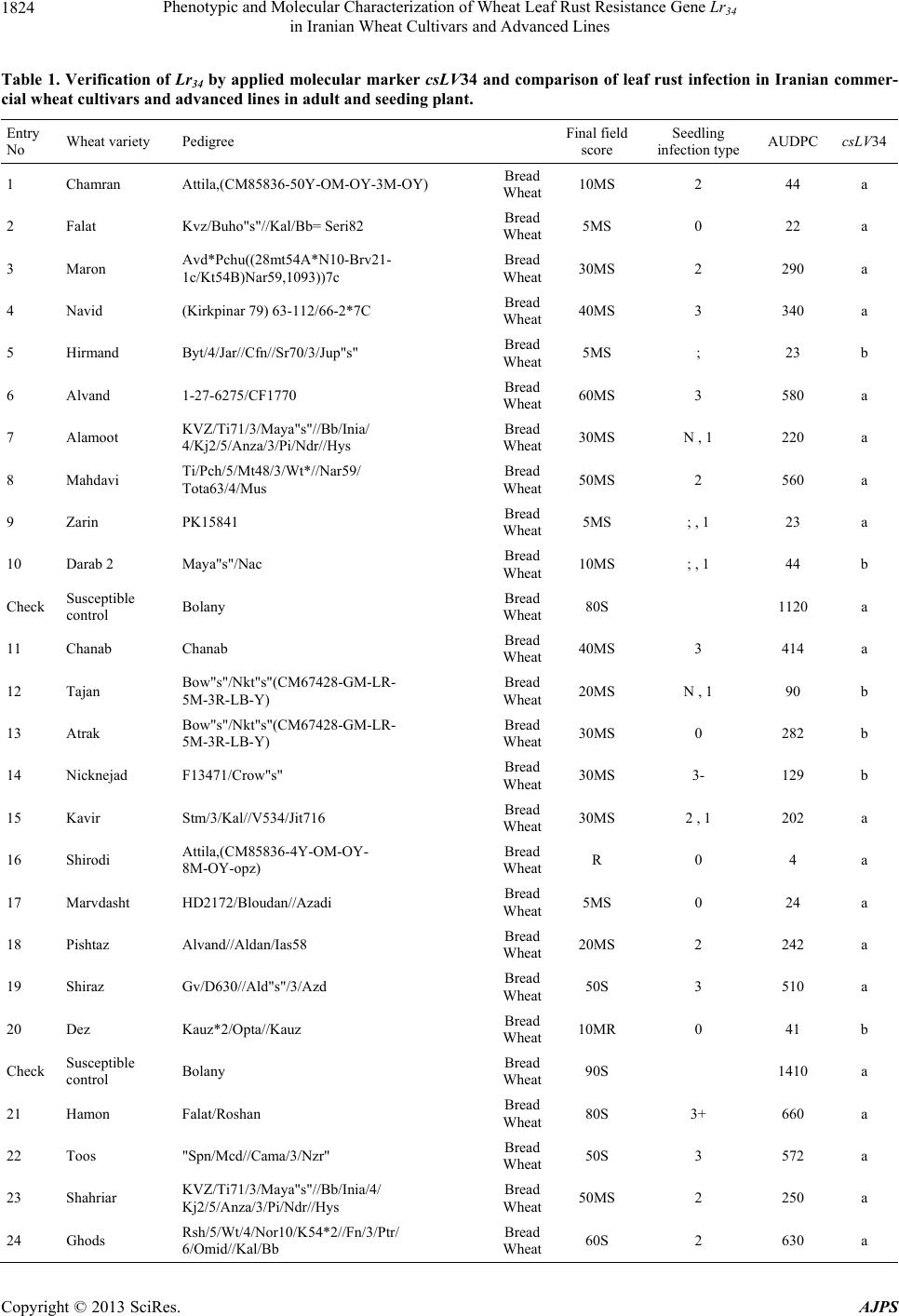 Phenotypic and Molecular Characterization of Wheat Leaf Rust Resistance Gene Lr34 in Iranian Wheat Cultivars and Advanced Lines 1824 Table 1. Verification of Lr34 by applied molecular marker csLV34 and comparison of leaf rust infection in Iranian commer- cial wheat cultivars and advanced lines in adult and seeding plant. Entry No Wheat variety Pedigree Final field score Seedling infection type AUDPC csLV34 1 Chamran Attila,(CM85836-50Y-OM-OY-3M-OY) Bread Wheat 10MS 2 44 a 2 Falat Kvz/Buho"s"//Kal/Bb= Seri82 Bread Wheat 5MS 0 22 a 3 Maron Avd*Pchu((28mt54A*N10-Brv21- 1c/Kt54B)Nar59,1093))7c Bread Wheat 30MS 2 290 a 4 Navid (Kirkpinar 79) 63-112/66-2*7C Bread Wheat 40MS 3 340 a 5 Hirmand Byt/4/Jar//Cfn//Sr70/3/Jup"s" Bread Wheat 5MS ; 23 b 6 Alvand 1-27-6275/CF1770 Bread Wheat 60MS 3 580 a 7 Alamoot KVZ/Ti71/3/Maya"s"//Bb/Inia/ 4/Kj2/5/Anza/3/Pi/Ndr//Hys Bread Wheat 30MS N , 1 220 a 8 Mahdavi Ti/Pch/5/Mt48/3/Wt*//Nar59/ Tota63/4/Mus Bread Wheat 50MS 2 560 a 9 Zarin PK15841 Bread Wheat 5MS ; , 1 23 a 10 Darab 2 Maya"s"/Nac Bread Wheat 10MS ; , 1 44 b Check Susceptible control Bolany Bread Wheat 80S 1120 a 11 Chanab Chanab Bread Wheat 40MS 3 414 a 12 Tajan Bow"s"/Nkt"s"(CM67428-GM-LR- 5M-3R-LB-Y) Bread Wheat 20MS N , 1 90 b 13 Atrak Bow"s"/Nkt"s"(CM67428-GM-LR- 5M-3R-LB-Y) Bread Wheat 30MS 0 282 b 14 Nicknejad F13471/Crow"s" Bread Wheat 30MS 3- 129 b 15 Kavir Stm/3/Kal//V534/Jit716 Bread Wheat 30MS 2 , 1 202 a 16 Shirodi Attila,(CM85836-4Y-OM-OY- 8M-OY-opz) Bread Wheat R 0 4 a 17 Marvdasht HD2172/Bloudan//Azadi Bread Wheat 5MS 0 24 a 18 Pishtaz Alvand//Aldan/Ias58 Bread Wheat 20MS 2 242 a 19 Shiraz Gv/D630//Ald"s"/3/Azd Bread Wheat 50S 3 510 a 20 Dez Kauz*2/Opta//Kauz Bread Wheat 10MR 0 41 b Check Susceptible control Bolany Bread Wheat 90S 1410 a 21 Hamon Falat/Roshan Bread Wheat 80S 3+ 660 a 22 Toos "Spn/Mcd//Cama/3/Nzr" Bread Wheat 50S 3 572 a 23 Shahriar KVZ/Ti71/3/Maya"s"//Bb/Inia/4/ Kj2/5/Anza/3/Pi/Ndr//Hys Bread Wheat 50MS 2 250 a 24 Ghods Rsh/5/Wt/4/Nor10/K54*2//Fn/3/Ptr/ 6/Omid//Kal/Bb Bread Wheat 60S 2 630 a Copyright © 2013 SciRes. AJPS 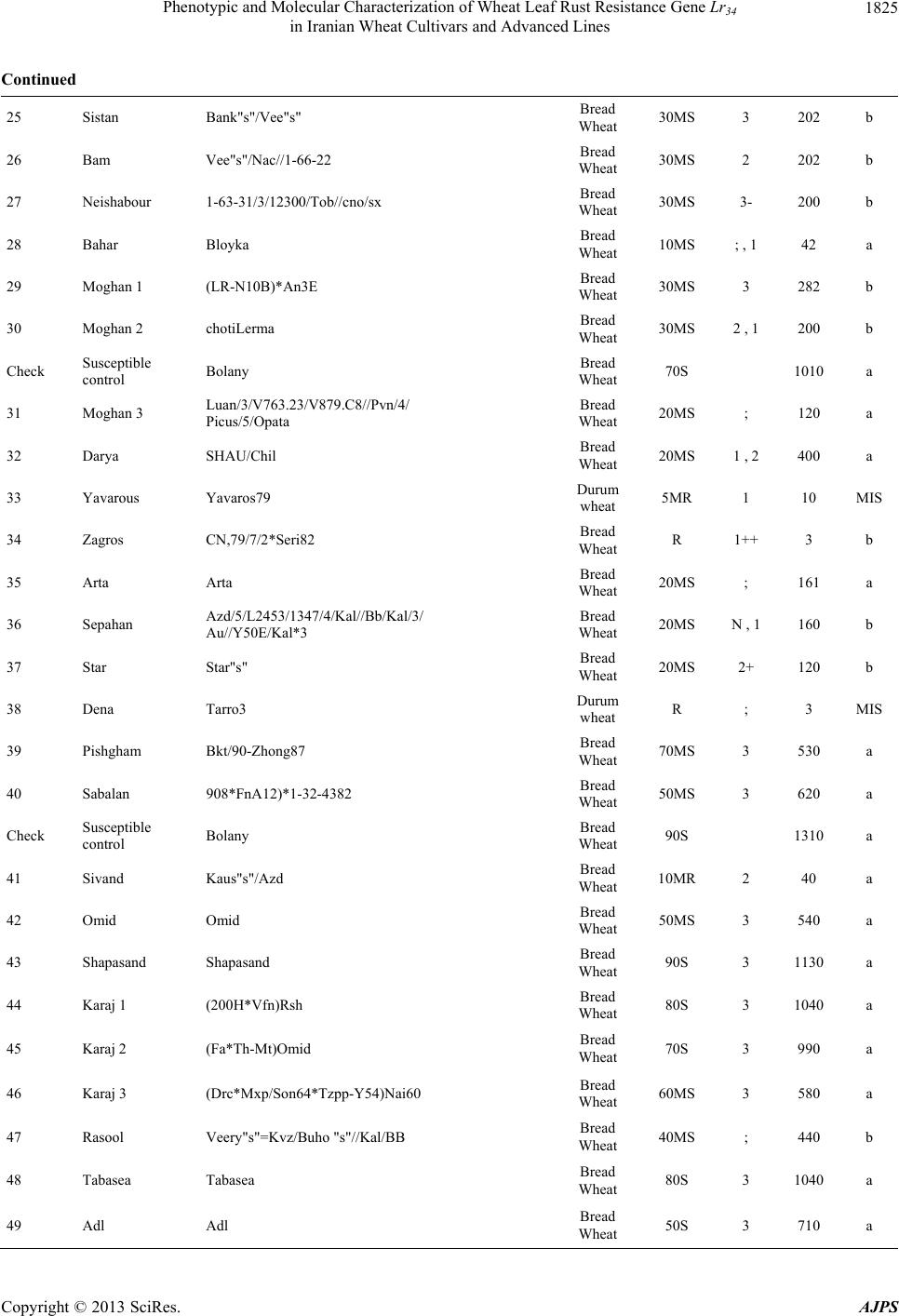 Phenotypic and Molecular Characterization of Wheat Leaf Rust Resistance Gene Lr34 in Iranian Wheat Cultivars and Advanced Lines 1825 Continued 25 Sistan Bank"s"/Vee"s" Bread Wheat 30MS 3 202 b 26 Bam Vee"s"/Nac//1-66-22 Bread Wheat 30MS 2 202 b 27 Neishabour 1-63-31/3/12300/Tob//cno/sx Bread Wheat 30MS 3- 200 b 28 Bahar Bloyka Bread Wheat 10MS ; , 1 42 a 29 Moghan 1 (LR-N10B)*An3E Bread Wheat 30MS 3 282 b 30 Moghan 2 chotiLerma Bread Wheat 30MS 2 , 1 200 b Check Susceptible control Bolany Bread Wheat 70S 1010 a 31 Moghan 3 Luan/3/V763.23/V879.C8//Pvn/4/ Picus/5/Opata Bread Wheat 20MS ; 120 a 32 Darya SHAU/Chil Bread Wheat 20MS 1 , 2 400 a 33 Yavarous Yavaros79 Durum wheat 5MR 1 10 MIS 34 Zagros CN,79/7/2*Seri82 Bread Wheat R 1++ 3 b 35 Arta Arta Bread Wheat 20MS ; 161 a 36 Sepahan Azd/5/L2453/1347/4/Kal//Bb/Kal/3/ Au//Y50E/Kal*3 Bread Wheat 20MS N , 1 160 b 37 Star Star"s" Bread Wheat 20MS 2+ 120 b 38 Dena Tarro3 Durum wheat R ; 3 MIS 39 Pishgham Bkt/90-Zhong87 Bread Wheat 70MS 3 530 a 40 Sabalan 908*FnA12)*1-32-4382 Bread Wheat 50MS 3 620 a Check Susceptible control Bolany Bread Wheat 90S 1310 a 41 Sivand Kaus"s"/Azd Bread Wheat 10MR 2 40 a 42 Omid Omid Bread Wheat 50MS 3 540 a 43 Shapasand Shapasand Bread Wheat 90S 3 1130 a 44 Karaj 1 (200H*Vfn)Rsh Bread Wheat 80S 3 1040 a 45 Karaj 2 (Fa*Th-Mt)Omid Bread Wheat 70S 3 990 a 46 Karaj 3 (Drc*Mxp/Son64*Tzpp-Y54)Nai60 Bread Wheat 60MS 3 580 a 47 Rasool Veery"s"=Kvz/Buho "s"//Kal/BB Bread Wheat 40MS ; 440 b 48 Tabasea Tabasea Bread Wheat 80S 3 1040 a 49 Adl Adl Bread Wheat 50S 3 710 a Copyright © 2013 SciRes. AJPS 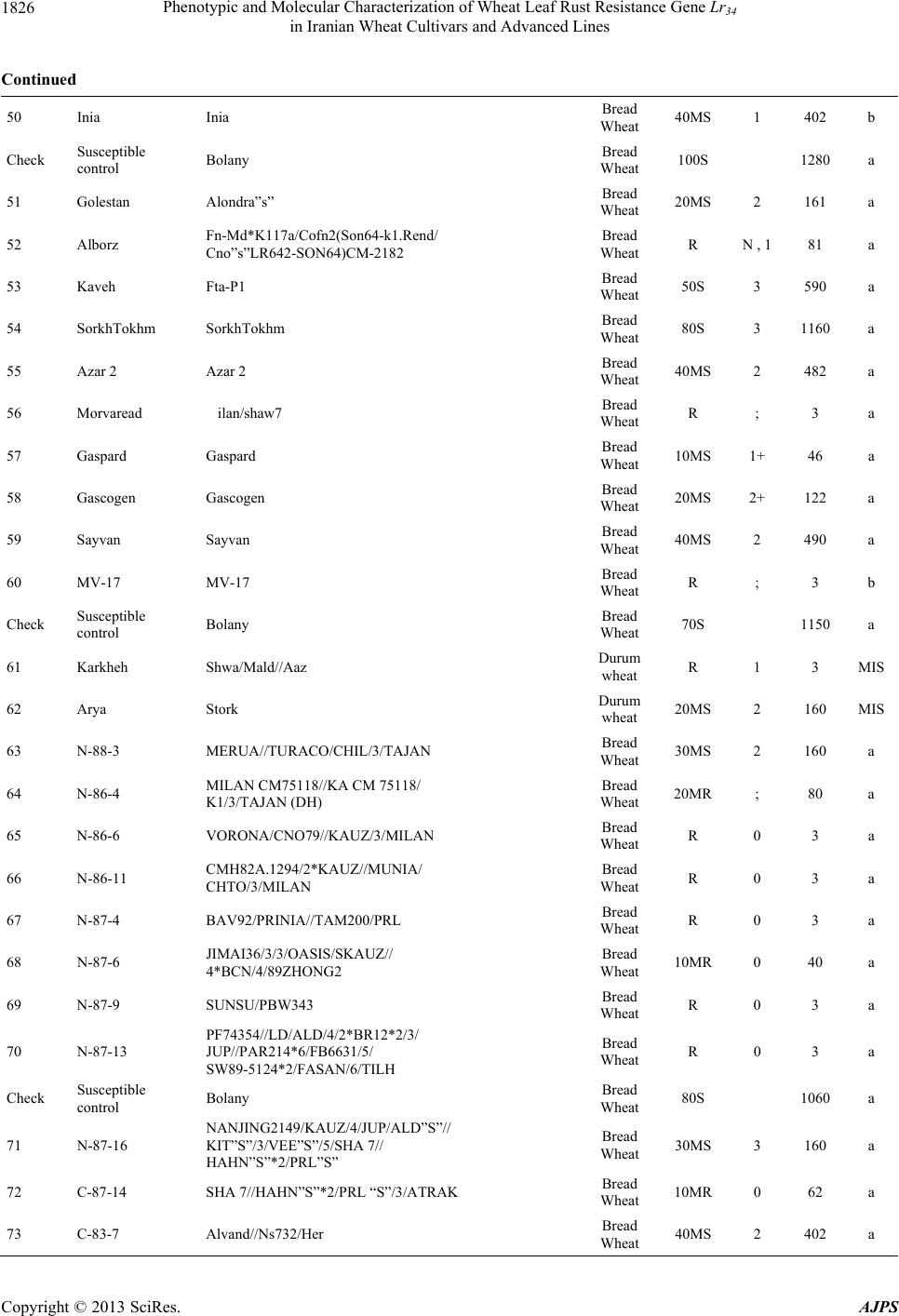 Phenotypic and Molecular Characterization of Wheat Leaf Rust Resistance Gene Lr34 in Iranian Wheat Cultivars and Advanced Lines 1826 Continued 50 Inia Inia Bread Wheat 40MS 1 402 b Check Susceptible control Bolany Bread Wheat 100S 1280a 51 Golestan Alondra”s” Bread Wheat 20MS 2 161 a 52 Alborz Fn-Md*K117a/Cofn2(Son64-k1.Rend/ Cno”s”LR642-SON64)CM-2182 Bread Wheat R N , 1 81 a 53 Kaveh Fta-P1 Bread Wheat 50S 3 590 a 54 SorkhTokhm SorkhTokhm Bread Wheat 80S 3 1160a 55 Azar 2 Azar 2 Bread Wheat 40MS 2 482 a 56 Morvaread ilan/shaw7 Bread Wheat R ; 3 a 57 Gaspard Gaspard Bread Wheat 10MS 1+ 46 a 58 Gascogen Gascogen Bread Wheat 20MS 2+ 122 a 59 Sayvan Sayvan Bread Wheat 40MS 2 490 a 60 MV-17 MV-17 Bread Wheat R ; 3 b Check Susceptible control Bolany Bread Wheat 70S 1150a 61 Karkheh Shwa/Mald//Aaz Durum wheat R 1 3 MIS 62 Arya Stork Durum wheat 20MS 2 160 MIS 63 N-88-3 MERUA//TURACO/CHIL/3/TAJAN Bread Wheat 30MS 2 160 a 64 N-86-4 MILAN CM75118//KA CM 75118/ K1/3/TAJAN (DH) Bread Wheat 20MR ; 80 a 65 N-86-6 VORONA/CNO79//KAUZ/3/MILAN Bread Wheat R 0 3 a 66 N-86-11 CMH82A.1294/2*KAUZ//MUNIA/ CHTO/3/MILAN Bread Wheat R 0 3 a 67 N-87-4 BAV92/PRINIA//TAM200/PRL Bread Wheat R 0 3 a 68 N-87-6 JIMAI36/3/3/OASIS/SKAUZ// 4*BCN/4/89ZHONG2 Bread Wheat 10MR 0 40 a 69 N-87-9 SUNSU/PBW343 Bread Wheat R 0 3 a 70 N-87-13 PF74354//LD/ALD/4/2*BR12*2/3/ JUP//PAR214*6/FB6631/5/ SW89-5124*2/FASAN/6/TILH Bread Wheat R 0 3 a Check Susceptible control Bolany Bread Wheat 80S 1060a 71 N-87-16 NANJING2149/KAUZ/4/JUP/ALD”S”// KIT”S”/3/VEE”S”/5/SHA 7// HAHN”S”*2/PRL”S” Bread Wheat 30MS 3 160 a 72 C-87-14 SHA 7//HAHN”S”*2/PRL “S”/3/ATRAK Bread Wheat 10MR 0 62 a 73 C-83-7 Alvand//Ns732/Her Bread Wheat 40MS 2 402 a Copyright © 2013 SciRes. AJPS 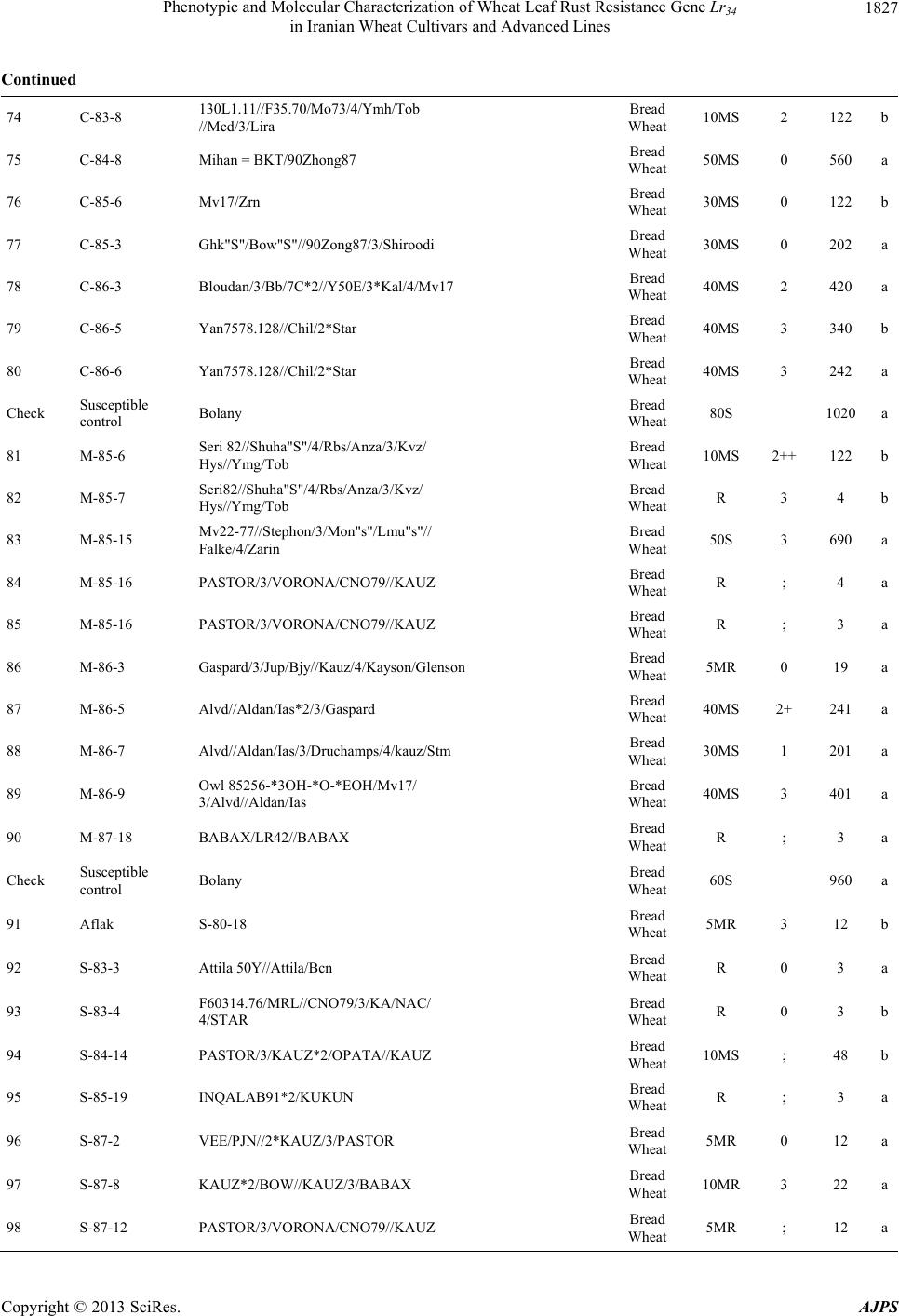 Phenotypic and Molecular Characterization of Wheat Leaf Rust Resistance Gene Lr34 in Iranian Wheat Cultivars and Advanced Lines 1827 Continued 74 C-83-8 130L1.11//F35.70/Mo73/4/Ymh/Tob //Mcd/3/Lira Bread Wheat 10MS 2 122 b 75 C-84-8 Mihan = BKT/90Zhong87 Bread Wheat 50MS 0 560 a 76 C-85-6 Mv17/Zrn Bread Wheat 30MS 0 122 b 77 C-85-3 Ghk"S"/Bow"S"//90Zong87/3/Shiroodi Bread Wheat 30MS 0 202 a 78 C-86-3 Bloudan/3/Bb/7C*2//Y50E/3*Kal/4/Mv17 Bread Wheat 40MS 2 420 a 79 C-86-5 Yan7578.128//Chil/2*Star Bread Wheat 40MS 3 340 b 80 C-86-6 Yan7578.128//Chil/2*Star Bread Wheat 40MS 3 242 a Check Susceptible control Bolany Bread Wheat 80S 1020 a 81 M-85-6 Seri 82//Shuha"S"/4/Rbs/Anza/3/Kvz/ Hys//Ymg/Tob Bread Wheat 10MS 2++ 122 b 82 M-85-7 Seri82//Shuha"S"/4/Rbs/Anza/3/Kvz/ Hys//Ymg/Tob Bread Wheat R 3 4 b 83 M-85-15 Mv22-77//Stephon/3/Mon"s"/Lmu"s"// Falke/4/Zarin Bread Wheat 50S 3 690 a 84 M-85-16 PASTOR/3/VORONA/CNO79//KAUZ Bread Wheat R ; 4 a 85 M-85-16 PASTOR/3/VORONA/CNO79//KAUZ Bread Wheat R ; 3 a 86 M-86-3 Gaspard/3/Jup/Bjy//Kauz/4/Kayson/Glenson Bread Wheat 5MR 0 19 a 87 M-86-5 Alvd//Aldan/Ias*2/3/Gaspard Bread Wheat 40MS 2+ 241 a 88 M-86-7 Alvd//Aldan/Ias/3/Druchamps/4/kauz/Stm Bread Wheat 30MS 1 201 a 89 M-86-9 Owl 85256-*3OH-*O-*EOH/Mv17/ 3/Alvd//Aldan/Ias Bread Wheat 40MS 3 401 a 90 M-87-18 BABAX/LR42//BABAX Bread Wheat R ; 3 a Check Susceptible control Bolany Bread Wheat 60S 960 a 91 Aflak S-80-18 Bread Wheat 5MR 3 12 b 92 S-83-3 Attila 50Y//Attila/Bcn Bread Wheat R 0 3 a 93 S-83-4 F60314.76/MRL//CNO79/3/KA/NAC/ 4/STAR Bread Wheat R 0 3 b 94 S-84-14 PASTOR/3/KAUZ*2/OPATA//KAUZ Bread Wheat 10MS ; 48 b 95 S-85-19 INQALAB91*2/KUKUN Bread Wheat R ; 3 a 96 S-87-2 VEE/PJN//2*KAUZ/3/PASTOR Bread Wheat 5MR 0 12 a 97 S-87-8 KAUZ*2/BOW//KAUZ/3/BABAX Bread Wheat 10MR 3 22 a 98 S-87-12 PASTOR/3/VORONA/CNO79//KAUZ Bread Wheat 5MR ; 12 a Copyright © 2013 SciRes. AJPS  Phenotypic and Molecular Characterization of Wheat Leaf Rust Resistance Gene Lr34 in Iranian Wheat Cultivars and Advanced Lines 1828 Continued 99 S-87-18 CBRD-3/STORK X DICOCCOIDES Bread Wheat 5MR ; 12 a 100 S-87-20 OASIS/SKAUZ//4*BCN/3/2*PASTOR Bread Wheat 30MS ; 122 b Check Susceptible control Bolany Bread Wheat 70S 1070 a 101 S-87-21 520- BABAX/LR42//BABAX*2/ 3/VIVITSI Bread Wheat R 0 3 a 102 DM-79-2 PORTO-7 Durum wheat 5MR 0 16 MIS 103 DM-81-6 PLATA-1/SNM//PLATA-9 Durum wheat R ; 3 MIS 104 DM-82-6 SOOTY-9/RASCON-37 Durum wheat R ; 3 MIS 105 DM-83-10 AUK/GUIL//GREEN Durum wheat R ; 3 MIS 106 DM-84-3 RASCON-37/BEJAH-7 Durum wheat R ; 4 MIS 107 DM-85-10 RASCON-37/BEJAH-7 Durum wheat 5MR ; 12 MIS 108 WS-85-10 PRL/2*PASTOR Bread Wheat 10MR 3 22 a 109 WS-85-15 PBW343*2/KONK Bread Wheat R 0 3 b 110 WS-86-5 Shi#4414/Crow"S"//Azd Bread Wheat R 0 3 a Check Susceptible control Bolany Bread Wheat 80S 1080 a 111 WS-86-8 SW89.5181/KAUZ Bread Wheat R 0 25 b 112 WS-86-11 MUNIA/3/RUFF/FGO//YAV79/4/ PASTOR Bread Wheat 20MS ; 83 a 113 WS-86-12 PJN/BOW//OPATA*2/3/CROC_1/ AE.SQUARROSA (224)//OPATA Bread Wheat 10MS 0 42 b 114 WS-86-13 VORONA/CNO79//KAUZ/3/MILAN Bread Wheat 5MS 0 22 a 115 WS-86-14 KAUZ/PASTOR Bread Wheat 20MS 0 82 b 116 MS-85-15 Ombu/Alamo//Mahooti/3/1-66-22 Bread Wheat 30MS 0 124 b 117 MS-85-12 Ombu/Alamo//Alvd/3/Kauz/Stm Bread Wheat 30MS 1 160 b 118 MS-84-13 GF-gy54/Attila Bread Wheat 40MS 3 242 b 119 MS-85-17 Sakha 8/Darab#2//1-66-22 Bread Wheat 30MS 3 204 b 120 MS-84-16 Gkzombor/Zrn Bread Wheat 30MR 3 300 a Check Susceptible control Bolany Bread Wheat 80S 1300 a 121 SS-85-6 1-66-22/3/GUP/BGY//kauz Bread Wheat 30MS 3 202 b 122 SS-85-10 OMBU/ALAMO//ALVD/3/1-66-22 Bread Wheat 30MS 3 202 b Copyright © 2013 SciRes. AJPS  Phenotypic and Molecular Characterization of Wheat Leaf Rust Resistance Gene Lr34 in Iranian Wheat Cultivars and Advanced Lines Copyright © 2013 SciRes. AJPS 1829 Continued 123 SS-85-11 OMBU/ALAMO//MAHOOTI/3/1-66-22 Bread Wheat 30MS ; 202 b 124 SS-85-14 SAKHA 8/DARAB#2//1-66-22 Bread Wheat 30MS ; 282 b 125 SS-85-20 OMBU/ALAMO//KAV/3/PASOR/ SORKHTOKHM.. Bread Wheat 30MS 0 202 b 126 DW-79-5 LAGOST-2 Durum wheat 20MS ; 89 b 127 DW-81-18 SORA/2*PLATA12 Durum wheat 20MS ; 85 b 128 DW-84-5 GREEN_14//YAV_10/AUK Durum wheat R ; 6 MIS 129 Bezostaya Bezostaya Bread Wheat 3 b 130 Veerynak Veerynak Bread Wheat 2 a Check Susceptible control Bolany Bread Wheat 100S 3+ 1280 a Check Positive control Thatcher (Lr34) Bread Wheat 30MS 3- 300 b Among commercial Iranian cultivars, 18 of 64 culti- vars contained csLV34b, indicating presence of Lr34; only “Hirmand” and “Bam” which were the result of national crossing program, which had origin in international germ- plasm. None of the nine advanced lines bread wheat for the warm and humid climate of northern Iran carried Lr34, i.e. they contained csLV34a. Among the nine advanced lines bread wheat for cold climates, three had Lr34; among the 11 advanced lines bread wheat for the warm climate of the south, five carried Lr34. From nine lines bread wheat for mild climates, two had the 150-bp band indicating the presence of Lr34. However, cultivars studied in environmental stress con- ditions and selected to assess their tolerance of these conditions were totally different as follows. Among the eight chosen advanced lines bread wheat for humid stress conditions, four had Lr34 (50%). Of the five selected ad- vanced lines bread wheat for saline conditions located in mild climate areas, four contained Lr34; among five ad- vanced lines bread wheat for saline conditions in the warm climate of the south, all five carried Lr34, i.e. 90% of advanced cultivars bread wheat to tolerate salinity contained Lr34. It seems that cultivars carrying Lr34 could better tolerate stress conditions, and most cultivars pre- viously introduced for areas with salinity stress in mild climates such as “Hirmand”, “Sistan”, “Neishabour”, “Nicknejad”, “Tajan”, and “Darab2” contained Lr34. “Inia” is a cultivar resistant to salinity in experiments and also contained Lr34. “Star” is a late maturity cultivar, and is presently widely cultivated in Khuzestan Province. In evaluation of salinity resistance of wheat cultivars in laboratory and field conditions, the percentage of germi- nation and seedling establishment of “Star” under saline conditions was good relative superiority. “Bam” was re- cently introduced for mild climate areas with soil and water salinity stress, and contains Lr34 [27]. Cultivars that were not resistant to environmental stress such as salinity lacked Lr34, e.g. lines bread wheat for the northern cli- mate or cultivars “Darya”, “Golestan”, “Alborz”, “Kaveh”, and “Bahar” (Table 1). In the present research, 13 genotypes of durum wheat were also investigated, four cultivars and seven lines of which did not produce any bands to confirm the presence or absence of Lr34. A separate experiment for these cul- tivars was repeatedly conducted with positive and nega- tive controls and a similar result was obtained. Absence of a reproduced band or piece in durum wheat was likely due to the lack of the D genome since Lr34 is located on the small arm of chromosome 7 of genome D and prim- ers should be placed on this part to be reproduced. Be- cause of the absence of this genome in tetraploid culti- vars (e.g. durum wheat), this piece was not reproduced in these cultivars. 3.2. Phenotypic Characterization About 44 cultivars gave R or MR reactions in field as- sessment, among which only eight carried Lr34. Most cultivars (35 genotypes) contained Lr34 or were MS. The estimated AUDPC for cultivars carrying Lr34 was within 3% - 440%/day, indicating that some cultivars may carry  Phenotypic and Molecular Characterization of Wheat Leaf Rust Resistance Gene Lr34 in Iranian Wheat Cultivars and Advanced Lines 1830 some other resistance genes as well as Lr34. This was confirmed by further studies conducted with other mark- ers of race-specific genes on the same cultivars (unpub- lished data). In wheat cultivars with a combination of resistance genes genetic infection type with the highest resistance conceals the impact of the type with lower in- fection; therefore, these cultivars that contain race-spe- cific resistance genes in addition to Lr34, an infection type of R or MR is seen instead of MS resistance type and the presence of Lr34 is masked by other main genes. Accordingly, methods such as molecular methods which can easily identify this gene are important. The pheno- typic method used currently relies on Ltn1 and makes it very difficult to recognize Lr34 from the visual phenotype of leaves since Ltn1 does not express equally in different environments. This method requires a lot of experience and the results are not always correct. AUDPC for the examined cultivars in the present study was within 3% - 1410%/day. AUDPC of control susceptible “Bolani”, which was repeated 13 times among the field-grown cultivars (was planted at the end of the experiment and after each plot of 10 cultivars as suscep- tible control), was calculated to be 960% - 1410%/d, this difference in AUDPC is the result of environment. AUDPC of other cultivars, except for that of the sus- ceptible control (Check), was 3% - 1160%/day. The lower AUDPC belonged to cultivars that were resistant due to their effective race-specific resistance genes and were discussed previously. The results showed that AUDPC of 500% - 800%/day indicated a susceptible cultivar and AUDPC > 800 indicated a too susceptible one. In this experiment AUDPC < 500 was regarded as ac- ceptable resistance because about two months after an epi- demic of the disease and at the time of maximum flag leaf efficiency in photosynthesis and grain filling, the maximum infection remained at 40 MS. The highest AUDPC for genotypes containing Lr34 was for “Rasool”, “Inia”, and line C-86-5 with values 440%, 402%, and 340%/day, respectively. Most lines and cultivars con- taining this gene had AUDPC of about 200%/day and “Thatcher” had 300%/day. Accordingly, AUDPC of 250 - 500 was considered as semi-susceptible or relatively re- sistant; AUDPC of 150 - 250 was considered semi-re- sistant, and AUDPC < 150 was considered resistant. The flag leaf plays a crucial role in grain filling. The surface of this leaf in susceptible cultivars can be rapidly covered with leaf rust pustules at the time of grain filling. As a result, the entire surface can be infected and so harms its function. However, cultivars containing Lr34 are resistant to rapid development of the pathogen and delay it. The flag leaf of such cultivars is more capable of grain filling and incurs less damage. In epidemics, leaf rusts do much damage to flag leaves; therefore, assessing resistance to leaf and yellow rust at the adult plant stage is very important in improvement programs. Most assessment of resistance to leaf rust is done on the flag leaf because severity of the disease on leaves reflects the primary growth of the pathogen and damage to the plant [24]. An obvious advantage of presence of Lr34/Yr18 in cul- tivars is the absence of high intensities of infection at the end of the wheat growing season. However, cultivars that do not contain this gene can be highly infected by leaf rust during the whole growing season. Cultivars with race-specific genes, which are widely used in cultivars, are expected not to show long-term resistance for patho- types that are virulent on the Lr9 resistance gene. These were previously discussed by Kolmer [28]. However, if resistance of a race-specific gene is broken, Lr34 prevents rapid epidemics of the disease and major damage. In all cultivars in which the presence of Lr34 was shown by molecular marker, infection rate was less than in culti- vars not containing this gene (Table 1). Field results con- firmed the analysis results concerning the csLV34b mar- ker (Table 1). Lr34 is believed to be dominant. The results clearly showed gradual rust resistance in heterozygous cultivars and with no difference for cultivars homozygous for this gene. Cultivars not containing Lr34 included 87 genotypes, divided into four groups according to resistance and sus- ceptibility in field and greenhouse as follows. The first group of seven genotypes was susceptible in the seedling stage and resistant in the adult plant stage: N-87-16, C-86-6, M-86-9, S-87-8, WS-85-10, and MS- 84-16. These genotypes are crucial since they carry a gene or genes of adult plant stage resistance other than Lr34. Markers are needed to verify and identify the pres- ence of these genes. The second group included 57 genotypes resistant in both adult plant and seedling stages. This group con- tained race-specific resistance genes to the utilized genes. These genotypes may contain non-specific race resis- tance genes other than Lr34 that are masked by the effect of specific resistance genes. The third group included 17 cultivars which were sus- ceptible in both seedling and adult plant stages. This group lacked adult plant stage genes and effective spe- cific race genes to the applied isolate. In the fourth group, five cultivars were resistant or immune in the seedling stage but susceptible in the adult plant stage. This showed that these cultivars lacked adult plant stage resistance genes; however, they were influ- enced by pathogen races other than those present in the seedling stage in the field. At the time of collecting spores and testing them in greenhouse condition, this Copyright © 2013 SciRes. AJPS  Phenotypic and Molecular Characterization of Wheat Leaf Rust Resistance Gene Lr34 in Iranian Wheat Cultivars and Advanced Lines 1831 race did not exist or was just part of the field’s patho- genic population. The population or race which could in- fect these cultivars was not present in the population ga- thered and used in the greenhouse, or alternatively their frequency was low. Thus, they did not have the opportu- nity to appear under greenhouse conditions but could have greater effect in the field due to the longer time available. Cultivars carrying Lr34 were categorized into three groups, based on their reaction to leaf rust in field and greenhouse. Cultivars in the first group included 33 ge- notypes that were resistant in both seedling stage and adult plant stages, indicating that they contained effective race-specific genes other than Lr34. The second category included two groups. The first group contains delight cultivars susceptible in the seed- ling stage and semi-resistant (MR) or semi-susceptible (MS) in the adult plant stage. This is characteristic of Lr34 and these cultivars apparently carried only Lr34. The second group was “Aflak” and the line M-85-7. They were totally susceptible in the seedling stage and com- pletely resistant in the adult plant stage. It seems that these cultivars lack the effective race-specific resistance gene to the utilized isolate. However, their high resis- tance in field conditions indicated that they contained a gene or genes of adult plant stage resistance other than Lr34. This makes them unique and they require further investigation. 4. Discussion In the present study, in cultivars of Iranian origin Lr34 was only present in cultivars and lines linked with the very old 22-66-1 lines of ill-defined pedigree. Almost all other Iranian cultivars containing this gene originated from international germplasm, especially from CYMMIT. Lr34was also present in cultivars of international germ- plasm. Introducing cultivars of CYMMIT origin into Iran has increased the frequency of Lr34in Iranian cultivars. Lr34 has received much attention in recent years, since this gene is present in high frequency in CIMMYT bread wheat germplasm and derived cultivars with CIMMYT origin [29]. Examination of 123 local cultivars (landraces) of Iran by Kolmer et al. [18] showed that only three cultivars (2.4%) had Lr34. Also, in other parts of the world, the csLV34b allele did not exist in most local cultivars and had a low frequency, compared with the general fre- quency in improved wheat cultivars. The incongruity of csLV34b occurring among improved and local cultivars may be directly or indirectly caused by improvement trials to combine Lr34/Yr18 into new cultivars. Among international cultivars, those from CYMMIT showed high frequency (30%) of csLV34b. The mentioned result was verified by assessing the in- fection, analysis of molecular markers, and data gathered through pedigree for presence of Lr34. Of 130 cultivars, which had Lr34 according to pedigree, specific bands were produced in only 43. Due to the high sensitivity of this marker in detecting the Lr34 gene allele, having pure seeds from the desired cultivars, and not mixing with other cultivars are critical to providing reliable results. Studies have shown that due to probable mistakes in data in pedigrees, it is crucial to apply specific molecular mar- kers to confirm the presence of resistant genes against leaf rust in wheat cultivars. Many researchers have con- cluded that molecular markers are better for this predic- tion than pedigree data [30,31]. In the present study, cultivars selected and investigated in stress conditions had the highest percentage presence of Lr34 and it seems that this gene was effective in in- creasing the tolerance of cultivars in environmental stress conditions. All chosen lines and cultivars of the warm and humid climate in the north of Iran lacked Lr34, and these cultivars were selected in environmental conditions without stresses such as drought, heat, cold, and salinity. The warm and dry climate of the south of Iran, followed by lines bread wheat lines for cold climates, had the highest frequency of Lr34. Lr34 belongs to the super family of ABC transporters that produce proteins connected to the plasma membrane, which plays an important role in transferring materials in and out of the membrane. ABC transporters can transport a wide range of materials that can be cytotoxic, including ions, so that they transport macromolecules against the diffusion gradient on both sides of the cell membrane [32,33]. Drug transporters were primarily recognized in cancer cells which were resistant to drugs. These trans- porters carry the consumed drugs out of cancer cells and make the cells resistant to drugs. This mechanism was also discovered in drug-resistant fungi such that, in the resistant mutant fungi, gene expression or drug trans- porter genes and accordingly related proteins greatly in- creased. Consequently, by discharging more and lower- ing fungicide concentrations below the fatality threshold in fungal cells this causes resistance to fungicides. In ad- dition to fungicide disposal, drug transporters can pass mycotoxin discharge of other fungi, natural antimicrobial compounds of other organisms, and plant defense com- pounds out of the cell and cause resistance in fungi [34]. It seems that ABC transporters are one effective factor in resistance to salinity in plants. This system is probably active in cultivars resistant to salinity that contain ABC transporters, and extra salt ions are actively pumped out of the cells. As a result, transporters enable salinity tol- erance in various cultivars or help the process of identi- Copyright © 2013 SciRes. AJPS  Phenotypic and Molecular Characterization of Wheat Leaf Rust Resistance Gene Lr34 in Iranian Wheat Cultivars and Advanced Lines 1832 fying salinity ions and prevent them entering the cytosol. Accordingly, these cultivars are probably resistant to sa- linity. This hypothesis was also strengthened by assess- ing durum cultivars, which lack the D genome and are more susceptible to salinity than wheat cultivars. There- fore, Lr34—in addition to providing resistance to leaf rust, yellow rust, powdery mildew, and barley yellow dwarf virus in wheat—plays an important role in improving tolerance to environmental stresses such as salinity. Per- haps one mechanism of Lr34 in providing relative resis- tance to leaf rust agent pathogen is removing toxins, me- tabolites, or other harmful substances discharged by pa- thogens into host cells. As an example, virulent factors, which are discharged by haustoria of pathogens and are vital for aiding pathogen growth in the host cell, are pumped out of the cell by these transporters. This makes the pathogen grow slowly compared to hosts which lack this gene. Additionally, the phenotype of fewer and smaller pustules, and a longer latent period, would con- sequently appear since these substances lower the growth of the out -of-cell pathogen and reduce its density in the environment. This material is harmful to plant cells and its removal causes the plant tolerance to increase with no negative impact on plant physiological functions. Pump- ing harmful substances out of plant cells actually in- crease the plant’s tolerance to biotic and abiotic stresses. A more accurate assessment of the relationship between this gene and the tolerance to environmental stresses such as salinity needs a further and more complete inves- tigation. REFERENCES [1] J. Huerta-Espino, R. P. Singh, S. S. Germa´n, B. D. Mc- Callum, R. F. Park, W. Q. Chen, S. C. Bhardwaj and H. Goyeau, “Global Status of Wheat Leaf Rust Caused by Puccinia triticina,” Euphytica, Vol. 179, No. 1, 2011, pp. 143-160. doi:10.1007/s10681-011-0361-x [2] J. A. Kolmer, “Genetics of Resistance to Leaf Rust,” An- nual Review of Phytopathology, Vol. 34, 1996, pp. 435- 455. doi:10.1146/annurev.phyto.34.1.435 [3] M. Torabi, V. Mardoukhi, A. Froutan, M. Aliramaei, S. T. Dadrezaie, H. AkbariMoghaddam, S. Rajaei and H. Azi- mi, “Virulence Genes of Pucciniareconditaf. sp. tritici, the Causal Agent of Wheat Leaf Rust in Some Regions of Iran during 1995-1999,” Seed and Plant, Vol. 18, No. 4, 2003, pp. 432-449. [4] S. T. Dadrezaei, E. Mohammadi Goltapeh, F. Afshari and K. Nazari, “Identification of Pathotypes and Physiological Races of Puccinia triticina Eriks, the Casual Agent of Wheat Leaf Rust in the Iran in 2009-2010,” Seed and Plant Improvement Journal, Vol. 28, No. 4, 2012, pp. 685-715. [5] M. D. Bolton, J. A. Kolmer and D. F. Garvin, “Wheat Leaf Rust Caused by Puccinia triticina,” Molecular Plant Pathology, Vol. 9, No. 5, 2008, pp. 563-575. doi:10.1111/j.1364-3703.2008.00487.x [6] E. S. Lagudah, “Molecular Genetics of Race Non-Speci- fic Rust Resistance in Wheat,” BGRI 2010 Technical Workshop, St Petersburg, 30-31 May 2010. [7] S. G. Krattinger, E. S. Lagudah, W. Spielmeyer, R. P. Singh, J. Huerta-Espino and H. McFadden, “A Putative ABC Transporter Confers Durable Resistance to Multiple Fungal Pathogens in Wheat,” Science, Vol. 323, No. 5919, 2009, pp. 1360-1363. doi:10.1126/science.1166453 [8] R. P. Singh, A. Mujeebkazi and J. Huerta-Espino, “Lr46: A Gene Conferring Slow Rusting Resistance to Leaf Rust in Wheat,” Phytopathology, Vol. 88, No. 9, 1998, pp. 890-894. doi:10.1094/PHYTO.1998.88.9.890 [9] S. A. Herrera-Foessel, E. S. Lagudah, J. Huerta-Espino, M. J. Hayden, H. S. Bariana, D. Singh and R. P. Singh, “New Slow-Rusting Leaf Rust and Stripe Rust Resistance Genes Lr67 and Yr46 in Wheat Are Pleiotropic or Closely Linked,” Theoretical and Applied Genetics, Vol. 122, No. 1, 2011, pp. 239-249. doi:10.1007/s00122-010-1439-x [10] P. L. Dyck, D. J. Samborski and R. G. Anderson, “In- heritance of Adult-Plant Leaf Rust Resistance Derived from the Common Wheat Varieties Exchange and Fron- tana,” Canadian Journal of Genetics and Cytology, Vol. 8, 1966, pp. 665-671. [11] P. L. Dyck, “The Association of a Gene for Leaf Rust Resistance with the Chromosome 7D Suppressor of Stem Rust Resistance in Common Wheat,” Genome, Vol. 29, 1987, pp. 467-469. doi:10.1139/g87-081 [12] R. Johnson, “Practical Breeding for Durable Resistance to Rust Diseases in Self-Pollinating Cereals,” Euphytica, Vol. 27, No. 2, 1978, pp. 529-540. doi:10.1007/BF00043179 [13] R. Johnson, “The Concept of Durable Resistance,” Phy- topathology, Vol. 69, 1979, pp. 198-199. doi:10.1094/Phyto-69-198 [14] J. A. Kolmer, D. L. Long, E. Kosman and M. E. Hughes, “Physiologic Specialization of Puccinia triticina on Wheat in the United States in 2001,” Plant Disease, Vol. 87, No. 7, 2003, pp. 859-866. doi:10.1094/PDIS.2003.87.7.859 [15] R. P. Singh, “Association between Gene Lr34 for Leaf Rust Resistance and Leaf Tip Necrosis in Wheat,” Crop Science, Vol. 32, No. 4, 1992, pp. 874-878. doi:10.2135/cropsci1992.0011183X003200040008x [16] M. Lillemo, B. Asalf, R. P. Singh, J. Huerta-Espino, X. M. Chen, Z. H. He and Å. Bjørnstad, “The Adult Plant Rust Resistance Loci Lr34/Y r18 and Lr46/Yr29 Are Important Determinants of Partial Resistance to Powdery Mildew in Bread Wheat Line Saar,” Theoretical and Applied Genet- ics, Vol. 116, No. 8, 2008, pp. 1155-1166. doi:10.1007/s00122-008-0743-1 [17] W. Spielmeyer, R. A. McIntosh, J. Kolmer and E. S. Lagudah, “Powdery Mildew Resistance and Lr34/Yr18 Genes for Durable Resistance to Leaf and Stripe Rust Cosegregate at a Locus on the Short Arm of Chromosome 7D of Wheat,” Theoretical and Applied Genetics, Vol. 111, 2005, pp. 731-735. doi:10.1007/s00122-005-2058-9 Copyright © 2013 SciRes. AJPS  Phenotypic and Molecular Characterization of Wheat Leaf Rust Resistance Gene Lr34 in Iranian Wheat Cultivars and Advanced Lines Copyright © 2013 SciRes. AJPS 1833 [18] J. A. Kolmer, R. P. Singh, D. F. Garvin, L. Viccars, H. M. William, J. Huerta-Espino, F. C. Ogbonnaya, H. Raman, S. Orford, H. S. Bariana and E. S. Lagudah, “Analysis of the Lr34/Yr18 Rust Resistance Region in Wheat Germ- plasm,” Crop Science, Vol. 48, 2008, pp. 1841-1852. doi:10.2135/cropsci2007.08.0474 [19] T. Schnurbusch, S. Paillard, A. Schori, M. Messmer, G. Schachermayr, M. Winzeler and B. Keller, “Dissection of Quantitative and Durable Leaf Rust Resistance in Swiss Winter Wheat Reveals a Major Resistance QTL in the Lr34 Chromosomal Region,” Theoretical and Applied Genetics, Vol. 108, No. 3, 2004, pp. 477-484. doi:10.1007/s00122-003-1444-4 [20] K. Suenaga, R. P. Singh, J. Huerta-Espino and H. M. Wil- liam, “Microsatellite Markers for Genes Lr34/Yr18 and Other Quantitative Trait Loci for Leaf Rust and Stripe Rust Resistance in Bread Wheat,” Phytopathology, Vol. 93, No. 7, 2003, pp. 881-890. doi:10.1094/PHYTO.2003.93.7.881 [21] E. S. Lagudah, H. McFadden, R. P. Singh, J. Huerta- Espino, H. S. Bariana and W. Spielmeyer, “Molecular Genetics Characterization of the Lr34/Yr18 Slow Rusting Resistance Gene Region in Wheat,” Theoretical and Ap- plied Genetics, Vol. 114, No. 1, 2006, pp. 21-30. doi:10.1007/s00122-006-0406-z [22] R. A. McIntosh, C. R. Wellings and R. F. Park, “Wheat Rusts: An Atlas of Resistance Genes,” Kluwer Academic Publishers, Amsterdam, 1995. doi:10.1007/978-94-011-0083-0 [23] R. F. Peterson, A. B. Campbell and A. E. Hannah, “A Diagrammatic Scale for Estimating Rust Intensity of Leaves and Stems of Cereals,” Canadian Journal of Re- search, Vol. 26, 1948, pp. 496-500. doi:10.1139/cjr48c-033 [24] A. P. Rolefs, R. P. Singh and E. E. Saari, “Rust Disease of Wheat: Concepts and Methods of Disease Manage- ment,” CIMMYT, Mexico, 1992. [25] G. Shaner and R. E. Finney, “The Effect of Nitrogen Fer- tilization on the Expression of Slow-Mildewing Resis- tance in Knox Wheat,” Phytopathology, Vol. 67, 1977, pp. 1051-1056. doi:10.1094/Phyto-67-1051 [26] CIMMYT, “Laboratory Protocols: CIMMYT Applied Molecular Genetics Laboratory,” 3rd Edition, CIMMYT, Mexico, 2005. [27] M. Vahabzadeh, E. MajidiHeravan, H. Haj AkhoundMey- bodi, M. T. Tabatabaei, R. Bozorgipour, F. Bakhtiar, A. Akbari, A. Pakdel, M. Sharif Alahosseini, D. Afyouni, H. Rostami, H. Azarmjou, S. H. Kouhkan, G. H. R. Amiri- JebalBarez, M. H. Saberi, H. Binabaji, A. Ghandi, S. Ba- hraei, M. Torabi, K. Nazari and B. Pirayeshfar, “Bam, a New Bread Wheat Cultivar for Moderate Zones with Sa- linity of Soil and Water (Cultivar Release),” Seed and Plant Improvement Journal, Vol. 25, No. 1, 2009, pp. 223-226. [28] J. A. Kolmer, “Tracking Wheat Rust on a Continental Scale,” Current Opinion in Plant Biology, Vol. 8, No. 4, 2005, pp. 441-449. doi:10.1016/j.pbi.2005.05.001 [29] R. P. Singh, J. Huerta-Espino, S. Bhavani, S. A. Herrera- Foessel, D. Singh, P. K. Singh, G. Velu, R. E. Mason, Y. Jin, P. Njau and J. Crossa, “Race Non-Specific Resistance to Rust Diseases in CIMMYT Spring Wheats,” Euphytica, Vol. 179, No. 1, 2011, pp. 175-186. doi:10.1007/s10681-010-0322-9 [30] L. Stępień, L. Golka and J. Chelkowski, “Leaf Rust Re- sistance Genes of Wheat: Identification in Cultivars and Resistance Sources,” Journal of Applied Genetics, Vol. 44, No. 2, 2003, pp. 139-149. [31] L. Błaszczyk, I. Krämer, F. Ordon, J. Chełkowski, M. Tyrka and G. Vida, “Validity of Selected DNA Markers for Breeding Leaf Rust Resistant Wheat,” Cereal Re- search Communications, Vol. 36, No. 2, 2008, pp. 201- 213. doi:10.1556/CRC.36.2008.2.1 [32] B. E. Bauer, H. Wolfger and K. Kuchler, “Inventory and Function of Yeast ABC Proteins: About Sex, Stress, Plei- otropic Drug and Heavy Metal Resistance,” Biochimicaet Biophysica Acta, Vol. 1461, 1999, pp. 217-236. [33] F. L. Theodoulou, “Plant ABC Transporters,” Biochimi- caet Biophysica Acta, Vol. 1465, No. 1-2, 2000, pp. 79- 103. [34] M. A. De Waard, A. C. Andrade, K. Hayashi, H. Schoon- beek, I. Stergiopoulos and L. H. Zwiers, “Impact of Fun- gal Drug Transporters on Fungicide Sensitivity, Mul- tidrug Resistance and Virulence,” Pest Management Sci- ence, Vol. 62, No. 3, 2006, pp. 195-207. doi:10.1002/ps.1150
|