Paper Menu >>
Journal Menu >>
 Vol.2, No.12, 1413-1420 (2010) Health doi:10.4236/health.2010.212210 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ Diagnostic accuracy of biochemical markers of fibrosis in black African patient s wit h c hron ic hepatitis B Alassan Kouamé Mahassadi1*, Alain Koffi Attia1, Fulgence M. Yao Bathaix1, Narcisse Baudouin Agbé1, Stanislas Doffou1, Henriette Ya Kissi1, Isidore Mouhenou Diomandé2, Paul Cales3, Thérese Ndri-Yoman1 1Service d’hépato-gastroentérologie, CHU de Yopougon, Abidjan, Côte d’Ivoire; *Corresponding Author: mahassadi@yahoo.com; 2Laboratoire d’anatomie pathologique, Faculté de médecine de Cocody, Abidjan, Côte d’Ivoire; 3Service d’hépato-gastroentérologie, CHU d’Angers, France. Received 15 August 2010; revised 6 October 2010; accepted 11 October 2010 ABSTRACT Contradictory results of the accuracy of bio- chemical markers to p redict th e st age of fibrosis in black Afri can p atients with chronic hepatitis B (CHB) were previously published. We con- ducted a prospective cohort study to determine the diagnostic accuracy of aspartate aminotr ans- ferase to platelet ratio (APRI), aspartate ami- notransferase to alanine aminotransferase ratio (AAR), platelet count, age-platelet (AP) index, and FIB-4 index for the prediction of significant fibrosis or cirrhosis in 117 black African p atient s (median age: 38 years, males: 73%) with CHB not previously treated. Among them, 45 had significant fibrosis and 18 had cirrhosis using the METAVIR score system. Factors associated either with significant fibrosis or cirrhosis were determined in logistic multivariate analysis. Areas under receiver operating curve were as- sessed and compared for APRI, AAR, AP index, FIB-4 index and platelet count. Sensitivity, specificity, positive and negative predictive values were determined for each biochemical markers. Multivariate analysis showed that as- partate aminotransferase (p < 0.0001) and platelets (p = 0.03) were the independent factors associated with significant fibrosis and only platelets (p = 0.01) were associated with cirrho- sis. APRI (cut-off > 1.1) and FIB-4 index (cut-off > 2.1) ruled out significant fibrosis with high specificity of 84.7% and 86.1% respectively and negative predictive values of 78.2% and 72.9% respectively. More accurately, APRI (cut-off > 0.63) or FIB-4 index (cut-off > 1.26) ruled out cirrhosis with high sensitivity of 94.4% and 88.9% and high negative predictive values of 98.1% and 96.3% respectively. In conclusion, APRI and FIB-4 index are simple readily avail- able markers to exclude significant fibrosis or more accurately cirrhosis in black African pa- tient s with CHB. Keywords: Non Invasive Models; Fibrosis; Cirrhosis; Hepatitis B; Su b-Saharan Africa 1. INTRODUCTION Chronic hepatitis B (CHB) is the major cause of chronic liver disease that affects approximately 350 mil- lions individuals worldwide leading to cirrhosis and hepatocellular carcinoma. Africa and Asia have the high- est prevalence of hepatitis B virus infection worldwide [1,2]. It is obvious that treatment of CHB is a challenge for clinicians in Africa. The assessment of liver disease in patients with CHB by the mean of liver biopsy is not a mandatory but needed at baseline to exclude others causes of liver disease or to evaluate the histologic dam- age in the liver before the initiation of treatment after long term follow up [2-4]. However, liver biopsy is an invasive, costly and not completely safe mean that can lead to severe complications with a potential risk of mor- tality. In addition, it is limited by sampling error and poor concordance between two observers [5,6]. Viral hepatitis C is the more prevalent chronic liver disease in western countries [7]. Non invasive means are constructed to determine the stage of fibrosis in patients with chronic hepatitis C with acceptable accuracies and therefore the need of liver biopsy can be obviated [8-14]. Several studies attempt to use some of these means in patients with CHB and yield unsatisfactorily results [15-17]. WAI et al. show that aspartate aminotransferase (AST) to platelet ratio (APRI), aspartate aminotrans- ferase to alanine aminotransferase (ALT) ratio (AAR) and platelet count were not accurate in predicting either significant fibrosis or cirrhosis among patients with CHB. These findings are confirmed by Kim et al. for the  A. K. Mahassadi et al. / Health 2 (2010) 1413-1420 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 1414 prediction of cirrhosis in patients with CHB. All these tests are validated in Asian patients [16,17]. The diagnostic accuracy of biochemical markers for the prediction of the stage of fibrosis or cirrhosis in black African patients was not evaluated enough. One study suggested that FIB-4 index was accurate to ex- clude significant fibrosis in black African patients with CHB [18]. Recently discordant results were published by Bonnard et al. that demonstrated low accuracy of FIB-4 index and APRI for the prediction of significant fibrosis or cirrhosis in this ethnic group of population [19]. The aim of this study was to evaluate the AAR, APRI, platelet count, age-platelet (AP) index, and FIB-4 index for the prediction of significant fibrosis and cirrhosis in black African patients with CHB attending the hepatol- ogy unit of the Teaching hospital of Yopougon in Abid- jan, Ivory Coast, West Africa. 2. MATERIALS AND METHODS 2.1. Patients All consecutive patients with positive serum hepatitis B surface antigen (HBsAg) and HIV-negative referred to the hepatology unit at Yopougon teaching hospital were eligible for the study. CHB infection was considered if HBsAg persists 6 months after the date of the onset of acute hepatitis B infection. For those with unknown date of the onset of acute hepatitis B infection, CHB was con- sidered if HBsAg is present in a secondary blood test performed 6 months after the date of the previous posi- tive blood test [1,2]. The exclusion criteria were con- comitant liver disease such hepatitis D superinfection, hepatitis C coinfection, autoimmune hepatitis, decom- pensated cirrhosis, hepatic schistosomiasis, if blood test were positive for antibodies against schistosomiasis or the presence in liver biopsy, granulomatous and fibrotic reaction related to hepatic schistosomiasis [20] concomi- tant treatment against HBV, and alcohol consumption over 50 g/l. All patients gave the consent for liver biopsy and data were used according to Helsinki declaration. 2.2. Methods 2.2.1. Clinical Dat a Following clinical data collected: age, sex, occupation, ethnicity, source of contamination, presumably date of contamination, past history of vaccination, alcohol con- sumption. 2.2.2. Biological Dat a Before liver biopsy, blood test were performed in the laboratory of the Yopougon teaching hospital and pro- vided following variables, haemoglobin, hematocrit, mean corpuscular volume, platelet count (Coulter T-540 Coulter Corporation Hialeah, Florida USA), prothrom- bin index (Coagulometer Option 4 plus Biomerieux Germany), AST, ALT (Automate Analyzer, Hitachi Ldt, Tokyo, Japan). The upper limit of normal of AST and ALT were different according to sex and regarding the normal values assigned by the laboratory AST: 31 and 36 IU/L, ALT: 34 and 43 IU/L, for respectively male and female. 2.2.3. Virological Assay HBsAg, hepatitis B e antigen (HBeAg), and antibody, antibody to hepatitis B core antigen (Anti-HBc) were performed using commercial assays (Cobas, Roche Di- agnostics, Mannheim) if treatment was needed according to the result of liver histology, ALT level or viral load [3,4]. All virological assays were assessed at the Centre Intégré de Recherche Bioclinique d’Abidjan (CIRBA) laboratory of Abidjan. 2.2.4. Histological Assessment Liver biopsies if not contraindicated were performed after liver ultrasonography within 7 days after blood testing. Needles of 1.6 mm diameter (Hepafix, Braun, Melsungen) were used for all patients and liver biopsy was performed according the Menghini technique [5]. Liver biopsy specimens were formalin-fixed, paraffin- embedded and stained with hematoxilin-eosin-safran and perls coloration for iron load. Necroinfammatory activity and fibrosis stage were assessed by one senior patholo- gist (DMI), who was blinded to the clinical examination and blood tests and according to the METAVIR semi quantitative system [21]. The fibrosis stage was as fol- low: F0: no fibrosis, F1: portal fibrosis, F2: fibrosis with few septa, F3: fibrosis with numerous septa, F4: cirrho- sis. The grade of activity was as follow: A0: no histo- logical activity, A1: mild activity, A2: moderate activity, A3: severe activity. The length of biopsy specimen was not recorded for all patients. 2.2.5. Diagnostic Target Two diagnostic targets (significant fibrosis and cirrho- sis) assessed by the histologic findings after liver biopsy were used to identify four groups of patients. Patients with significant fibrosis (F2, F3, and F4) were compared to those without significant fibrosis (F0 and F1) and pa- tients with cirrhosis (F4) were compared with those without cirrhosis (F0, F1, F2, and F3). 2.2.6. Statisti cal Analysis Continuous variables were expressed as median and interquartile range and qualitative variables were ex- pressed as percentage. Mann-Withney U test and χ2 or Fischer test (if appropriated) were used to compare  A. K. Mahassadi et al. / Health 2 (2010) 1413-1420 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 1415 quantitative and qualitative variables. The correlations between AST, ALT and platelet count with the stage of fibrosis were assessed by the non parametric Spearman’s rho test (rho). The AAR, APRI, AP index and FIB-4 in- dex were determined by formula previously published and resumed in Table 1 [8,11,13,14]. Variables signifi- cantly associated with significant fibrosis or cirrhosis in univariate analysis were tested using a multivariate lo- gistic regression. The diagnostic accuracy with the best cut-off point that maximizes the sensitivity and the specificity and express as sensitivity, specificity, predic- tive positive and negative values were determined by the receiver operating characteristic curve (ROC) for platelet count, AAR, APRI, AP index and FIB-4 index. The areas under ROC of platelet count, AAR, APRI, AP index and FIB-4 index of each group were compared according to the Henley and Mac Neil test [22]. Value of area under ROC of 1.0 indicated an ideal test whereas value of 0.5 indicated that the test was not significant. All tests were two-tailed and performed by SPSS for Windows version 11.0 (SPSS Inc., Chicago, IL). P value under 0.05 was considered as significant. 3. RESULTS 3.1. Study Sample Between January 2002 and December 2005, a total of 136 patients were recruited. Twenty four patients were excluded (7 patients with chronic hepatitis C, 12 patients with both CHB and C, 3 patients with hepatic steatosis and 2 patients with sclerosing cholangitis). The remain- ing 117 patients were included (73.3% were male). All of them were Ivoirians and black Africans with a median age (interquartile range) of 38 years (30-46). The base- line characteristics are summarised in Tab le 2. Signifi- cant fibrosis and cirrhosis were found respectively in 45 (42.2%) and 18 (40%) patients. 3.2. Factors Associated with Significant Fibrosis or Cirrhosis Patients with significant fibrosis or cirrhosis had Table 1. Formulas of biochemical markers for the prediction of necroinflammatory activity and fibrosis stage. Tests Formulas AAR AST/ALT APRI (AST [ULN]/platelet count [103/mL]) × 100 AP index Age (years): <30 = 0; 30-39 = 1; 40-49 = 2; 50-59 = 3; 60-69 = 4; ≥ 70 = 5 Platelet count (× 103/mL): ≥ 225 = 0; 200-224 = 1; 175-199 = 2; 150-174 = 3; 125-149 = 4; < 125 = 5 FIB-index Age[years] × AST [IU/L]/(platelet count [103/mL] × (ALT1/2 [IU/L]) AAR: aspartate aminotransférase to alanine aminotransferase ratio, APRI: Aspartate aminotransferase to platelet count ratio, AP index: age-platelet index. Table 2. Baseline characteristic of 117 patients included. all patients (n = 117) Age (years ) [median (IQR)] 38 (30-46) Black Africans [n (%)] 117 (100) Sex (male) [n (%)] 86 (73.5) Blood parameters ALT (IU/L) [median (IQR)] 45 (29-105) ALT (ULN) [median (IQR)] 1.1 (0.7-2.6) AST (IU/L) [median (IQR)] 44 (27-86) AST (ULN) [median (IQR)] 1.3 (0.8-2.5) Platelet count (× 103/mL) [median (IQR)] 196 (140.5-250) Prothrombin time (%) [median (IQR)] 90 (80-100) Metavir fibrosis stage No fibrosis (F0) [n (%)] 52 (44.4) Portal fibrosis (F1) [n (%)] 20 (17.1) Few septa (F2) [n (%)] 16 (13.7) Numerous septa (F3) [n (%)] 11 (9.4 ) Cirrhosis (F4) [n (%)] 18 (15.4) Metavir histologic activity No activity (A0) [n (%)] 45 (38.5) Mild activity (A1) [n (%)] 30 (25.6) Moderate activity (A2) [n (%)] 21 (17.9) Severe activity (A3) [n (%)] 21 (17.9) AST: aspartate aminotransferase, ALT: alanine aminotransferase, ULN: upper limit of normal, IQR: interquartile range. higher level of ALT (ULN) or AST (ULN) and lower platelet count than those without significant fibrosis or cirrhosis in univariate analysis (Table 3). AST (ULN) and ALT (ULN) levels correlated positively (both corre- lation coefficients rho = 0.4, p < 0.0001) whereas plate- let count correlated negatively (rho = –0.28, p = 0.002) with the stage of fibrosis. Overall comparison demon- strated significant difference between AST or Platelets count and the stage of fibrosis (Figure 1). In logistic multivariate analysis, AST (p < 0.0001) and platelet count (p = 0.03) were independent predictors of signifi- cant fibrosis whereas platelet count was the only inde- pendent factor associated with cirrhosis (p = 0.01). The median values (with interquartile range) of biochemical markers are summarised in Table 4. Besides AAR for significant fibrosis, biochemical markers showed sig- nificant but modest area under ROC either for the pre- diction of significant fibrosis (fibrosis stage ≥ F2) or cirrhosis (F4) in our study (Table 5). 3.3. Comp ari son of Biochemical Pa rameters for the Prediction of Significant Fibrosis As illustrated in Figure 2(a), APRI, platelet count, FIB-4 index and AP index had similar diagnostic accu- 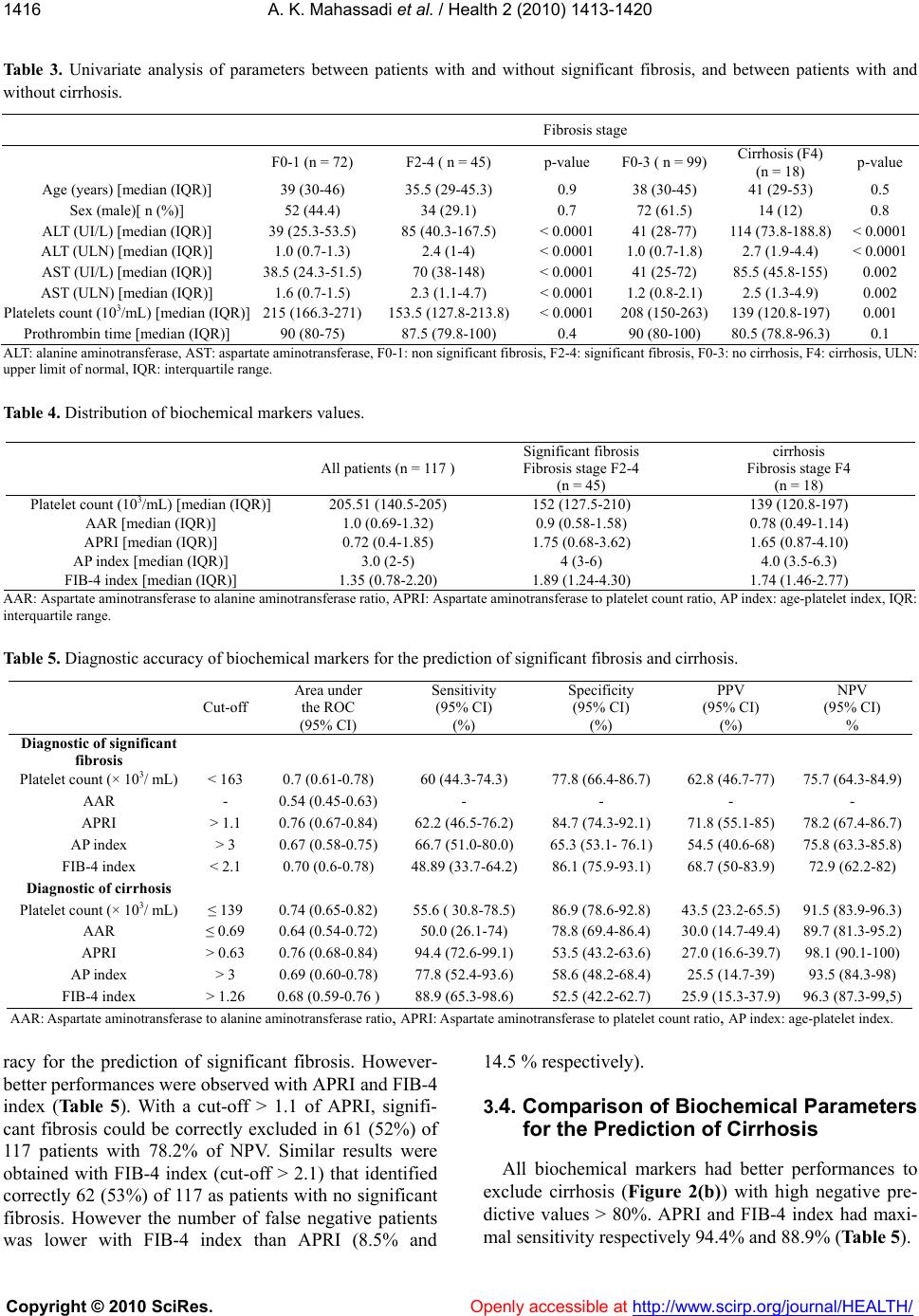 A. K. Mahassadi et al. / Health 2 (2010) 1413-1420 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 1416 Table 3. Univariate analysis of parameters between patients with and without significant fibrosis, and between patients with and without cirrhosis. Fibrosis stage F0-1 (n = 72) F2-4 ( n = 45) p-value F0-3 ( n = 99) Cirrhosis (F4) (n = 18) p-value Age (years) [median (IQR)] 39 (30-46) 35.5 (29-45.3) 0.9 38 (30-45) 41 (29-53) 0.5 Sex (male)[ n (%)] 52 (44.4) 34 (29.1) 0.7 72 (61.5) 14 (12) 0.8 ALT (UI/L) [median (IQR)] 39 (25.3-53.5) 85 (40.3-167.5) < 0.0001 41 (28-77) 114 (73.8-188.8)< 0.0001 ALT (ULN) [median (IQR)] 1.0 (0.7-1.3) 2.4 (1-4) < 0.0001 1.0 (0.7-1.8) 2.7 (1.9-4.4) < 0.0001 AST (UI/L) [median (IQR)] 38.5 (24.3-51.5) 70 (38-148) < 0.0001 41 (25-72) 85.5 (45.8-155) 0.002 AST (ULN) [median (IQR)] 1.6 (0.7-1.5) 2.3 (1.1-4.7) < 0.0001 1.2 (0.8-2.1) 2.5 (1.3-4.9) 0.002 Platelets count (103/mL) [median (IQR)] 215 (166.3-271) 153.5 (127.8-213.8) < 0.0001 208 (150-263) 139 (120.8-197)0.001 Prothrombin time [median (IQR)] 90 (80-75) 87.5 (79.8-100) 0.4 90 (80-100) 80.5 (78.8-96.3)0.1 ALT: alanine aminotransferase, AST: aspartate aminotransferase, F0-1: non significant fibrosis, F2-4: significant fibrosis, F0-3: no cirrhosis, F4: cirrhosis, ULN: upper limit of normal, IQR: interquartile range. Table 4. Distribution of biochemical markers values. AAR: Aspartate aminotransferase to alanine aminotransferase ratio, APRI: Aspartate aminotransferase to platelet count ratio, AP index: age-platelet index, IQR: interquartile range. Table 5. Diagnostic accuracy of biochemical markers for the prediction of significant fibrosis and cirrhosis. Cut-off Area under the ROC (95% CI) Sensitivity (95% CI) (%) Specificity (95% CI) (%) PPV (95% CI) (%) NPV (95% CI) % Diagnostic of significant fibrosis Platelet count (× 103/ mL) < 163 0.7 (0.61-0.78) 60 (44.3-74.3) 77.8 (66.4-86.7) 62.8 (46.7-77) 75.7 (64.3-84.9) AAR - 0.54 (0.45-0.63) - - - - APRI > 1.1 0.76 (0.67-0.84) 62.2 (46.5-76.2) 84.7 (74.3-92.1) 71.8 (55.1-85) 78.2 (67.4-86.7) AP index > 3 0.67 (0.58-0.75) 66.7 (51.0-80.0) 65.3 (53.1- 76.1) 54.5 (40.6-68) 75.8 (63.3-85.8) FIB-4 index < 2.1 0.70 (0.6-0.78) 48.89 (33.7-64.2) 86.1 (75.9-93.1) 68.7 (50-83.9) 72.9 (62.2-82) Diagnostic of cirrhosis Platelet count (× 103/ mL) ≤ 139 0.74 (0.65-0.82) 55.6 ( 30.8-78.5) 86.9 (78.6-92.8) 43.5 (23.2-65.5) 91.5 (83.9-96.3) AAR ≤ 0.69 0.64 (0.54-0.72) 50.0 (26.1-74) 78.8 (69.4-86.4) 30.0 (14.7-49.4) 89.7 (81.3-95.2) APRI > 0.63 0.76 (0.68-0.84) 94.4 (72.6-99.1) 53.5 (43.2-63.6) 27.0 (16.6-39.7) 98.1 (90.1-100) AP index > 3 0.69 (0.60-0.78) 77.8 (52.4-93.6) 58.6 (48.2-68.4) 25.5 (14.7-39) 93.5 (84.3-98) FIB-4 index > 1.26 0.68 (0.59-0.76 ) 88.9 (65.3-98.6) 52.5 (42.2-62.7) 25.9 (15.3-37.9) 96.3 (87.3-99,5) AAR: Aspartate aminotransferase to alanine aminotransferase ratio, APRI: Aspartate aminotransferase to platelet count ratio, AP index: age-platelet index. racy for the prediction of significant fibrosis. However- better performances were observed with APRI and FIB-4 index (Tab le 5 ). With a cut-off > 1.1 of APRI, signifi- cant fibrosis could be correctly excluded in 61 (52%) of 117 patients with 78.2% of NPV. Similar results were obtained with FIB-4 index (cut-off > 2.1) that identified correctly 62 (53%) of 117 as patients with no significant fibrosis. However the number of false negative patients was lower with FIB-4 index than APRI (8.5% and 14.5 % respectively). 3.4. Comparison of Biochemical Parameters for the Prediction of Cirrhosis All biochemical markers had better performances to exclude cirrhosis (Figure 2(b)) with high negative pre- dictive values > 80%. APRI and FIB-4 index had maxi- mal sensitivity respectively 94.4% and 88.9% (Table 5). All patients (n = 117 ) Significant fibrosis Fibrosis stage F2-4 (n = 45) cirrhosis Fibrosis stage F4 (n = 18) Platelet count (103/mL) [median (IQR)] 205.51 (140.5-205) 152 (127.5-210) 139 (120.8-197) AAR [median (IQR)] 1.0 (0.69-1.32) 0.9 (0.58-1.58) 0.78 (0.49-1.14) APRI [median (IQR)] 0.72 (0.4-1.85) 1.75 (0.68-3.62) 1.65 (0.87-4.10) AP index [median (IQR)] 3.0 (2-5) 4 (3-6) 4.0 (3.5-6.3) FIB-4 index [median (IQR)] 1.35 (0.78-2.20) 1.89 (1.24-4.30) 1.74 (1.46-2.77)  A. K. Mahassadi et al. / Health 2 (2010) 1413-1420 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 1417 Figure 1. Box plots of AST (a) or platelet count (b) according to the stage of fibrosis defined by META- VIR score system. The box represents the interquartile range; the top and the bottom of the box are re- spectively the 25 th and 75 th percentile. The line across the box is the median. The lower and upper values are indicated by the whiskers. The circles represent the outliers. Figure 2. Receiver operating characteristic curve of the five biochemical markers for the prediction of sig- nificant fibrosis (a) or cirrhosis (b) according to the METAVIR stage of fibrosis in black African patients with chronic hepatitis B. APRI: aspartate aminotransferase to platelet count ratio, AAR: aspartate ami- notransferase to alanine aminotransferase ratio, AP: age-platelet index and FIB-4 index. With a cut-off > 0.63 of APRI, cirrhosis was correctly excluded in 52 (52.5%) patients among 117 with a NPV of 98.1%. Among 18 patients with histological proven cirrhosis, 17 (94.4%) were correctly classified and 1 (1.9 %) patient was false negative. Applying a cut-off > 1.26 of FIB-4 index, we found similar results, 51 (51.5%) patients of 117 correctly classified as patients with no cirrhosis. Thus 16 (88.9%) patients of 18 with cirrhosis were diagnosed and 2 (3.8%) were false negative. 4. DISCUSSION We demonstrated in this study that biochemical mark- ers previously assessed in chronic hepatitis C such as APRI, AAR, FIB-4 index, platelet count and AP index had low accuracy regarding their respective areas under ROC, to predict the presence of significant fibrosis or cirrhosis among black Africans with CHB. However some of them could be used to exclude more accurately cirrhosis in low medicalized countries of Africa. Indeed we found that in patient with cirrhosis, APRI and FIB-4 index enabled to eliminate cirrhosis with high degree of certainty better than that published by Bonnard et al., in Burkina Faso [19]. This is probably related to the size of our sample twice larger. Indeed our patients (a) (b) (a) (b)  A. K. Mahassadi et al. / Health 2 (2010) 1413-1420 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 1418 were slightly older than those from Mayotte and some of them had platelet count in normal range despite moder- ate to severe fibrosis (Figure 1(b)) leading to misclassi- fication of FIB-4 index. The length of liver biopsy frag- ment was probably not large enough in our study to en- hance the accuracy of FIB-4 index as previously demon- strated [18]. In other hand the interaction of parasitosis or other blood transmitted diseases as malaria and CHB both prevalent in sub-Saharan African countries could reduce the global performance of these biochemical markers in African patients [24]. Nevertheless APRI and FIB-4 index could be used to monitor patient with CHB when liver biopsy is difficult to perform. Both were ac- curate to exclude cirrhosis among black Africans with high degree of NPV. This probably means that APRI and FIB-4 index are more reliable when cirrhosis occurs in sub Saharan African patients with CHB than westerners or other black people. Recent studies conducted in Asia demonstrated discordant results of APRI and FIB-4 in- dex for the prediction of stage of fibrosis. Wu et al., founded low accuracy of APRI and FIB-4 index to pre- dict significant fibrosis comparable to our findings [25]. However these two biochemical markers showed better performances to predict severe fibrosis. Kim et al. dem- onstrated that FIB-4 allowed detecting cirrhosis with high degree of certainty than APRI, and AAR [26]. These recent findings emphasized the difficulty to pro- vide definite and invariable performance of biochemical markers in patients with CHB whatever the geographical area. This study was conducted in West Africa where the endemicity of HBV is high and enrolled only black Af- ricans. Most of them acquired HBV infection perinataly or during childhood which is the most common route of transmission of HBV in this area [1]. Liver damage (fi- brosis and cirrhosis) occurs mainly in adulthood with a high risk of onset of hepatocellular carcinoma. This ex- plained that 45% of patients in our study had significant fibrosis or cirrhosis and were eligible for treatment [1,2]. We were not able to seek the date of the onset of acute hepatitis B infection, to determine viral load or HBeAg and antibody for most of patients because of the high cost of their determination and high number of missing values. These parameters were not included in the analy- sis. Despite these limitations, this study pointed out some specificities in a population of black African pa- tients. Firstly, AST and platelet count were independent predictors of significant fibrosis and only platelet count were associated with cirrhosis in multivariate analysis. Secondly, biochemical markers assessed in this study with significant values of area under ROC had high abil- ity to exclude patients with cirrhosis. Each of them showed high NPV > 85%. Regarding the area under ROC, our study confirmed the findings made by Wai et al. and Kim et al. in Asian patients [16,17]. Furthermore our study is consistent with that of Hongo et al., in which the areas under the ROC of APRI and AP index were also modest respectively 0.76 and 0.74 [23]. In contrast to the study of Wai et al. in which only platelet count were an independent factor associated either with significant fibrosis or cirrhosis in multivariate analysis in a cohort of Asian patients [16], our study suggest that black African patients experience more flares of hepatitis B with intermittent elevation of transaminases because of immune pressure and high prevalence of HBeAg negative CHB that lead to severe liver damage [2,27,28]. Indeed, cirrhosis and hepatocellular carcinoma occurred more rapidly in patients with HBeAg negative CHB and hepatocellular carcinoma related to HBV is more preva- lent among blacks Africans [29,30]. Similar to others studies, in patients with CHB even in those with chronic hepatitis C, we found an association between platelet count and cirrhosis as demonstrated by others [31,32]. Chronic hepatitis B is a public health concern in West Africa where the vaccination against hepatitis B infec- tion is not widely implemented in most sub-Saharan Af- rican countries. Antiviral treatments are expensive for most patients with severe liver damage. Pathologists with a high experience in liver histologic examination are not widely available. There is a need for accurate, readily available and routinely feasible blood markers for the prediction of significant fibrosis or cirrhosis in Africa. Previous reports showed that combination of biochemical markers that included hyaluronidase, alpha2 macroglobulin or serum globulin are accurate to predict significant fibrosis or cirrhosis in patients with CHB but most of then have low values of area under the ROC or are not routinely incorporated in the panel of blood tests in most hospital laboratories even in Europe than in Af- rica [15,33,34]. Hui et al. demonstrate that a score in- cluding body mass index, platelet count, serum albumin and total bilirubin was accurate in predicting the absence of significant fibrosis but needs complicated calculation [35]. Mohamadnejad et al., published a score to assess significant fibrosis only in patient with HBe Ag negative CHB [36]. Hongbo et al. also demonstrated that combi- nation of biochemical markers may enhance diagnosis accuracy for either the prediction (AP index with AST in parallel interpretation) or the exclusion (AP index with gammaglutamyltransferase in serial interpretation) of significant fibrosis in patient with CHB [23]. However, we think that the procedure of calculation and interpreta- tion is not easy to fulfil in clinical practice. Bonnard et al. found that Elastometry were more reliable than APRI and FIB-4 index to predict significant fibrosis in black  A. K. Mahassadi et al. / Health 2 (2010) 1413-1420 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 1419 African patients but the device for it determination is expensive and not available in most hospitals of sub Sa- haran African countries [19]. Liver biopsy remains in this area the only means to quantify liver fibrosis in pa- tients with CHB. Guidelines and consensus conferences recommend to monitor patients with cirrhosis by routine alpha-fetoprotein determination and liver ultrasonogra- phy every 6 months [2,3]. Close surveillance is recom- mended to detect the onset of oesophageal varices if absent at the time of the diagnosis of cirrhosis by routine endoscopy [37,38]. Lamivudine, a nucleoside analogue is widely used in Africa as part of highly antiretroviral therapy in HIV infected patients. It is a cost effective drug affordable to most Africans for the treatment of CHB. However, patients with cirrhosis treated by lami- vudine need close surveillance because of the risk of hepatitis flares and liver decompensation during treat- ment [2,39]. Most of these recommendations are difficult to fulfil in developing countries of Africa. Biochemical markers such as APRI, and FIB-4 index easy to determine and cost effective could be used to identify African patients with CHB who do not have cirrhosis, and in whom close surveillance could be delayed during treatment by lami- vudine. 5. CONCLUSION This study demonstrated that biochemical markers currently used in chronic hepatitis C, had low accuracy regarding their areas under ROC. However APRI and FIB-4 index could be used to exclude cirrhosis in black African patients with CHB with high certainty. Further studies enrolling a large sample of black African patients with CHB are needed to establish the clinical relevance of the accuracy of biochemical markers in African pa- tient. REFERENCES [1] Lavanchy, D. (2004) Hepatitis B virus epidemiology, disease burden, treatment and current and emerging prevention and control measures. Journal of viral hepatitis, 11, 97-107. [2] Lok, S.F. and Mc Mahon, B.J. (2007) Chronic hepatitis B. Hepatology, 45, 507-539. [3] EASL (2009) Clinical practice guidelines: management. Journal of Hepatology, 50, 227-242. [4] Keffe, E.B., Dieterich, D.T., Han, S.H., Jacobson, I.M., Martin, P., Schiff, E.R. and Tobias, H.A. (2008) A treatment algorithm for the management of chronic hepatitis B virus in the United States: 2008 update. Clinical G astroe nter olog y and H ep atolo gy, 6, 1315-1341. [5] Bravo, A., Sheth, S.G. and Chopra, S. (2001) Liver biopsy. New England Journal of Medicine, 344, 495-500. [6] Skripenova, S., Trainer, D.T., Krawitt L.E. and Blaszyk H. (2007) Variability of grade and stage in simultaneous paired liver biopsies in patients with hepatitis C. Journal of Clinical Pathology, 60, 321-324. [7] Lauer, M.G. and Walker, B.D. (2001) Hepatitis C virus infection. New England Journal of Medicine, 345, 41-50. [8] Poynard T., Bedossa, P. and METAVIR, CLINIVIR, cooperative study. (1997) Age and platelet count: A simple index for predicting the presence of histological lesions in patients with antibodies to hepatitis C virus. Journal of Viral Hepatitis, 4, 199-208. [9] Imbert-Bismuth, F., Ratzui, V., Pieroni, L., Charlotte, F., Benhamou, Y. and Poynard T. (2001) Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet, 357, 1069-1075. [10] Pohl, A., Behling C., Oliver D., Kilani, M., Monson P. and Hassanein, T. (2001) Serum aminotransferase levels and platelets counts as predictors of degree of fibrosis in chronic hepatitis C infection. American Journal of Gastroenterology, 96, 3142-3146. [11] Myer,s R.P., De Torres, M., Imbert-Bismut, F., Ratziu, V., Charlotte, F. and Poynard T. (2003) Biochemical markers of fibrosis in patients with chronic hepatitis C. A comparison with prothrombin time, platelet count and age-platelet index. Digestive Diseases and Sciences, 48, 146-153. [12] Forns, X., Ampurdanes, S., Llovet, J.M., Aponte, J., Quinto, L., Bauer-Martinez, E., Bruguera, M., Sanchez-Tapias, J.M. and Rodes R. (2002) Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology, 36, 987-992. [13] Wai, C-T., Greenson, J.K., Fontana, R.J., Kalbfleisch, J.D., Marrero, J.A., Conjeevaram, H.S. and Lok, A.S.F. (2003). A simple non-invasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology, 38,518-526. [14] Vallet-Pichard, A., Mallet, V., Nalpas, B.,Verkarre,V., Nalpas, A., Dhalluim-Venier, V., Fontaine, H. and Pol, S. (2007). FIB-4 index an inexpensive and accurate markers of liver fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology,46, 32-35. [15] Myers, R.P., Tainturier, M-H., Ratziu, V., Piton, A., Thibault, V., Imbert-Bismut, F., Messous, D., Charlotte, F., Di-Martino,V., Benhamou Y. and Poynard T. (2003) Prediction of liver histological lesions with biochemical markers in patients with chronic hepatitis B. Journal of Hepatology, 39, 222-230. [16] Wai, C-T., Cheng, C.L., Wee, A., Dan , Y.Y., Chua, W., Mak, B., AM, Oo. and Lim, S.G. (2006). Non-invasive models for predicting histology in patients with chronic hepatitis B. Liver international, 26, 666-672. [17] Kim, B.K., Kim, S.A., Park, Y.N., Cheong, J.Y., Kim, H.S., Park, J.Y., Cho, S.W., Han, K.-H., Chon, C.Y., Moon, Y.M. and Ahan, S.H. (2007) Non invasive models to predict liver cirrhosis in patients with chronic hepatitis B. Liver international, 27, 969-976. [18] Mallet, V., Dhalluin-Venier, V., Roussin, C., Bourlière M., Pettinelli, M.E., Giry, C., Vallet-Pichard, A., Fontaine, H. and Pol, S. (2009) The accuracy of FIB-4 for the diagnostic of mild fibrosis in chronic hepatitis B. Alimentary Pharmacology and Th erapeutics , 29, 409-415.  A. K. Mahassadi et al. / Health 2 (2010) 1413-1420 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 1420 [19] Bonnard, P., Sombié, R., Lescure, F.X., Bougouma, A., Guiard-Schmid, J.B., Poynard, T., Cales, P., Housset, C., Callard, P., Le Pendeven, C., Drabo, J., Carrat, F. and Pialoux, G. (2010) Comparison of elastography, serum marker scores, and histology for the assessment of liver fibrosis in hepatitis B virus (HBV)-infected patients in Burkina Faso. American Journal of Tropical Medicine and Hygiene, 82, 454-458. [20] Plorde, J.J. and Jong, C.E. (1983) Schistomiasis. In: Petersdrof, R., Adams, R.D., Braunwald, E., Isselbacher, K.J., Martin, J.D., et al., Eds., Harrison’s Principles of Internal Medecine, MacGrw-Hill, Inc., New York, 1217- 1222. [21] The French METAVIR Cooperative Study Group (1994) Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology, 20, 15-20. [22] Hanley J.A. and McNeil, B.J. (1983) A method of comparing the areas under receveir operating characteristic curves derived from the same cases. Radi ology, 148, 839-843. [23] Hongbo, L., Xiaohui, L., Hong, K., Wei, W. and Yon, Z. (2007) Assessing routine and serum markers ol liver fibrosis in chronic hepatitis B patients using parallel and serial interpretation. Clinical biochemistry, 40,562-566. [24] Ignatus, C.M., Emeka, E.N., and Blessing, N.E. (2008) Effect of malaria parasitemia on liver enzyme tests. International Journal of Tropical Medecine, 3, 49-52. [25] Wu, S.D., Wang, J.Y. and Li, L. (2010) Staging of liver fibrosis in chronic hepatitis B patients with a composite predictive model: A comparative study. World Journal of Gastroenterology, 16, 501-517. [26] Kim, B.K., Kim do, Y., Park, J.Y., Ahn, S.H., Chon, C.Y., Kim, J.K. Paik, Y.H., Lee, K.S., Park, Y.N. and Han, K.H. (2010) Validation of FIB-4 and comparison with other simple noninvasive indices for predictiong liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver international, 45, 355-360. [27] Zarsky, J.P., Marcellin, P., Leroy, V., Trepo, C., Samuel, D., Ganne-Carrie N., Barange, K., Canva, V., Doffoel M., Cales, P. and Fédération Nationale des Pôles de Références et des Réseaux Hépatites. (2006) Characteristics of patients with chronic hepatitis Bin France: Predominant frequency of HBe antigen negative cases. Journal of hepatology, 45, 355-360. [28] Suzuki, S., Sugauchi F., Orito, E., Kato, H., Usuda, S., Siransy, L., Arita, I., Sakamoto, Y., Yoshihara, N; El- Gohary, A., Ueda, R. and Mizokami, M. (2003) Distribution of hepatitis B virus (HBV) genotype among HBV carriers in the Côte d’Ivoire: Complete genome sequence and phylogenetic relatedness of HBV genotype E. Journal of Medical Virology, 69, 946-953. [29] Baptista, M., Kramvis, A., and Kew, MC. (1999) High prevalence of 1762 (T) 1764(A) mutations in the basic core promoter of hepatitis B virus isolated from black Africans with hepatocellular carcinoma compared with asymptomatic carriers. Hepatology, 29, 946-953. [30] Kew, M.C. (2002) Epidemiology of hepatocellular carcinoma. Toxicology, 27, 35-38. [31] Karasu, Z., Tekin, F., Ersoz, G., Gunsar, F., Batur, Y., Ilter, T. and Akarca, U.S. (2007) Liver fibrosis is associated with decreased peripheral platelet count in patients with chronic hepatitis B and C. Digestive Diseases and Sciences, 52, 1535-1539. [32] Goulis, J., Chau, T.N., Jordan. S, Metha, A.B., Watkinson, A., Rolles, K. and Burroughs, A.K. (1999) Thrombopoietin concentrations are low in patients with cirrhosis and thrombocytopenia and are restored after orthotopic liver transplantation. Gut, 44, 754-758. [33] Zeng, M.D., Lu, L.G., Mao, Y.M., Qui, D.K., Li, M.B., Wan, C.W., Chen, J.Y., Wang, X., Gao, C.F. and Zhou, X.Q. (2005) Prediction of significant fibrosis in HbeAg- positive patients with chronic hepatitis B by a non- invasive model. Hepatology, 42, 1437-1445. [34] Schmilovitz-Weiss, H., Tovar, A., Halpern, M., Sulkes, J., Braun, M. and Rotman, Y. (2006) Predictive value of serum globulin levels for the extent of hepatic fibrosis in patients with chronic hepatitis B infection. Journal of viral hepatitis, 13, 671-677. [35] Hui, A.Y., Chan, H.L., Wong, V.W., Liew, C.T., Chim, A.M., Chan, F.K. and Sung, J.J. (2005) Identification of chronic hepatitis B patients without significant liver fibrosis by a simple non invasive predictive model. American Journal of Gastroenterology, 100, 616-623. [36] Mohamadnejad, M., Montazeri, G., Fazlollahi, A., Zamani, F., Nasiri, J., Nobakht, H., Forouzanfar, M.H., Abedian, S., Tavangar, S.M., Mohamadkhani, A., Ghoujeghi, F., Estakhri, A., Nouri, N., Farzadi, Z., Najjari, A. and Malekzadeh, R. (2006) Noninvasive markers of liver fibrosis and inflammation in chronic hepatitis B-virus related liver disease. American Journal of Gastroenterology, 101, 2537-2545. [37] Cales, P., Desmorat, H., Vinel, J.P., Caucanas, J.P., Ravaud, P., Gerin, P., Brouet, P. and Pascal, J.P. (1990) Incidence of large oesophageal varices in patients with cirrhosis: Application to prophylaxis of first bleeding. Gut, 31, 1298-1302. [38] de Franchis, R. and Baveno, V. (2010) Faculty Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. Journal of hepatology, 53, 762-768. [39] Marrone, A., Zampino, R., Karayannis, P., Cirillo, G., Cesaro, G., Guerrera, B., Riccioti, R., Del Giudice, E.M., Utili, R., Adinolfi, L.E. and Ruggiero, G. (2005) Clinical reactivation during lamivudine treatment correlates with mutations in the precore/core promoter and polymerase regions of hepatitis B virus in patients with anti-Hepatitis B e-positive chronic hepatitis. Alimentary Pharmacology and Therapeutics, 22, 707-714. |

