 Advances in Chemical Engineering and Science, 2013, 3, 10-16 http://dx.doi.org/10.4236/aces.2013.33A3004 Published Online August 2013 (http://www.scirp.org/journal/aces) Improved Hemolytic Performance of Blood Pump with Fluorine-Doped Hydr ogenated Amorphous Carbon Coating Yasuharu Ohgoe1*, Masanori Hiratsuka2, Hirohito Sumikura3, Kazuyoshi Fukunaga4, Akihiko Homma1, Kenji K. Hirakuri5, Akio Funakubo1, Yasuhiro Fukui1 1Division of Electronic and Mechanical Engineering, Tokyo Denki University, Saitama, Japan 2Nanotec Corporation, Nanotec Corporation, Chiba, Japan 3Department of Artificial Organs, National Cerebral and Cardiovascular Center Research Institute, Osaka, Japan 4Department of Clinical Engineering, Faculty of Health Sciences, Kyorin University, Tokyo, Japan 5Department of Electrical and Electronic Engineering, Tokyo Denki University, Tokyo, Japan Email: *yasuharu@mail.dendai.ac.jp Received May 10, 2013; revised June 10, 2013; accepted July 10, 2013 Copyright © 2013 Yasuharu Ohgoe et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Fluorine-doped hydrogenated amorphous carbon (a-C:H:F) film was deposited on a flow-straightener, impeller and dif- fuser surface (SUS 304) of an enclosed-impeller type flow blood pump using the ionization deposition method with a source gas of C6F5H. The surface characteristics of the a-C:H:F film were examined using atomic force microscopy, X-ray photoelectron spectroscopy, and measurements of surface roughness, friction and surface potential. The a-C:H:F film tends to increase surface roughness and the negative surface charge. In addition, the surface energy and friction decrease with fluorine dopant in the a-C:H film. To estimate the hemolytic performance of a blood pump with the a-C:H:F film coating, the amount of hemolysis was measured using a mock circulatory system (in vitro test) with 500 mL of pig blood containing sodium citrate. In vitro test was conducted for 180 min with the blood flow and pump head maintained at 5 L/min and 100 mmHg, respectively. The a-C:H:F film coating reduced the amount of hemolysis and improved the hemolytic performance. Decreasing the surface energy and negative surface charge of the a-C:H:F film contributes to the improvement of the hemolytic performance. The a-C:H:F film coating is thus expected to be utilized in medical technology as a surface coating technology for artificial heart blood pumps. Keywords: Fluorine-Doped a-C:H Film; Hemolytic Performance; Artificial Heart Blood Pump 1. Introduction A ventricular assist device (VAD) is an important in- strument in treating heart failure. With the development of heart surgery, VADs have been used as a bri- dge-to-transplant or destination therapy [1-3]. The VAD provides effective blood circulation support until a donor heart becomes available for transplant, improves other organ function, improves exercise performance, and en- ables participation in cardiac rehabilitation [4]. In recent years, the VAD has also been considered for long-term implantation as a destination therapy. The circulatory support can provide symptomatic relief and improved survival for those who do not have access to cardiac transplantation [5-7]. A VAD generally consists of a pump unit that is either implanted in the abdomen or is outside the body, a control system and an energy supply. VADs can be divided into two main categories: dis- placement pumps and rotary pumps [1,8,9]. Energy transfer in displacement pumps is characterized by peri- odic changes of the working space as a pulsation, whereas in rotary pumps, the energy transfer to the fluid is established by velocity changes within the impeller vanes. Rotary pumps are designed to work in a constant speed mode of mechanical circulatory support, thus, in principle, produce non-pulsatile flow. Compared with displacement pumps, the rotary type pumps have advan- tages of compactness, no valves, simpler control aspects, lower power consumption, lower relative cost, and a more simplistic design, which results in smaller size and increased reliability [2,8,10]. However, the fluid dynamic forces in centrifugal blood pump impellers contribute to the destruction of red blood cells because the rotational speed leads to harsh interactions between the impeller and red blood cells [11-13]. Thus, hemolysis is caused by *Corresponding author. C opyright © 2013 SciRes. ACES 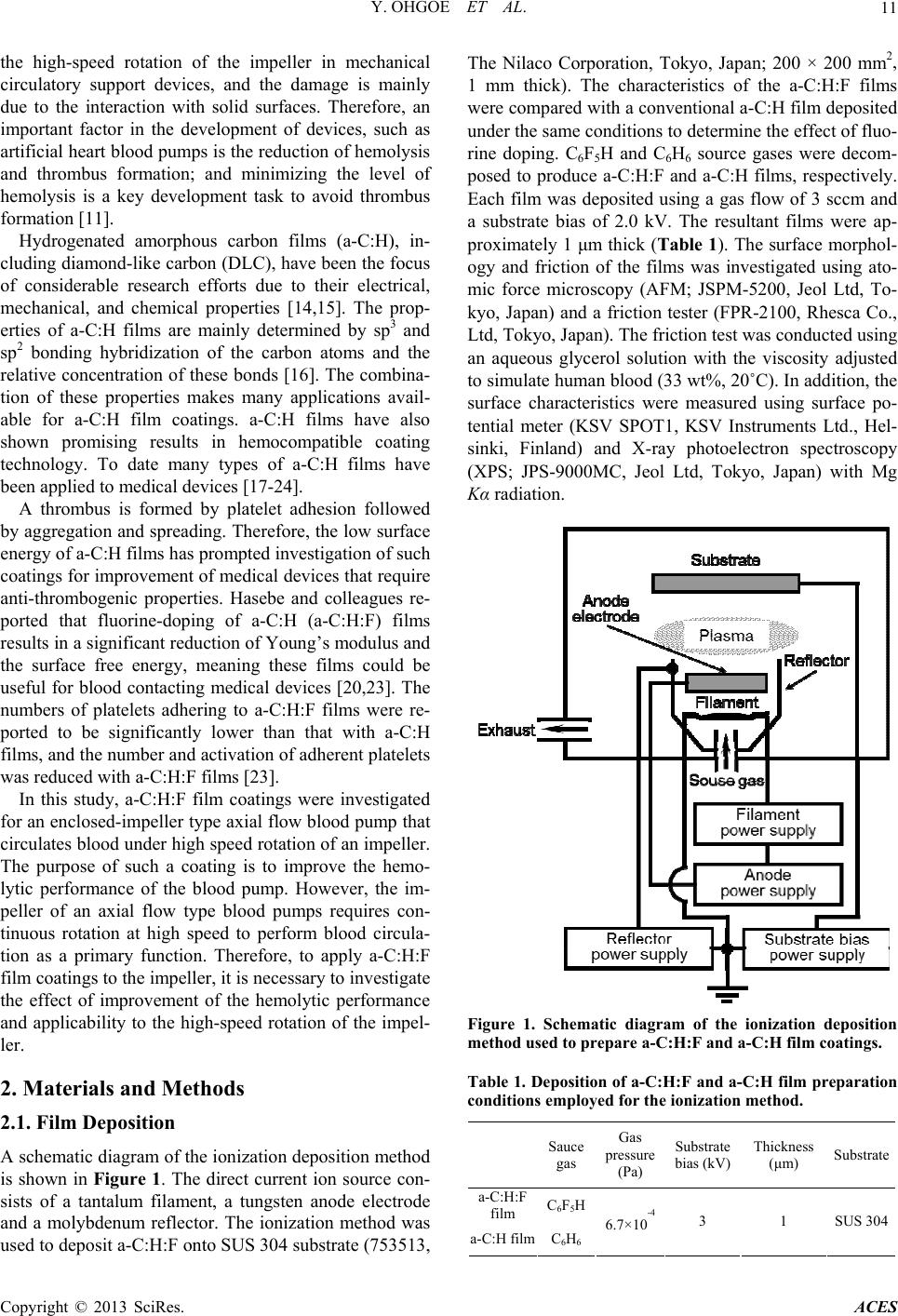 Y. OHGOE ET AL. 11 the high-speed rotation of the impeller in mechanical circulatory support devices, and the damage is mainly due to the interaction with solid surfaces. Therefore, an important factor in the development of devices, such as artificial heart blood pumps is the reduction of hemolysis and thrombus formation; and minimizing the level of hemolysis is a key development task to avoid thrombus formation [11]. Hydrogenated amorphous carbon films (a-C:H), in- cluding diamond-like carbon (DLC), have been the focus of considerable research efforts due to their electrical, mechanical, and chemical properties [14,15]. The prop- erties of a-C:H films are mainly determined by sp3 and sp2 bonding hybridization of the carbon atoms and the relative concentration of these bonds [16]. The combina- tion of these properties makes many applications avail- able for a-C:H film coatings. a-C:H films have also shown promising results in hemocompatible coating technology. To date many types of a-C:H films have been applied to medical devices [17-24]. A thrombus is formed by platelet adhesion followed by aggregation and spreading. Therefore, the low surface energy of a-C:H films has prompted investigation of such coatings for improvement of medical devices that require anti-thrombogenic properties. Hasebe and colleagues re- ported that fluorine-doping of a-C:H (a-C:H:F) films results in a significant reduction of Young’s modulus and the surface free energy, meaning these films could be useful for blood contacting medical devices [20,23]. The numbers of platelets adhering to a-C:H:F films were re- ported to be significantly lower than that with a-C:H films, and the number and activation of adherent platelets was reduced with a-C:H:F films [23]. In this study, a-C:H:F film coatings were investigated for an enclosed-impeller type axial flow blood pump that circulates blood under high speed rotation of an impeller. The purpose of such a coating is to improve the hemo- lytic performance of the blood pump. However, the im- peller of an axial flow type blood pumps requires con- tinuous rotation at high speed to perform blood circula- tion as a primary function. Therefore, to apply a-C:H:F film coatings to the impeller, it is necessary to investigate the effect of improvement of the hemolytic performance and applicability to the high-speed rotation of the impel- ler. 2. Materials and Methods 2.1. Film Deposition A schematic diagram of the ionization deposition method is shown in Figure 1. The direct current ion source con- sists of a tantalum filament, a tungsten anode electrode and a molybdenum reflector. The ionization method was used to deposit a-C:H:F onto SUS 304 substrate (753513, The Nilaco Corporation, Tokyo, Japan; 200 × 200 mm2, 1 mm thick). The characteristics of the a-C:H:F films were compared with a conventional a-C:H film deposited under the same conditions to determine the effect of fluo- rine doping. C6F5H and C6H6 source gases were decom- posed to produce a-C:H:F and a-C:H films, respectively. Each film was deposited using a gas flow of 3 sccm and a substrate bias of 2.0 kV. The resultant films were ap- proximately 1 μm thick (Table 1). The surface morphol- ogy and friction of the films was investigated using ato- mic force microscopy (AFM; JSPM-5200, Jeol Ltd, To- kyo, Japan) and a friction tester (FPR-2100, Rhesca Co., Ltd, Tokyo, Japan). The friction test was conducted using an aqueous glycerol solution with the viscosity adjusted to simulate human blood (33 wt%, 20˚C). In addition, the surface characteristics were measured using surface po- tential meter (KSV SPOT1, KSV Instruments Ltd., Hel- sinki, Finland) and X-ray photoelectron spectroscopy (XPS; JPS-9000MC, Jeol Ltd, Tokyo, Japan) with Mg Kα radiation. Figure 1. Schematic diagram of the ionization deposition method used to prepare a-C:H:F and a-C:H film coatings. Table 1. Deposition of a-C:H:F and a-C:H film preparation conditions employed for the ionization method. Sauce gas Gas pressure (Pa) Substrate bias (kV) Thickness (μm) Substrate a-C:H:F film C6F5H a-C:H filmC6H6 6.7×10 -4 3 1 SUS 304 Copyright © 2013 SciRes. ACES 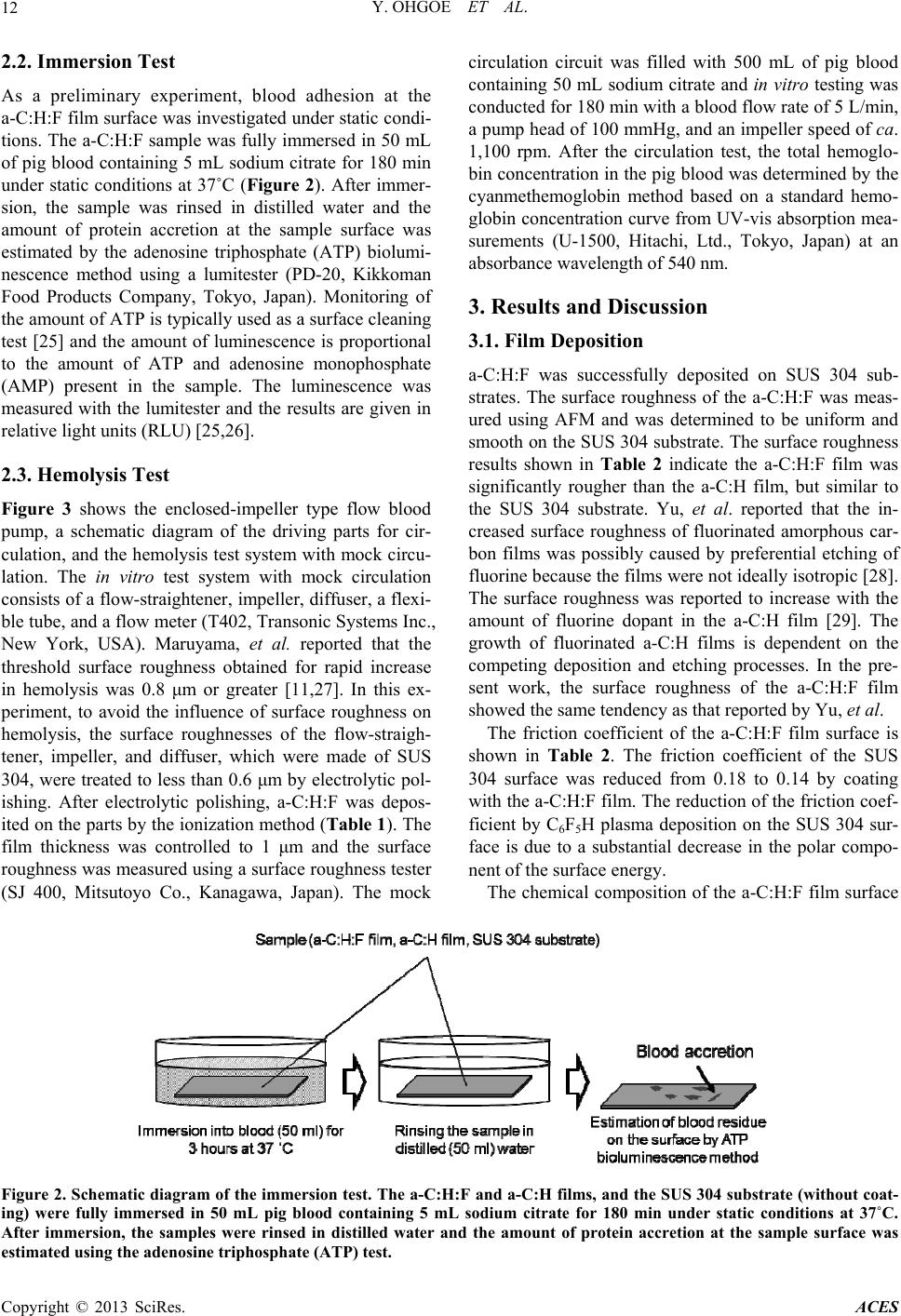 Y. OHGOE ET AL. Copyright © 2013 SciRes. ACES 12 2.2. Immersion Test circulation circuit was filled with 500 mL of pig blood containing 50 mL sodium citrate and in vitro testing was conducted for 180 min with a blood flow rate of 5 L/min, a pump head of 100 mmHg, and an impeller speed of ca. 1,100 rpm. After the circulation test, the total hemoglo- bin concentration in the pig blood was determined by the cyanmethemoglobin method based on a standard hemo- globin concentration curve from UV-vis absorption mea- surements (U-1500, Hitachi, Ltd., Tokyo, Japan) at an absorbance wavelength of 540 nm. As a preliminary experiment, blood adhesion at the a-C:H:F film surface was investigated under static condi- tions. The a-C:H:F sample was fully immersed in 50 mL of pig blood containing 5 mL sodium citrate for 180 min under static conditions at 37˚C (Figure 2). After immer- sion, the sample was rinsed in distilled water and the amount of protein accretion at the sample surface was estimated by the adenosine triphosphate (ATP) biolumi- nescence method using a lumitester (PD-20, Kikkoman Food Products Company, Tokyo, Japan). Monitoring of the amount of ATP is typically used as a surface cleaning test [25] and the amount of luminescence is proportional to the amount of ATP and adenosine monophosphate (AMP) present in the sample. The luminescence was measured with the lumitester and the results are given in relative light units (RLU) [25,26]. 3. Results and Discussion 3.1. Film Deposition a-C:H:F was successfully deposited on SUS 304 sub- strates. The surface roughness of the a-C:H:F was meas- ured using AFM and was determined to be uniform and smooth on the SUS 304 substrate. The surface roughness results shown in Table 2 indicate the a-C:H:F film was significantly rougher than the a-C:H film, but similar to the SUS 304 substrate. Yu, et al. reported that the in- creased surface roughness of fluorinated amorphous car- bon films was possibly caused by preferential etching of fluorine because the films were not ideally isotropic [28]. The surface roughness was reported to increase with the amount of fluorine dopant in the a-C:H film [29]. The growth of fluorinated a-C:H films is dependent on the competing deposition and etching processes. In the pre- sent work, the surface roughness of the a-C:H:F film showed the same tendency as that reported by Yu, et al. 2.3. Hemolysis Test Figure 3 shows the enclosed-impeller type flow blood pump, a schematic diagram of the driving parts for cir- culation, and the hemolysis test system with mock circu- lation. The in vitro test system with mock circulation consists of a flow-straightener, impeller, diffuser, a flexi- ble tube, and a flow meter (T402, Transonic Systems Inc., New York, USA). Maruyama, et al. reported that the threshold surface roughness obtained for rapid increase in hemolysis was 0.8 μm or greater [11,27]. In this ex- periment, to avoid the influence of surface roughness on hemolysis, the surface roughnesses of the flow-straigh- tener, impeller, and diffuser, which were made of SUS 304, were treated to less than 0.6 μm by electrolytic pol- ishing. After electrolytic polishing, a-C:H:F was depos- ited on the parts by the ionization method (Table 1). The film thickness was controlled to 1 μm and the surface roughness was measured using a surface roughness tester (SJ 400, Mitsutoyo Co., Kanagawa, Japan). The mock The friction coefficient of the a-C:H:F film surface is shown in Table 2. The friction coefficient of the SUS 304 surface was reduced from 0.18 to 0.14 by coating with the a-C:H:F film. The reduction of the friction coef- ficient by C6F5H plasma deposition on the SUS 304 sur- face is due to a substantial decrease in the polar compo- nent of the surface energy. The chemical composition of the a-C:H:F film surface Figure 2. Schematic diagram of the immersion test. The a-C:H:F and a-C:H films, and the SUS 304 substrate (without coat- ing) were fully immersed in 50 mL pig blood containing 5 mL sodium citrate for 180 min under static conditions at 37˚C. After immersion, the samples were rinsed in distilled water and the amount of protein accretion at the sample surface was estimated using the adenosine triphosphate (ATP) test. 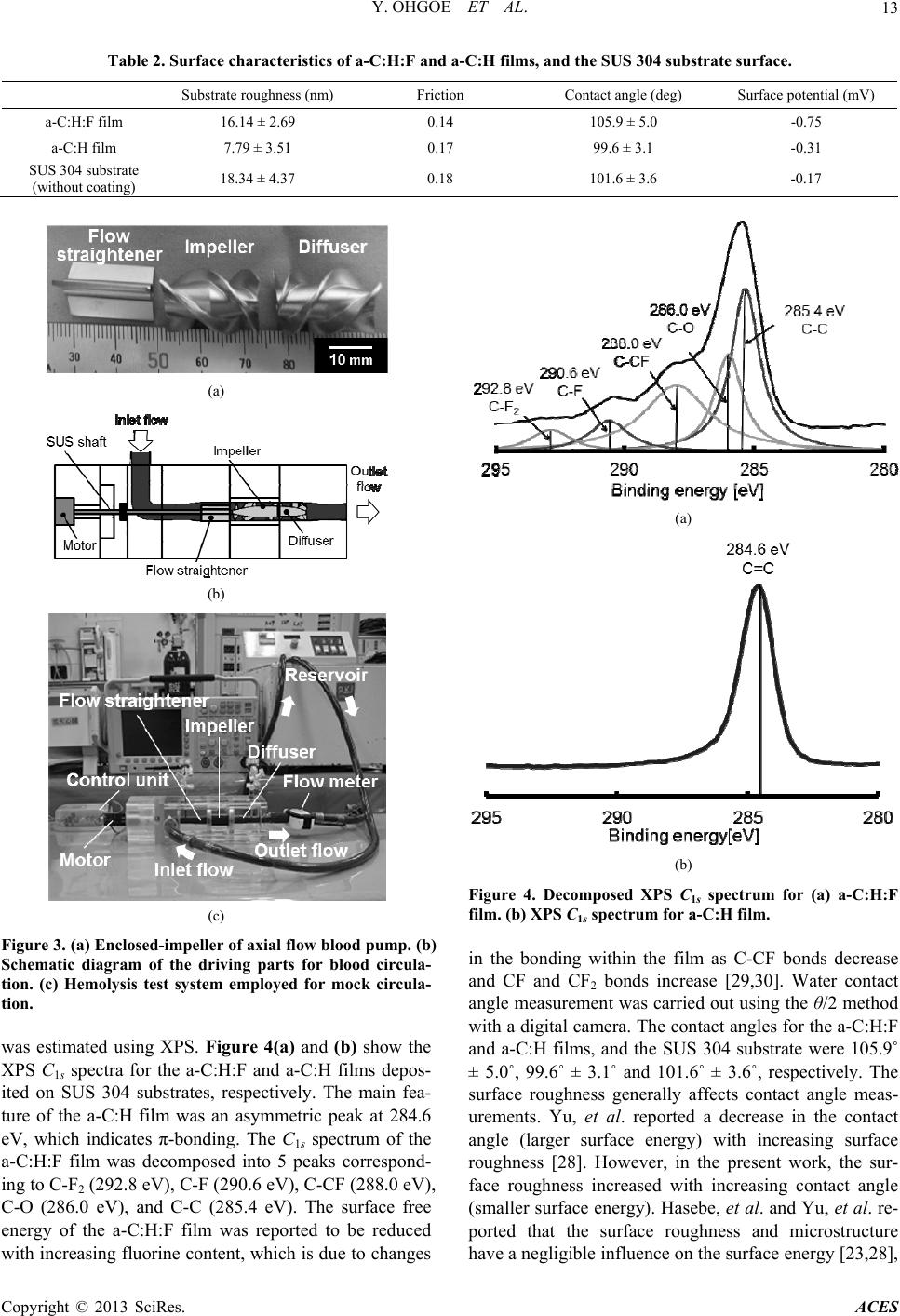 Y. OHGOE ET AL. 13 Table 2. Surface characteristics of a-C:H:F and a-C:H films, and the SUS 304 substrate surface. Substrate roughness (nm) Friction Contact angle (deg) Surface potential (mV) a-C:H:F film 16.14 ± 2.69 0.14 105.9 ± 5.0 -0.75 a-C:H film 7.79 ± 3.51 0.17 99.6 ± 3.1 -0.31 SUS 304 substrate (without coating) 18.34 ± 4.37 0.18 101.6 ± 3.6 -0.17 (a) (a) (b) (b) Figure 4. Decomposed XPSs spectrum for (a) a-C:H:F the bonding within the film as C-CF bonds decrease (c) Figure 3. (a) Enclosed-impeller of axial flow blood pump. (b) Schematic diagram of the driving parts for blood circula- tion. (c) Hemolysis test system employed for mock circula- tion. was estimated using XPS. Figure 4(a) and (b) show the XPS C1s spectra for the a-C:H:F and a-C:H films depos- ited on SUS 304 substrates, respectively. The main fea- ture of the a-C:H film was an asymmetric peak at 284.6 eV, which indicates π-bonding. The C1s spectrum of the a-C:H:F film was decomposed into 5 peaks correspond- ing to C-F2 (292.8 eV), C-F (290.6 eV), C-CF (288.0 eV), C-O (286.0 eV), and C-C (285.4 eV). The surface free energy of the a-C:H:F film was reported to be reduced C 1 film. (b) XPS C1s spectrum for a-C:H film. in and CF and CF2 bonds increase [29,30]. Water contact angle measurement was carried out using the θ/2 method with a digital camera. The contact angles for the a-C:H:F and a-C:H films, and the SUS 304 substrate were 105.9˚ ± 5.0˚, 99.6˚ ± 3.1˚ and 101.6˚ ± 3.6˚, respectively. The surface roughness generally affects contact angle meas- urements. Yu, et al. reported a decrease in the contact angle (larger surface energy) with increasing surface roughness [28]. However, in the present work, the sur- face roughness increased with increasing contact angle (smaller surface energy). Hasebe, et al. and Yu, et al. re- ported that the surface roughness and microstructure have a negligible influence on the surface energy [23,28], with increasing fluorine content, which is due to changes Copyright © 2013 SciRes. ACES  Y. OHGOE ET AL. 14 which is consistent with the present results. Fluorine is a termination radical in C-C networks and co 3.2. Immersion Test TP bioluminescence for protein 3.3. Hemolysis Test sited on the surface of the flow- nsequently decreases cross-linking, which leads to new, more open structural arrangements that can lead to a de- crease in the density of a film [29-31]. The addition of fluorine also results in a decrease in the hardness and stress of a film [29,30]. These results indicate that a-C:H:F film becomes more graphitic with increasing fluorine concen- tration [28]. In addition, fluorine atoms are very electro- negative and so will carry some degree of negative charge. The surface of the a-C:H:F film was increased to more negative potentials, as shown in Table 2. Figure 5 shows the A accretion on the a-C:H:F and a-C:H films, and on the SUS 304 substrate after the immersion test. The surface of a-C:H:F film had a low luminescence level, and thus a low ATP concentration, which indicates that the a-C:H:F film tends to weaken the protein adhesion of blood com- pared with the other samples is due to negative charge polarity. The high electronegativity of fluorine gives car- bon-fluorine bonds a significant polarity or dipole mo- ment. Therefore, it was expected that the van der Waals interaction of the pig blood with the a-C:H:F surface is reduced by the carbon-fluorine bonds. Platelet adhesion and activation on the surface of a biomaterial is the most important factor in determining the hemocompatibility of a biomaterial, and low platelet adhesion denotes good hemocompatibility, while a higher degree of platelet ad- hesion tends to result in the formation of a thrombus [20]. a-C:H:F film was depo Figure 5. ATP bioluminescence results for the a-C:H:F and a-C:H films, and the SUS 304 substrate. effect of a-C:H:F film coating on the ance of a blood pump was investigated. straightener, enclosed-impeller, and the diffuser. The surface roughness of these parts was 0.48 μm, while that for the SUS 304 substrate and the a-C:H film were 0.47 and 0.46 μm, respectively (Table 3). Therefore, it was expected that there was no influence of surface rough- ness on the hemolytic performance. The hemolytic per- formance of a blood pump with a-C:H:F film coating was determined according to the amount of hemolysis meas- ured for a mock circulatory system (in vitro tests). Fig- ure 6 shows the amount of free hemoglobin in the blood that was released in a hemolysis reaction for the blood pump with a-C:H:F and a-C:H film coatings, and the SUS 304 without a coating. The rotational speed of the impeller in the blood pump was around 11,000 rpm. The amount of hemolysis was reduced from 1110 mg/dL to 925 mg/dL with the a-C:H:F film coating. There was a significant difference between the a-C:H:F film coating and the SUS 304 surface without a coating (P < 0.05). According to the ATP bioluminescence of the a-C:H:F film surface, it was expected that the amount of hemoly- sis was reduced by 20% with the decrease in the surface energy of the blood contact surface. In addition, the a-C:H:F film had good stability during the in vitro test. The negatively charged polarity suppresses platelet adhesion and fibrinogen adsorption as the platelets and proteins tend to have a net negative zeta potential [20,23]. In this study, the a-C:H:F film surface resulted in a higher degree of negative charge polarity. Roy, et al. [20] studied the effect of fluorine incorporation into a-C:H films on hemocompatibility. They reported that platelet adhesion was suppressed as the fluorine concentration increased and the surface charge became more negative. This observation is closely related with the C-F2, C-F, and C-CF surface bonds, because the negative charge of the surface increased with the fluorine concentration [20,29]. Yu, et al. also reported that the polar component of the surface energy was reduced with increasing fluo- rine concentration [28]. Thus, it is possible to have higher surface polarization in films with higher fluorine concentration. Their observations support the present suggestion that the negatively charged polar component caused by C-F2, C-F and C-CF surface bonds improves the hemolytic performance under dynamic high-speed rotation of the impeller. 4. Conclusion In this study, the hemolytic perform The results indicate that the a-C:H:F film tends to in- crease the surface roughness and negative charge of the surface. In addition, the surface friction is decreased with respect to fluorine doping in the a-C:H film. In in vitro test, it has been indicated that fluorine in the a-C:H film Copyright © 2013 SciRes. ACES 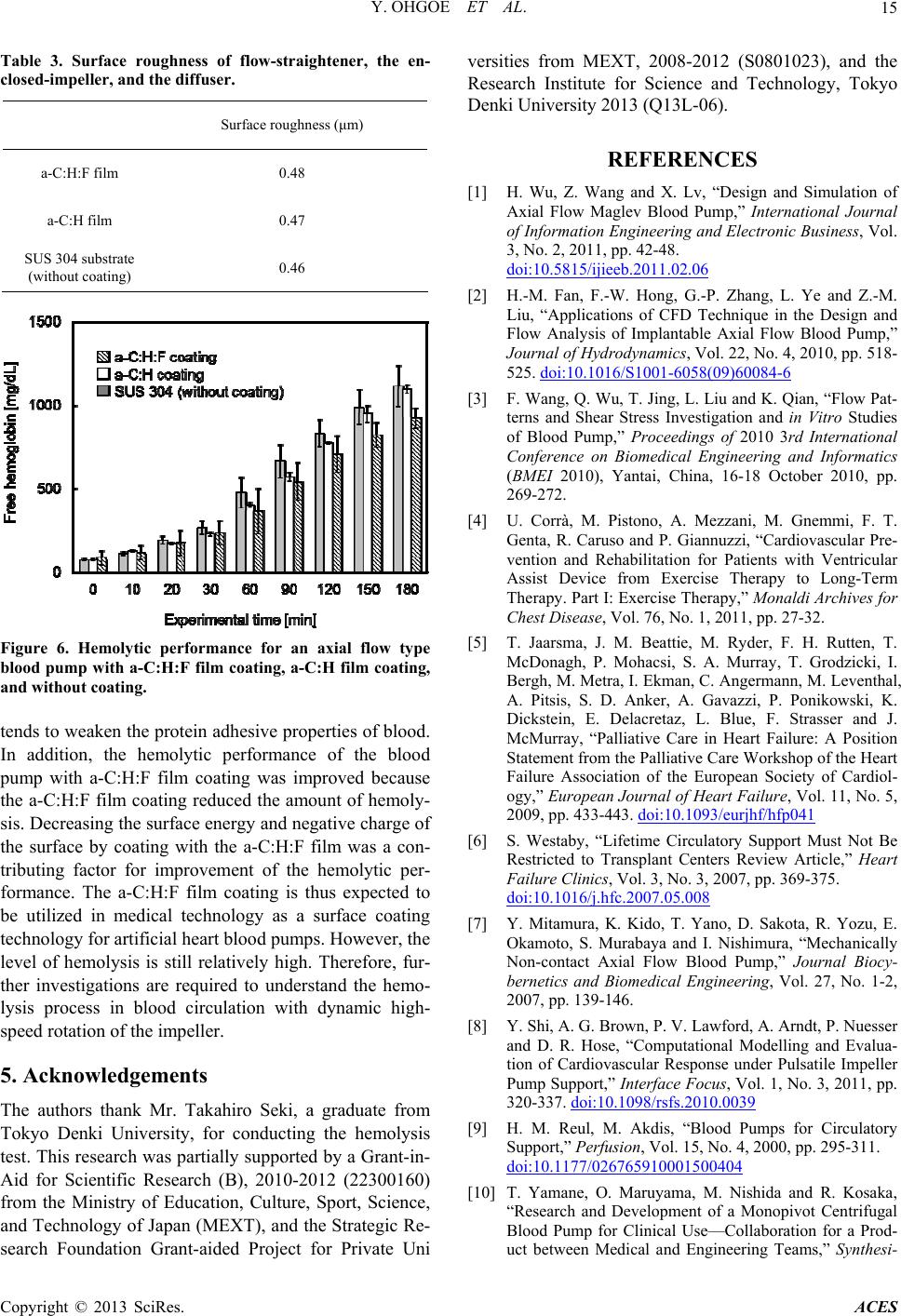 Y. OHGOE ET AL. 15 Table 3. Surface roughness of flow-straightener, the en- closed-impeller, and the diffuser. Surface roughness (μm) a-C:H:F film 0.48 a-C:H film 0.47 SUS 304 substrate (wg) ithout coatin0.46 Figure 6. Hemolytic performance for an axial flow type blood pump with a-C:H:F film coating, a-C:H film coating and without coating. molytic performance of the blood hiro Seki, a graduate r conducting the hemolysis [1] H. Wu, Z. Wang and X. Lv, “Design and Simulation of Axial Flow Mag of Informationonic Business, Vol. , tends to weaken the protein adhesive properties of blood. addition, the heIn pump with a-C:H:F film coating was improved because the a-C:H:F film coating reduced the amount of hemoly- sis. Decreasing the surface energy and negative charge of the surface by coating with the a-C:H:F film was a con- tributing factor for improvement of the hemolytic per- formance. The a-C:H:F film coating is thus expected to be utilized in medical technology as a surface coating technology for artificial heart blood pumps. However, the level of hemolysis is still relatively high. Therefore, fur- ther investigations are required to understand the hemo- lysis process in blood circulation with dynamic high- speed rotation of the impeller. 5. Acknowledgements The authors thank Mr. Taka Tokyo Denki University, fo from test. This research was partially supported by a Grant-in- Aid for Scientific Research (B), 2010-2012 (22300160) from the Ministry of Education, Culture, Sport, Science, and Technology of Japan (MEXT), and the Strategic Re- search Foundation Grant-aided Project for Private Uni versities from MEXT, 2008-2012 (S0801023), and the Research Institute for Science and Technology, Tokyo Denki University 2013 (Q13L-06). REFERENCES lev Blood Pump,” International Journal Engineering and Electr 3, No. 2, 2011, pp. 42-48. doi:10.5815/ijieeb.2011.02.06 [2] H.-M. Fan, F.-W. Hong, G.-P. Zhang, L. Ye and Z.-M. Liu, “Applications of CFD Technique in the Design and Flow Analysis of Implantable Axial Flow Blood Pump,” Journal of Hydrodynamics, Vol. 22, No. 4, 2010, pp. 518- 525. doi:10.1016/S1001-6058(09)60084-6 [3] F. Wang, Q. Wu, T. Jing, L. Liu and K. Qian, “Flow Pat- terns and Shear Stress Investigation and in Vitro Studies of Blood Pump,” Proceedings of 2010 3rd International Conference on Biomedical Engineering and Informatics (BMEI 2010), Yantai, China, 16-18 October 2010, pp. 269-272. [4] U. Corrà, M. Pistono, A. Mezzani, M. Gnemmi, F. T. Genta, R. Caruso and P. Giannuzzi, “Cardiovascular Pre- vention and Rehabilitation for Patients with Ventricular Assist Device from Exercise Therapy to Long-Term Therapy. Part I: Exercise Therapy,” Monaldi Archives for Chest Disease, Vol. 76, No. 1, 2011, pp. 27-32. [5] T. Jaarsma, J. M. Beattie, M. Ryder, F. H. Rutten, T. McDonagh, P. Mohacsi, S. A. Murray, T. Grodzicki, I. Bergh, M. Metra, I. Ekman, C. Angermann, M. Leventhal, A. Pitsis, S. D. Anker, A. Gavazzi, P. Ponikowski, K. Dickstein, E. Delacretaz, L. Blue, F. Strasser and J. McMurray, “Palliative Care in Heart Failure: A Position Statement from the Palliative Care Workshop of the Heart Failure Association of the European Society of Cardiol- ogy,” European Journal of Heart Failure, Vol. 11, No. 5, 2009, pp. 433-443. doi:10.1093/eurjhf/hfp041 [6] S. Westaby, “Lifetime Circulatory Support Must Not Be Restricted to Transplant Centers Review Article,” Heart Failure Clinics, Vol. 3, No. 3, 2007, pp. 369-375. doi:10.1016/j.hfc.2007.05.008 [7] Y. Mitamura, K. Kido, T. Yano, D. Sakota, R. Yozu, E. Okamoto, S. Murabaya and I. Nishimura, “Mechanically Non-contact Axial Flow Blood Pump,” Journal Biocy- bernetics and Biomedical Engineering, Vol. 27, No. 1-2, 2007, pp. 139-146. [8] Y. Shi, A. G. Brown, P. V. Lawford, A. Arndt, P. Nuesser and D. R. Hose, “Computational Modelling and Evalua- tion of Cardiovascular Response under Pulsatile Impeller Pump Support,” Interface Focus, Vol. 1, No. 3, 2011, pp. 320-337. doi:10.1098/rsfs.2010.0039 [9] H. M. Reul, M. Akdis, “Blood Pumps for Circulatory Support,” Perfusion, Vol. 15, No. 4, 2000, pp. 295-311. doi:10.1177/026765910001500404 [10] T. Yamane, O. Maruyama, M. Nishida and R. Kosaka, “Research and Development of a Monopivot Centrifugal Blood Pump for Clinical Use—Collaboration for a Prod- uct between Medical and Engineering Teams,” Synthesi- Copyright © 2013 SciRes. ACES  Y. OHGOE ET AL. Copyright © 2013 SciRes. ACES 16 ology, Vol. 5, No. 1, 2012, pp. 17-24. doi:10.5571/synth.5.16 [11] O. Maruyama, M. Nishida, T. Yamane, I. Oshima, Y. Adachi and T. Masuzawa, “Hemolysis Resulting from Surface Roughness Under Shear Flow C Rotational Shear Stresso onditions Using r,” Artificial Organs, Vol. 30, No a . 5, 2006, pp. 365-370. doi:10.1111/j.1525-1594.2006.00227.x [12] W. K. Chan, Y. W. Wong, Y. Ding, L. P. Chua and S. C. Yu, “Numerical Investigation of the Effect of Blade Ge- ometry on Blood Trauma in a Centrifug Artificial Organs, Vol. 26, No. 9, 2002, p al Blood Pump,” p. 785-793. doi:10.1046/j.1525-1594.2002.06954.x [13] L. B. Leverett, J. D. Hellums, C. P. Alfrey and E. C. Lynch, “Red Blood Cell Damage by Shear Stress,” Bio- physical Journal, Vol. 12, No. 3, 1972, pp. 257-273. doi:10.1016/S0006-3495(72)86085-5 [14] W. J. Ma, A. J. Ruys, R. S. Mason, P. J. Martin, A. Ben- david, Z. Liu, M. Ionescu and H. Zreiqat, “DLC Coatings: Effects of Physical and Chemical Properties on Biologi Response,” Biomaterials, Vol. 28, No. cal 9, 2007, pp. 1620- 1628. doi:10.1016/j.biomaterials.2006.12.010 [15] Y. Cheng and Y. F. Zheng, “The Corrosion Behavior and Hemocompatibility of TiNi Alloys Coated with DLC by Plasma Based Ion Implantation,” Surface and Coatings Technology, Vol. 200, No. 14-15, 2006, pp. 4543-4548. doi:10.1016/j.surfcoat.2005.03.039 [16] J. Robertson, “Diamond-Like Amorphous Carbon,” Ma- terials Science and Engineering R, Vol. 37, No. 4-6, 2002, 129-281. doi:10.1016/S0927-796X(02)00005-0 [17] M. Jelínek, T. Kocourek, J. Remsa, J. Mikšovský, J. Ze- mek, K. Smetana Jr., B. Dvoˇránková and T. Luxbacher, “Diamond/Graphite Content and Biocompatibility of DLC Films Fabricated by PLD,” Applied Physics A, Vol. 101, No. 4, 2010, pp. 579-583. doi:10.1007/s00339-010-5912-9 [18] J. M. Lackner and W. Waldhauser, “Diamond and Dia- mond-Like Carbon Coated Surfaces as Biomaterials,” BHM, Vol. 155, No. 11, 2010, pp. 528-533. 9, 2009, pp. [19] R. K. Roy, Sk. F. Ahmed, J. W. Yi, M.-W. Moon, K.-R. Lee and Y. Jun, “Improvement of Adhesion of DLC Coating on Nitinol Substrate by Hybrid Ion Beam Depo- sition Technique,” Vacuum, Vol. 83, No. 1179-1183. doi:10.1016/j.vacuum.2009.03.005 [20] R. K. Roy, H. W. Choi, J. W. Yi, M. W. Moon, K. R. Lee, D. K. Han, J. H. Shin, A. Kamijo and T. Hasebe, “Hemo- compatibility of Surface-Modified, Silicon-Incorporated, Diamond-like Carbon Films,” Acta Biomaterialia, Vol. 5, No. 1, 2009, pp. 249-256. doi:10.1016/j.actbio.2008.07.031 [21] H. C. Cheng, S. Y. Chiou, C. M. Liu, M. H. Lin, C. C. Chen and K. L. Ou, “Effect of Plasma Energy on En- hancing Biocompatibility and Hem mond-Like Carbon Film with Various Titanium ocompatibility of Dia- Concen- trations,” Journal of Alloys and Compounds, Vol. 477, No. 1-2, 2009, pp. 931-935. doi:10.1016/j.jallcom.2008.11.043 [22] B. O’Brien and W. Carroll, “The Evolution of cular Stent Materials and Surfaces i Cardiovas- n Response to Clinical Drivers: A Review,” Acta Biomaterialia, Vol. 5, No. 1, 2009, pp. 945-958. doi:10.1016/j.actbio.2008.11.012 [23] T. Hasebe, S. Yohena, A. Kamijo, Y. Okazaki, A. Hotta, K. Takahashi and T. Suzuki, “Fluorine Doping into Dia- mond-Like Carbon Coatings Inhibits Protein Adsorption and Platelet Activation,” Journal of Biomedical Materials Research Part A, Vol. 83, No. 4, 2007, pp. 1192-1199. doi:10.1002/jbm.a.31340 [24] R. K. Roy and K. R. Lee, “Biomedical Applications o Diamond-Like Carbon Co f atings: A Review,” Journal of Biomedical Materials Research Part B: Applied Biomate- rials, Vol. 83B, No. 1, 2007, pp. 72-84. doi:10.1002/jbm.b.30768 [25] T. Lewis, C. Griffith, M. Gallo and M Modified ATP Benchmark . Weinbren, “A for Evaluating the Cleaning of Some Hospital Environmental Surfaces,” Journal of Hos- pital Infection, Vol. 69, No. 2, 2008, pp. 156-163. doi:10.1016/j.jhin.2008.03.013 [26] N. Hattori, M. O. Nakajima, K. O’hara and T. S “Novel Antibiotic Susceptibility awai, Tests by the ATP-Biolu- d T. Masuzawa, “Hemolysis Cau- minescence Method Using Filamentous Cell Treatment,” Antimicrobial Agents and Chemotherapy, Vol. 42, No. 6, 1998, pp. 1406-1411. [27] O. Maruyama, Y. Numata, M. Nishida, T. Yamane, I. Oshima, Y. Adachi an sed by Surface Roughness under Shear Flow,” Journal of Artificial Organs, Vol. 8, No. 4, 2005, pp. 228-236. doi:10.1007/s10047-005-0316-x [28] G. Q. Yu, B. K. Tay, Z. Sun and L. K. Pan, “Propertie Fluorinated Amorphous Diamond s of Like Carbon Films by PECVD,” Applied Surface Science, Vol. 219, No. 3-4, 2003, pp. 228-237. doi:10.1016/S0169-4332(03)00644-5 [29] M. H. Ahmed, J. A. Byrne and J. McLaughlin, “Evalua- tion of Glycine Adsorption on Diamond Like Carbon (DLC) and Fluorinated DLC Deposited by Plasma-En- hanced Chemical Vapour Deposition (PECVD),” Surface and Coatings Technology, Vol. 209, 2012, pp. 8-14. doi:10.1016/j.surfcoat.2012.07.011 [30] A. Bendavid, P.J. Martin, L. Randeniya, M.S. Amin R. Rohanizadeh, “The Properties of and Fluorine-Containing Diamond-Like Carbon Films Prepared by Pulsed DC Pla- sma-Activated Chemical Vapour Deposition,” Diamond and Related Materials, Vol. 19, No. 12, 2010, pp. 1466- 1471. doi:10.1016/j.diamond.2010.10.001 [31] S. C. Trippea, R. D. Mansano, F. M. Costa and R. F. Sil- va, “Mechanical Properties Evaluation of Fluor-Doped Diamond-Like Carbon Coatings by Nanoindentation,” Thin Solid Films, Vol. 446, No. 1, 2004, pp. 85-90. doi:10.1016/j.tsf.2003.08.069
|