 Journal of Environmental Protection, 2013, 4, 65-73 http://dx.doi.org/10.4236/jep.2013.48A1009 Published Online August 2013 (http://www.scirp.org/journal/jep) 65 Effects of Gasoline Inhalation on Menstrual Characteristics and the Hormonal Profile of Female Petrol Pump Workers Christopher E. Ekpenyong, Koofreh Davies, Nyebuk Daniel Department of Physiology, Faculty of Basic Medical Sciences, University of Uyo, Uyo, Nigeria. Email: chrisvon200@yahoo.com Received May 15th, 2013; revised June 28th, 2013; accepted July 30th, 2013 Copyright © 2013 Christopher E. Ekpenyong et al. This is an open access article distributed under the Creative Commons Attribu- tion License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Increasing numbers of young women are employed as gasoline station attendants in most developing countries despite the lack of empirical data on the adverse reproductive health effect of this solvent. This study therefore sought to assess the effects of gasoline inhalation on the serum sex hormone profile and menstrual characteristics of female gasoline station attendants in Nigeria, given the global increase in the rate of infertility and the existing evidence on the repro- ductive toxicity of gasoline constituents. A site-by-site cross-sectional study of 117 female gasoline pump attendants and 118 age-matched controls was carried out between September 2011 and November 2012. The following 3 instru- ments were used for data collection: a semi-structured questionnaire, a female sex hormone profile assay and exposure status measures. The prevalence of menstrual disorders among the exposed and unexposed women was 37.2% and 28.5% respectively. Exposure to gasoline was significantly associated with disorders in both menstrual cycle length and quantity of flow. Specifically, exposed women had a greater than threefold increased risk of a menstrual disorder, with an odds ratio (OR) of 3.25 for abnormal cycle length and OR of 4.16 for abnormal quantity of flow. In addition, longer duration of exposure (>1 year) was significantly associated with higher likelihood of menstrual disorders. There were also persistent low serum levels of estradiol, and fluctuating levels of other reproductive hormones. Gasoline inhalation may interfere with ovarian functions leading to disordered menstrual characteristics and female sex hormone profiles, as well as future reproductive impairment. Keywords: Adverse Reproductive Outcome; Female Worker; Gasoline Inhalation 1. Introduction In recent years, there has been a significant influx of fe- male workers in petrochemical firms, and a considerable proportion of these workers are of reproductive age [1]. This scenario carries significant public health concern and has been an important issue in occupational medicine [2] due to the potential adverse effects of occupational exposure to toxic gasoline constituents on female repro- ductive endpoints, especially given the increased rate of infertility in both developed [3] and developing coun- tries. Among the numerous constituents of petroleum prod- ucts, gasoline constituents (benzene, toluene, ethyl ben- zene and xylene (BTEX)) are designated as the most toxic compounds to humans [4]. These compounds are volatile and lipophilic in nature, and workers may be exposed through inhalation, ingestion or dermal routes [5]. These exposures can be accidental or intentional and are insidious in nature [6,7]. It is generally considered that dermal absorption of gasoline does not contribute significantly to systemic toxicity [8]. Evidence, however, favours the view that BTEX in gasoline may be important human reproductive toxins. In addition, the relationship between exposure to these or- ganic solvents and adverse reproductive outcomes, such as congenital malformation, chromosomal abnormalities and altered fertility, has been repeatedly tested and documented in experimental animals [9]. However, the epidemiologic data associating gasoline with human reproductive health, with particular reference to its effect on female sex hormones and menstrual char- Copyright © 2013 SciRes. JEP  Effects of Gasoline Inhalation on Menstrual Characteristics and the Hormonal Profile of Female Petrol Pump Workers 66 acteristics, are limited. Gasoline constituents (BTEX) are regarded as reproductive toxicants [10], and as such, they can act as environmental endocrine disruptors [11], which are defined as exogenous agents that can modulate or interfere with the production, release, transport, me- tabolism, binding, actions and elimination of the natural hormones in the body [12,13]. The ovaries are the target organs for injury caused by these reproductive and endo- crine toxicants [14], although their effects on other endo- crine organs such as the hypothalamus, pituitary, adre- nals, thyroid, parathyroid and pancreas are also well documented [15]. For instance, evidence has accumu- lated that benzene and its analogues affect the luteal functions of female workers in petrochemical industries [16]. In addition, benzene fume inhalation has been shown to affect the hypophysis and the extension of ovarian functional status in rats [17]. Moreover, the documented adverse health effects of gasoline include female infertility, spontaneous abortion, birth defects, and intrauterine growth retardation. These adverse reproductive health effects may initially be signalled by the presence of altered menstrual charac- teristics, and the study by Rowland et al. [18] identified long and/or irregular cycles as being associated with in- fertility and foetal loss during the most recent pregnancy [18]. Menstrual disorders have also been reported among women in occupations such as farmers, health workers, dancers, athletes, semiconductor manufacturers and liq- uid crystal display workers [19,20], although scant data exist concerning the menstrual characteristics of women working as petrol pump attendants in gasoline stations, despite the influx of a large number of women (at the peak of reproductive age) into this occupation in Nigeria. Menstrual outcomes are important potential indicators or markers of other health outcomes, such as infertility, osteoporosis, breast and lung cancer, anaemia, pancyto- paenia, increased rate of leukaemia, neurological distur- bances, and breathlessness, which are also known to be associated with exposure to BTEX compounds [20-22]. Also, in most cases, individuals are unaware of reproduc- tive hazards resulting from these occupational exposures until they are interested in child birth [1]. It was therefore felt that a study to investigate the effect of gasoline inha- lation on the menstrual characteristics and hormonal pro- files of female petrol pump workers, who receive con- tinuous occupational exposure and whose future repro- ductive performance depends on the integrity of their reproductive organs, would be valuable. 2. Material/Methods 2.1. Subject and Methods This study took place in the Uyo metropolis of South- South Nigeria between September 2011 and November 2012. Several commercial petrol stations were selected as study sites based on their geographical location to represent the entire study area. Two hundred and thirty- five female respondents (117 petrol pump workers and 118 age-matched control subjects) participated in this survey. The inclusion criteria were as follows: age between 18 and 40 years, not pregnant or breast feeding, no use of oral contraceptives during the previous year, no history of menstrual disorder/irregularity prior to employment as a gasoline pump worker, no history of treatment for menstrual disorders prior to employment, no sexually transmitted infections or pelvic inflammatory disease, fertility, no history of any abdominal surgery, good die- tary intake and moderate physical activity status for the previous year, normal body mass index and the absence undue perceived stress. 2.2. Survey Methods Three survey instruments were used in this study: a semi- structured questionnaire adapted from previous studies on menstrual cycle characteristics [23,24]; assays to measure plasma female sex and other related hormones; and an exposure assessment tool. The questionnaire con- sisted of two sections and contained open-ended ques- tions and probes aimed at exploring the socio-demo- graphic profile and menstrual characteristics of the re- spondents. Section one contained seven open-ended questions structured to obtain information regarding the partici- pants’ age (years), educational status, marital status, du- ration on the job, physical activity status, nutritional habit and presence or absence of perceived stress. The age of the respondents was stratified into 3 groups: 18 - 25, 26 - 35 and 36 - 40 years. Educational status was divided into 3 groups according to the level of education attained, i.e., primary, secondary or tertiary education. Marital status was classified as single, married or di- vorced. Body mass index was calculated as weight/height (kh/m2), and subjects were defined as normal (<25), overweight (25.0 - 29.9) or obese (≥30) according to the WHO classification [25]. Perceived stress was assessed using the perceived stress scale; the values were scored on a 4-point Likert scale and interpreted as no stress (0 - 1), low stress (2 - 3) or high stress (≥4). The duration on the job was stratified into two groups: those who had worked for less than one year and those who had worked for more than one year. Part two of the questionnaire consisted of ten ques- tions aimed at obtaining information on the respondents’ past and present obstetric and gynecologic history, with Copyright © 2013 SciRes. JEP  Effects of Gasoline Inhalation on Menstrual Characteristics and the Hormonal Profile of Female Petrol Pump Workers 67 special emphasis placed on the age at menarche, contra- ceptive history and menstrual cycle characteristics (length/duration and quantity of flow) for the previous year. To categorise the respondents’ menstrual cycle length, they were asked “how many days are there from the first day of one menstrual period to the first day of the next period?” The answer categories included 24 days or less, 25 - 30 days, 31 - 35 days and greater than 35 days. Based on the answers given, the participants were divided into three groups, including those with a short cycle length (defined as a cycle that last for less than 24 days), those with a long cycle (>35 days) and those with a normal cycle length (24 - 35 days). The choice of these cut-off points was based on previous re- search into menstrual cycles [23,24]. To estimate the quantity of the menstrual flow, the participants were asked how many menstrual hygiene pads they typically needed to change per day and whether there had been an increase or decrease within the past three months or more. Those with a noticeable decrease in the number of pads required due to a reduced quantity of flow were grouped as having a scant/light flow, whereas those with a noticeable increase in the number of pads required due to an increase in quantity of flow were grouped as having a heavy menstrual flow. Participants with a normal flow were those with no indication to increase or decrease the number of sanitary pad required during menstruation. For the female sex hormone profile assay, participant blood samples were obtained from the cubital vein on the arm in the Department of Chemical Pathology of the University of Uyo Teaching Hospital, Uyo, Nigeria. The menstrual cycle phase of the participants when the blood was taken was noted, as the normal levels of these hor- mones vary according to the cycle phase. Based on the serum levels of the hormones and in comparison to the normal range, according to the cycle phase, the partici- pants were classified as having normal, high or low lev- els of sex hormones. The concentration of the BTEX compounds at each of the gasoline stations was assessed and used to monitor the attendants’ exposure. Personal samples were col- lected in sorbent tubes containing activated charcoal (80 - 100 mesh), which were mounted in the breathing zone of the gasoline pump attendants. The air around the breathing zone was drawn through the activated charcoal; it was then absorbed by the carbon disulphide and ana- lysed using gas chromatography (Perkin Elmer Auto System XL GL) with a flame ionisation detector. The instrument was positioned to established contact with the air inhaled by the exposed participants. Similar assess- ments were repeated few kilometres away from gasoline stations. 2.3. Statistical Analysis Frequencies and percentages were computed for the demographic characteristics of the subjects. The Chi- squared test was employed to test the association be- tween menstrual characteristics and exposure to petro- leum products. Furthermore, a logistic regression model was also used to examine the association between exposure to petro- leum products and menstrual disorders without and with adjustment for other possible confounders. This analysis was also performed to investigate the effect of exposure duration on menstrual disorders among exposed indi- viduals. Hence, crude and adjusted odd ratios and 95% confidence intervals were estimated. All of the statistical computations were enhanced using the statistical package for social sciences (SPSS 20.0). P values < 0.05 were considered to be statistically significant. 3. Results The socio-demographic variables of the 235 women who participated in this study revealed that 117 (49.8%) were exposed and 118 (50.2%) were unexposed. In addition, 37.9% were between the ages of 18 and 25 years, 54.5% were between 26 to 35 years, and 7.7% were between 36 and 40 years. Of the total participants, 10.6% had a primary level of education, 85.1% had a secondary level of education, and 4.3% had a tertiary level of education. In addition, 71.1% were single, 27.7% were married, and 1.3% was divorced. Those with a perceived high level of stress constituted 51.5% of the women, whereas 48.5% demonstrated a low perceived level of stress. In addition, 73.2% had a normal BMI, 20.0% were overweight, and 6.8% were obese (Ta- ble 1). The overall prevalence of menstrual disorders among the exposed and unexposed women was 37.2% and 28.5%, respectively. The results of the univariate analy- sis showed that exposure to gasoline inhalation was sig- nificantly associated with disorders in both menstrual cycle length (P = 0.009) and quantity of flow (P = 0.002). The prevalence of abnormal cycle length and quantity of flow among the exposed and unexposed women was 27.3% and 36.8% for exposed women and 11.9% and 20.3% for unexposed women, respectively (Table 2). The results of the reproductive hormone profile assay showed that the serum levels of estradiol were consis- tently low in most respondents with menstrual irregulari- ties regardless of the duration of exposure, whereas the levels of other reproductive hormones (FSH, LH, pro- gesterone and prolactin) fluctuated (Table 3). The multiple logistic regression analysis showed sig- nificant associations between exposure to gasoline and Copyright © 2013 SciRes. JEP 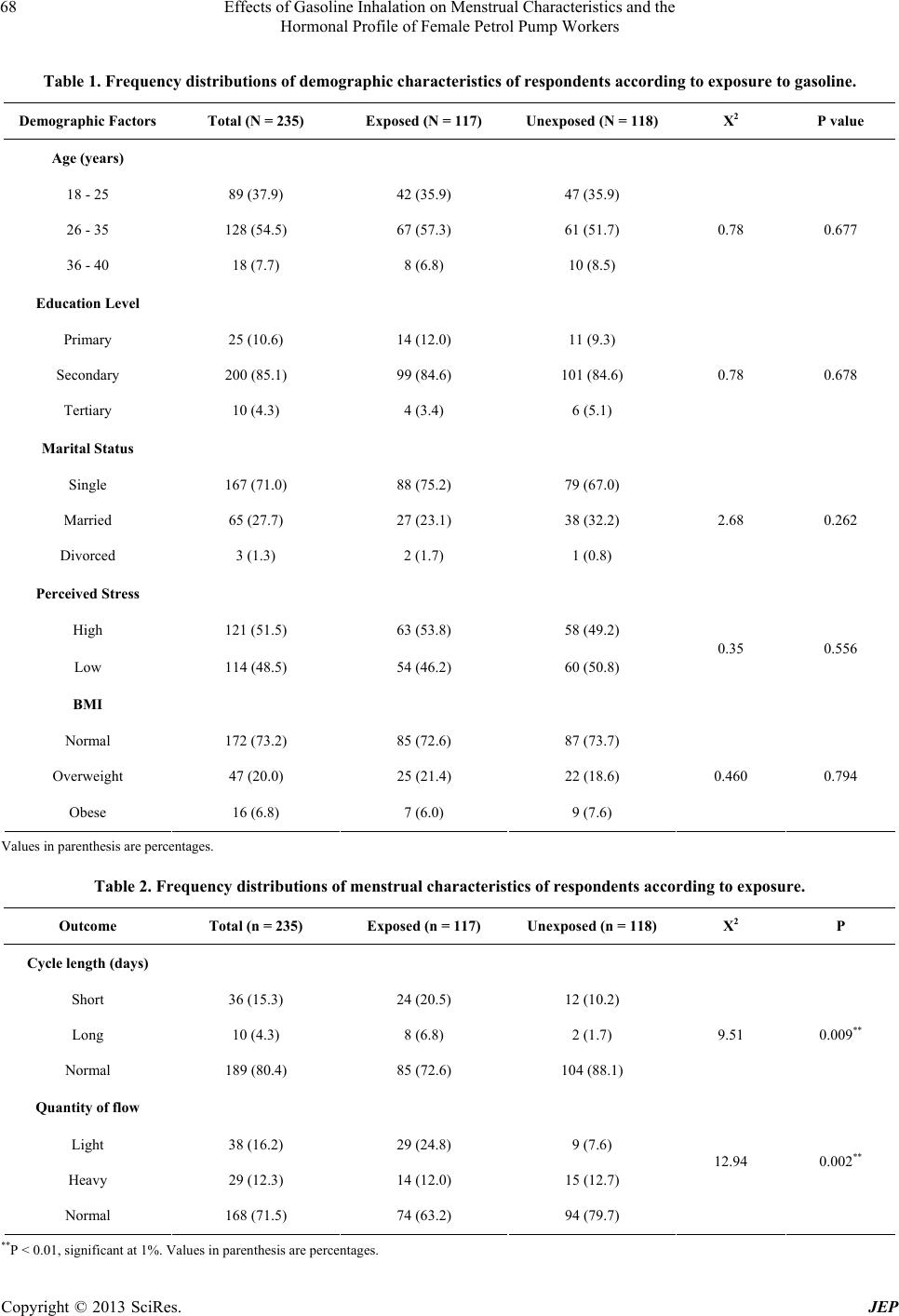 Effects of Gasoline Inhalation on Menstrual Characteristics and the Hormonal Profile of Female Petrol Pump Workers Copyright © 2013 SciRes. JEP 68 Table 1. Frequency distributions of demogr aphic characteristics of respondents according to exposure to gasoline. Demographic Factors Total (N = 235) Exposed (N = 117) Unexposed (N = 118) X2 P value Age (years) 18 - 25 89 (37.9) 42 (35.9) 47 (35.9) 26 - 35 128 (54.5) 67 (57.3) 61 (51.7) 36 - 40 18 (7.7) 8 (6.8) 10 (8.5) 0.78 0.677 Education Level Primary 25 (10.6) 14 (12.0) 11 (9.3) Secondary 200 (85.1) 99 (84.6) 101 (84.6) Tertiary 10 (4.3) 4 (3.4) 6 (5.1) 0.78 0.678 Marital Status Single 167 (71.0) 88 (75.2) 79 (67.0) Married 65 (27.7) 27 (23.1) 38 (32.2) Divorced 3 (1.3) 2 (1.7) 1 (0.8) 2.68 0.262 Perceived Stress High 121 (51.5) 63 (53.8) 58 (49.2) Low 114 (48.5) 54 (46.2) 60 (50.8) 0.35 0.556 BMI Normal 172 (73.2) 85 (72.6) 87 (73.7) Overweight 47 (20.0) 25 (21.4) 22 (18.6) Obese 16 (6.8) 7 (6.0) 9 (7.6) 0.460 0.794 Values in parenthesis are percentages. Table 2. Frequency distributions of menstr ual characteristics of respondents according to exposure. Outcome Total (n = 235) Exposed (n = 117) Unexposed (n = 118) X2 P Cycle length (days) Short 36 (15.3) 24 (20.5) 12 (10.2) Long 10 (4.3) 8 (6.8) 2 (1.7) Normal 189 (80.4) 85 (72.6) 104 (88.1) 9.51 0.009** Quantity of flow Light 38 (16.2) 29 (24.8) 9 (7.6) Heavy 29 (12.3) 14 (12.0) 15 (12.7) Normal 168 (71.5) 74 (63.2) 94 (79.7) 12.94 0.002** **P < 0.01, significant at 1%. Values in parenthesis are percentages. 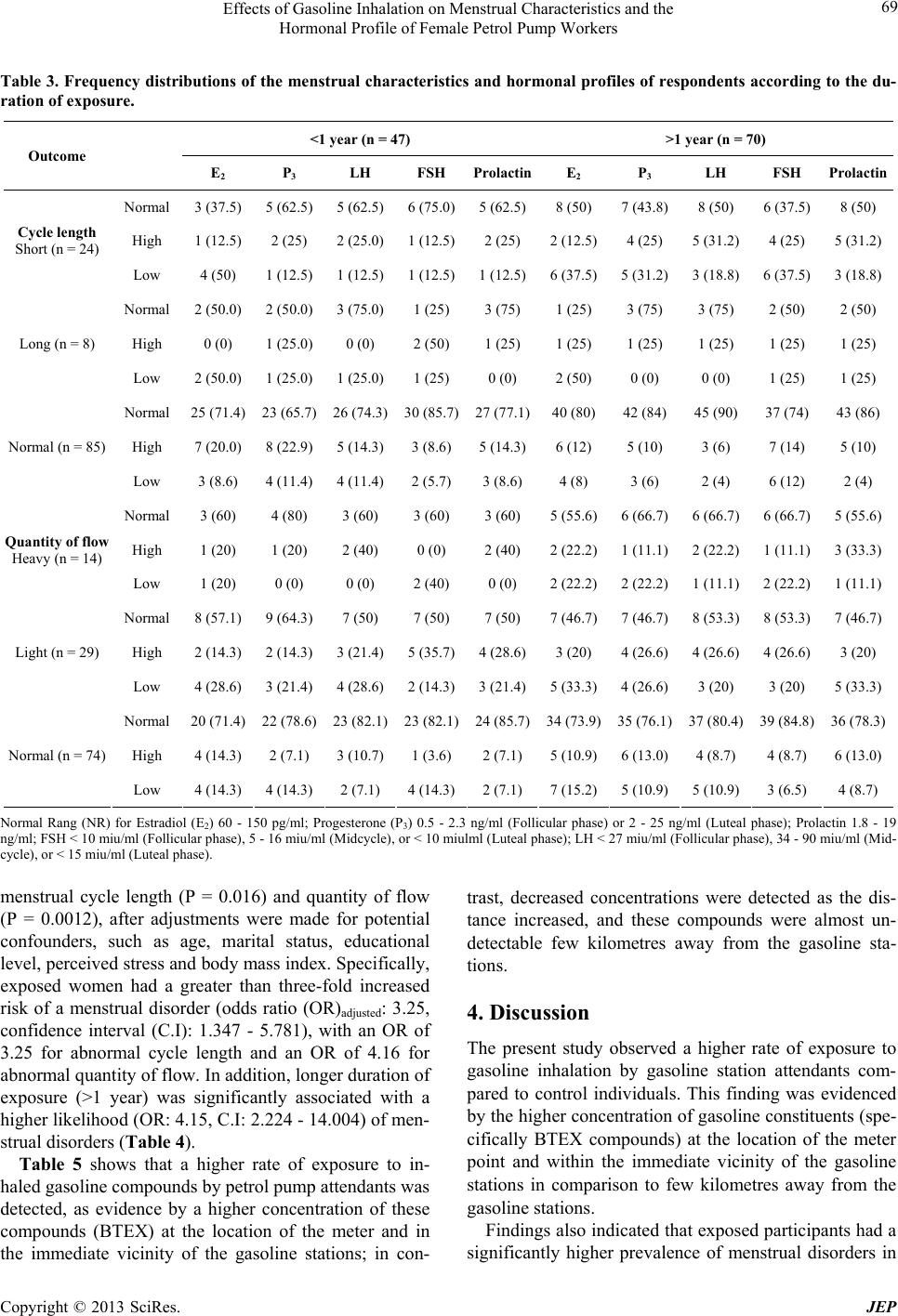 Effects of Gasoline Inhalation on Menstrual Characteristics and the Hormonal Profile of Female Petrol Pump Workers 69 Table 3. Frequency distributions of the menstrual characteristics and hormonal profiles of respondents accor ding to the du- ration of exposure. <1 year (n = 47) >1 year (n = 70) Outcome E2 P3 LH FSH ProlactinE2 P3 LH FSH Prolactin Normal 3 (37.5) 5 (62.5) 5 (62.5)6 (75.0)5 (62.5)8 (50) 7 (43.8)8 (50) 6 (37.5)8 (50) High 1 (12.5) 2 (25) 2 (25.0)1 (12.5)2 (25) 2 (12.5)4 (25) 5 (31.2) 4 (25) 5 (31.2) Cycle length Short (n = 24) Low 4 (50) 1 (12.5) 1 (12.5)1 (12.5)1 (12.5)6 (37.5)5 (31.2)3 (18.8) 6 (37.5)3 (18.8) Normal 2 (50.0) 2 (50.0) 3 (75.0)1 (25) 3 (75) 1 (25) 3 (75) 3 (75) 2 (50) 2 (50) High 0 (0) 1 (25.0) 0 (0) 2 (50) 1 (25) 1 (25) 1 (25) 1 (25) 1 (25) 1 (25) Long (n = 8) Low 2 (50.0) 1 (25.0) 1 (25.0)1 (25) 0 (0) 2 (50) 0 (0) 0 (0) 1 (25) 1 (25) Normal 25 (71.4) 23 (65.7) 26 (74.3)30 (85.7)27 (77.1)40 (80)42 (84)45 (90) 37 (74) 43 (86) High 7 (20.0) 8 (22.9) 5 (14.3)3 (8.6) 5 (14.3)6 (12) 5 (10) 3 (6) 7 (14) 5 (10) Normal (n = 85) Low 3 (8.6) 4 (11.4) 4 (11.4)2 (5.7) 3 (8.6) 4 (8) 3 (6) 2 (4) 6 (12) 2 (4) Normal 3 (60) 4 (80) 3 (60) 3 (60) 3 (60) 5 (55.6)6 (66.7)6 (66.7) 6 (66.7)5 (55.6) High 1 (20) 1 (20) 2 (40) 0 (0) 2 (40) 2 (22.2)1 (11.1)2 (22.2) 1 (11.1)3 (33.3) Quantity of flow Heavy (n = 14) Low 1 (20) 0 (0) 0 (0) 2 (40) 0 (0) 2 (22.2)2 (22.2)1 (11.1) 2 (22.2)1 (11.1) Normal 8 (57.1) 9 (64.3) 7 (50) 7 (50) 7 (50) 7 (46.7)7 (46.7)8 (53.3) 8 (53.3)7 (46.7) High 2 (14.3) 2 (14.3) 3 (21.4)5 (35.7)4 (28.6)3 (20) 4 (26.6)4 (26.6) 4 (26.6)3 (20) Light (n = 29) Low 4 (28.6) 3 (21.4) 4 (28.6)2 (14.3)3 (21.4)5 (33.3)4 (26.6)3 (20) 3 (20) 5 (33.3) Normal 20 (71.4) 22 (78.6) 23 (82.1)23 (82.1)24 (85.7)34 (73.9)35 (76.1)37 (80.4) 39 (84.8)36 (78.3) High 4 (14.3) 2 (7.1) 3 (10.7)1 (3.6) 2 (7.1) 5 (10.9)6 (13.0)4 (8.7) 4 (8.7) 6 (13.0) Normal (n = 74) Low 4 (14.3) 4 (14.3) 2 (7.1) 4 (14.3)2 (7.1) 7 (15.2)5 (10.9)5 (10.9) 3 (6.5) 4 (8.7) Normal Rang (NR) for Estradiol (E2) 60 - 150 pg/ml; Progesterone (P3) 0.5 - 2.3 ng/ml (Follicular phase) or 2 - 25 ng/ml (Luteal phase); Prolactin 1.8 - 19 ng/ml; FSH < 10 miu/ml (Follicular phase), 5 - 16 miu/ml (Midcycle), or < 10 miulml (Luteal phase); LH < 27 miu/ml (Follicular phase), 34 - 90 miu/ml (Mid- cycle), or < 15 miu/ml (Luteal phase). menstrual cycle length (P = 0.016) and quantity of flow (P = 0.0012), after adjustments were made for potential confounders, such as age, marital status, educational level, perceived stress and body mass index. Specifically, exposed women had a greater than three-fold increased risk of a menstrual disorder (odds ratio (OR)adjusted: 3.25, confidence interval (C.I): 1.347 - 5.781), with an OR of 3.25 for abnormal cycle length and an OR of 4.16 for abnormal quantity of flow. In addition, longer duration of exposure (>1 year) was significantly associated with a higher likelihood (OR: 4.15, C.I: 2.224 - 14.004) of men- strual disorders (Table 4). Table 5 shows that a higher rate of exposure to in- haled gasoline compounds by petrol pump attendants was detected, as evidence by a higher concentration of these compounds (BTEX) at the location of the meter and in the immediate vicinity of the gasoline stations; in con- trast, decreased concentrations were detected as the dis- tance increased, and these compounds were almost un- detectable few kilometres away from the gasoline sta- tions. 4. Discussion The present study observed a higher rate of exposure to gasoline inhalation by gasoline station attendants com- pared to control individuals. This finding was evidenced by the higher concentration of gasoline constituents (spe- cifically BTEX compounds) at the location of the meter point and within the immediate vicinity of the gasoline stations in comparison to few kilometres away from the gasoline stations. Findings also indicated that exposed participants had a significantly higher prevalence of menstrual disorders in Copyright © 2013 SciRes. JEP 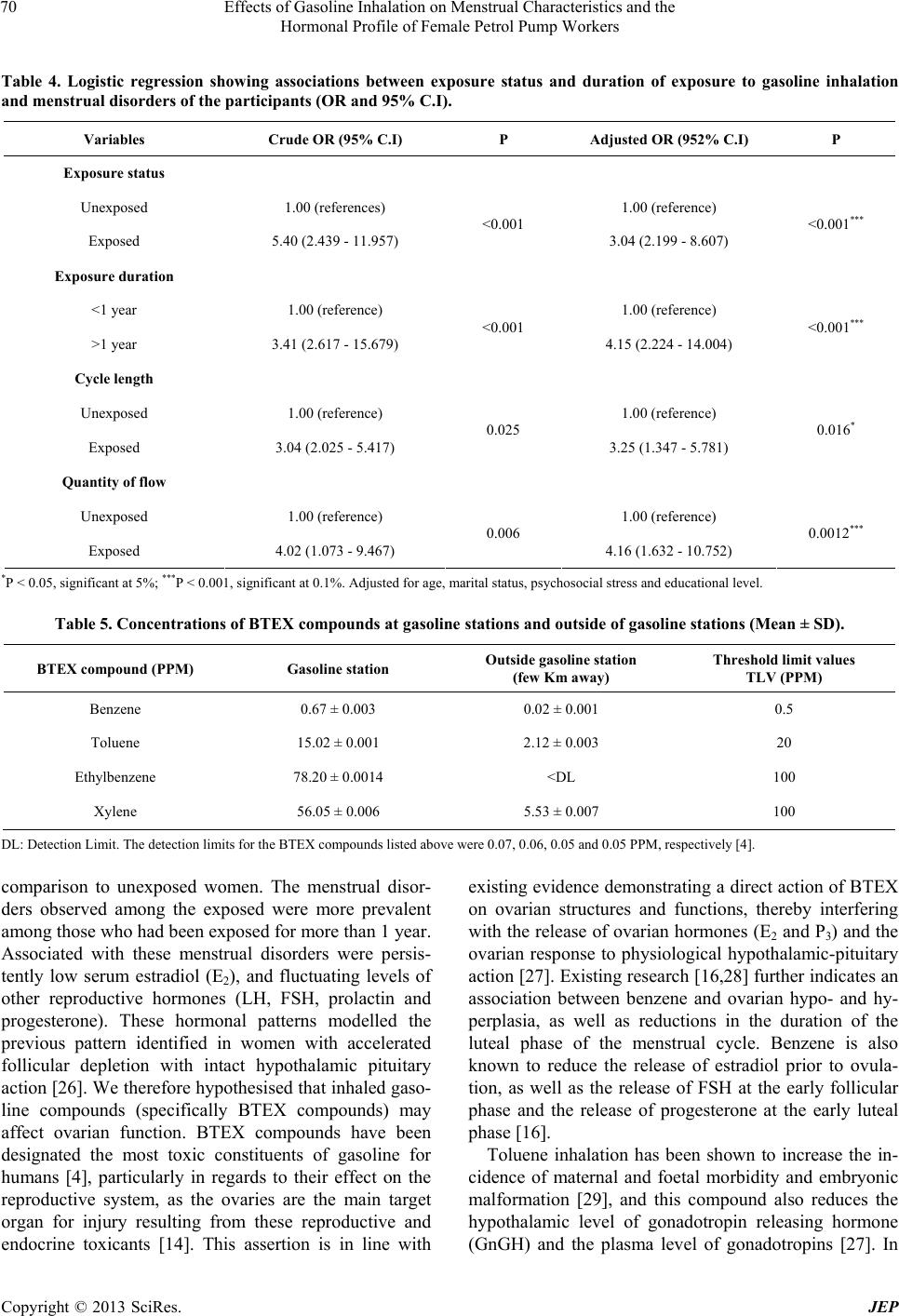 Effects of Gasoline Inhalation on Menstrual Characteristics and the Hormonal Profile of Female Petrol Pump Workers 70 Table 4. Logistic regression showing associations between exposure status and duration of exposure to gasoline inhalation and menstrual disorders of the participants (OR and 95% C.I). Variables Crude OR (95% C.I) P Adjusted OR (952% C.I) P Exposure status Unexposed 1.00 (references) 1.00 (reference) Exposed 5.40 (2.439 - 11.957) <0.001 3.04 (2.199 - 8.607) <0.001*** Exposure duration <1 year 1.00 (reference) 1.00 (reference) >1 year 3.41 (2.617 - 15.679) <0.001 4.15 (2.224 - 14.004) <0.001*** Cycle length Unexposed 1.00 (reference) 1.00 (reference) Exposed 3.04 (2.025 - 5.417) 0.025 3.25 (1.347 - 5.781) 0.016* Quantity of flow Unexposed 1.00 (reference) 1.00 (reference) Exposed 4.02 (1.073 - 9.467) 0.006 4.16 (1.632 - 10.752) 0.0012*** *P < 0.05, significant at 5%; ***P < 0.001, significant at 0.1%. Adjusted for age, marital status, psychosocial stress and educational level. Table 5. Concentrations of BTEX compounds at gasoline stations and outside of gasoline stations (Me an ± SD). BTEX compound (PPM) Gasoline station Outside gasoline station (few Km away) Threshold limit values TLV (PPM) Benzene 0.67 ± 0.003 0.02 ± 0.001 0.5 Toluene 15.02 ± 0.001 2.12 ± 0.003 20 Ethylbenzene 78.20 ± 0.0014 <DL 100 Xylene 56.05 ± 0.006 5.53 ± 0.007 100 DL: Detection Limit. The detection limits for the BTEX compounds listed above were 0.07, 0.06, 0.05 and 0.05 PPM, respectively [4]. comparison to unexposed women. The menstrual disor- ders observed among the exposed were more prevalent among those who had been exposed for more than 1 year. Associated with these menstrual disorders were persis- tently low serum estradiol (E2), and fluctuating levels of other reproductive hormones (LH, FSH, prolactin and progesterone). These hormonal patterns modelled the previous pattern identified in women with accelerated follicular depletion with intact hypothalamic pituitary action [26]. We therefore hypothesised that inhaled gaso- line compounds (specifically BTEX compounds) may affect ovarian function. BTEX compounds have been designated the most toxic constituents of gasoline for humans [4], particularly in regards to their effect on the reproductive system, as the ovaries are the main target organ for injury resulting from these reproductive and endocrine toxicants [14]. This assertion is in line with existing evidence demonstrating a direct action of BTEX on ovarian structures and functions, thereby interfering with the release of ovarian hormones (E2 and P3) and the ovarian response to physiological hypothalamic-pituitary action [27]. Existing research [16,28] further indicates an association between benzene and ovarian hypo- and hy- perplasia, as well as reductions in the duration of the luteal phase of the menstrual cycle. Benzene is also known to reduce the release of estradiol prior to ovula- tion, as well as the release of FSH at the early follicular phase and the release of progesterone at the early luteal phase [16]. Toluene inhalation has been shown to increase the in- cidence of maternal and foetal morbidity and embryonic malformation [29], and this compound also reduces the hypothalamic level of gonadotropin releasing hormone (GnGH) and the plasma level of gonadotropins [27]. In Copyright © 2013 SciRes. JEP  Effects of Gasoline Inhalation on Menstrual Characteristics and the Hormonal Profile of Female Petrol Pump Workers 71 addition, xylene was shown to decrease plasma levels of progesterone and estradiol. Although these adverse re- productive health effects are known and well docu- mented, it is noteworthy that they are not always present in all exposed individuals. For example, and similar to previous studies, we found that not all exposed gasoline workers developed abnormal menstrual characteristics or deranged reproductive hormone profiles. There are mul- tiple interpretations for this finding, including inter-indi- vidual differences due to variations in the pulmonary absorption, distribution, action, metabolism and excre- tion of inhaled gasoline compounds, which further de- pend on blood gas values and the fat-blood partition co- efficient [30]. Nonetheless, it is recognised that such differences could be due to the effects of other un-modifiable con- founders, including the age of the participants. In the present study, 36%, 6.8% and 57.3% of the exposed par- ticipants were within the perimenarchial, premenopausal and peak reproductive ages, respectively. Several lines of evidence have shown that changes in hormonal patterns, and hence menstrual irregularities with age, may be a consequence of the age-related de- cline in ovarian follicle reserve, leading to a decrease in ovarian factors (e.g., inhibin B) important for the regula- tion of ovarian pituitary feedback and a secondary de- cline in luteal function [31]. Such changes are common among women near the perimenarchial and premeno- pausal ages. The effects of inhaled solvents may also have been exacerbated in subjects within these age limits, with less of an effect for those within peak reproductive age. In a similar manner, earlier studies [27,32,33] showed that the deleterious effects of a potentially toxic solvent on the endocrine system generally require longer dura- tions of exposure and repeated absorption [15]. This may provide an explanation for the positive correlation be- tween the duration of exposure and the severity of the reproductive dysfunction present in a subset of the fe- male workers in the present study. Moreover, as in pre- vious studies that compared other constituents of gaso- line, benzene was found to be present in concentrations high enough to cause adverse reproductive health out- comes, and the plausibility of these findings is supported by the study of Sexton et al. [34]. According to that study, exposure to benzene above the threshold limit value was associated with higher ad- verse reproductive effects [34]. This assertion was later supported by the study of Mehrjerdi et al. [35], which was conducted at gas stations in the Yaza province. These authors found that, compared to other constituents of BTEX compounds, benzene was present in concentra- tions above the threshold limit value (0.5 PPM). In con- trast, toluene, ethylbenzene and xylene demonstrated mean concentrations below the threshold limit [4], as observed in the present study. Previous studies have proposed several conflicting opinions regarding the putative mechanisms of action of endocrine disrupters such as BTEX compounds that lead to adverse reproductive outcomes. While some of these authors have speculated on the direct effect of endocrine disrupters on steroidogenesis [2], by directly interfering with steroidogenic enzymes and acting as potent enzyme inhibitors to produce a low concentration of female sex hormones, others found less consistent or no effect on steroidogenic enzymes and hence hormone synthesis [13]. These apparently conflicting observations can be re- solved when certain facts are considered, including dif- ferences in chemical properties and the molecular prop- erties and pharmacodynamics of different endocrine dis- rupters. Other possible mechanisms include solvent in- terference with the hepatic microsomal enzyme system responsible for endogenous hormone metabolism [15]. In addition, Guisti et al. [35] proposed the alteration of uterine structure as a potential mechanism, and according to Kim et al. [2], BTEX compounds may exert their ac- tions at the level of the hypothalamic-pituitary axis to interfere with trophic hormones secretion and lead to down-regulation of gonadotropin releasing hormone (GnRH), GnRH receptors and pituitary-1 receptor mRNA levels. In particular, these authors [2] demon- strated that injection of toluene and xylene led to a sig- nificant reduction in the serum concentrations of GnRH, GnRH receptor and pituitary-1 mRNA in experimental animals [2]. Furthermore, knowledge of the different levels of action of endocrine disrupters may help differ- entiate hypothalamic-pituitary dysfunction from direct germ cell injury [19]; in hypothalamic-pituitary dysfunc- tion, the serum levels of gonadotropin hormones (FSH and LH) are low, whereas in germ cell affectation, high FSH levels with associated low E2 serum concentrations are typical. In support of the persistently low levels of E2 but fluc- tuating levels of FSH and LH observed in most exposed participants with menstrual disorders in the present study, it may be accurate to conclude, in agreement with previ- ous studies [27], that BTEX compounds interfere with the ovarian cycle (particularly during the follicular phase) in individuals exposed to gasoline fumes. The differences in the serum levels of FSH and LH observed among ex- posed participants may be ascribed to differences in the regulation of FSH and LH secretion and diminished E2 concentrations at the hypothalamic or pituitary levels or differential sensitivity to gonadotropin releasing hor- mones [36]. Copyright © 2013 SciRes. JEP  Effects of Gasoline Inhalation on Menstrual Characteristics and the Hormonal Profile of Female Petrol Pump Workers 72 5. Conclusion The present study provides additional evidence to sup- port the positive association between exposure to gaso- line inhalation and the development of adverse reproduc- tive endpoints, including menstrual and reproductive hormone profile abnormalities suggestive of ovarian functional affectations, specifically during the follicular phase. However, this association is likely influenced by several modifiable and un-modifiable confounders. REFERENCES [1] S. Kumar, “Occupational Exposure Association with Re- productive Dysfunction,” Journal of Occupational Health, Vol. 46, No. 1, 2004, pp. 1-19. doi:10.1539/joh.46.1 [2] D. H. Kim, H. Lee, C. K. Lee, D. S. Kang, J. H. Kin, J. T. Lee, J. H. Chun and C. K. Lee, “Effects of Toluene, Xy- lene and Trichloroethylene on the Regulation of GnRH, RnRH Reception and Pit-1 Gene Expression in male Rat Hypothalamus and Pituitary,” Korean Journal of Occu- pational and Environmental Medicine, Vol. 10, No. 2, 1998, pp. 267-281. [3] B. Runnebaum, T. Rabe, M. Sillem and W. Eggert-Kruse, “Infertility,” In: B. Runnebaum and T. Rabe, Eds., Gyne- cological Endocrinology and Reproductive Medicine, Springer-Verlag, New York, 1997, pp. 107-164. doi:10.1007/978-3-642-60390-7 [4] M. R. Azari, Z. N. Konjin, F. Z. Salehpour and M. D. Seyedi, “Occupational Exposure of Petroleum Depot workers to BTEX Compounds,” International Journal of Occupational and Environmental Medicine, Vol. 3, No. 1, 2012, pp. 39-44. [5] R. Cecil, R. J. Ellison, K. Larminaa, S. A. Margary, J. M. Mata and L. Morcillo, “Exposure Profile: Gasoline,” CONCAWE Report, CONCAWE, Brussels, 1997. [6] S. C. Edminster and M. J. Bayer, “Recreational Gasoline Sniffing, Acute Gasoline Intoxication and Latent Or- ganolead Poisoning: Case Reports and Literature Review,” Journal of Emergency Medicine, Vol. 3, No. 5, 1985, pp. 365-370. doi:10.1016/0736-4679(85)90321-X [7] S. Cairney, P. Maruff, C. Burns and B. Currie, “The Neurobehavioural Consequences of Petrol (Gasoline) Sniffing,” Neuroscience and Biobehavioural Reviews, Vol. 26, No. 1, 2002, pp. 81-89. doi:10.1016/S0149-7634(01)00040-9 [8] W. Machle, “Gasoline Intoxication,” Journal of the American Medicinal Association, Vol. 177, No. 23, 1941, pp. 1965-1971. doi:10.1001/jama.1941.02820490039013 [9] C. C. Ugwoke, E. D. Nwobodo, P. Unekwe, M. Odike, S. T. Chukwuma and G. Amilo, “The Reproductive Dys- function Effects of Gasoline Inhalation in Albino Rats,” Nigerian Journal of Physiological Sciences, Vol. 20, No. 1-2, 2005, pp. 54-57. [10] L. Fraier and M. L. Hage, “Reproductive Hazard of Workplaces,” Wiley, New York, 1997. [11] C. N. Humfrey and L. L. Smith, “Endocrine Disrupting Chemicals,” In: P. W. Harvey, K. C. Rush and A. Cock- born, Eds., The Evidence for Human Health Effects in Endocrine and Hormonal Toxicity, John Wiley and Sons, New York, 1999, p. 421. [12] R. J. Kavlock, et al., “Research Needs for the Risk As- sessment of Health and Environmental Disruptors: A Report of U.S. EPA-Sponsored Workshop,” Environ- mental Health Perspectives, Vol. 104, No. S4, 1996, pp. 715-740. [13] P. Nicolopoulou-Stamati and M. A. Pitsos, “The Impact of Endocrine Disrupters on the Female Reproductive Sys- tem,” Human Reproductive Update, Vol. 7, No. 3, 2000, pp. 323-330. doi:10.1093/humupd/7.3.323 [14] D. R. Mattison, D. R. Plowehalk, M. J. Meadow, A. Z. Al-Juburi, J. Gardy and A. Malek, “Reproductive Toxic- ity: Male and Female Reproductive Systems as Targets for Chemical Injury,” Medical Clinics of North America, Vol. 74, No. 2, 1990, pp. 391-411. [15] Y. Verma and S. V. Rana, “Endocrinal Toxicity of Indus- trial Solvents—A Mini Review,” Indian Journal of Ex- perimental Biology, Vol. 47, No. 7, 2009, pp. 537-549. [16] H. Chen, L. Song, X. Wang and S. Wang, “Effect of Ex- posure to Low Concentration of Benzene and Its Ana- logues on Luteal Function of Female Workers,” Wei Sheng Yan Jiu, Vol. 29, No. 6, 2000, pp. 351-353. [17] V. G. Matysiak, “The Effect of Benzene Fumes on the Functional Activity of Hypophysis, Adrenals and Ovaries of White Rats under Experimental Condition,” Godisen Zbornik na Medicinskiot Fakultet vo Skopje, Vol. 14, 1968, pp. 98-100. [18] S. Rowland, D. D. Barid, G. Longs, S. D. Wegienka, M. Harlow and D. P. Alavanja, “Influence of Medical Condi- tions and Life Style Factors on the Menstrual Cycle,” Epidemiology, Vol. 13, No. 6, 2002, pp. 668-674. doi:10.1097/00001648-200211000-00011 [19] M. Paul, “Occupational Reproductive Hazards,” The Lan- cet, Vol. 349, No. 9062, 1997, pp. 1385-1388. doi:10.1016/S0140-6736(96)07217-0 [20] C. C. Lin, C. N. Huang, Y. H. Hwang, J. D. Wang, S. P. Weng, R. H. Shie and P. C. Chem, “Shortened Menstrual Cycles in LCD Manufacturing Workers,” Occupational Medicine, Vol. 63, No. 1, 2013, pp. 45-52. doi:10.1093/occmed/kqs172 [21] G. Pandya, A. Gavane and V. Kondawar, “Assessment of Occupational Exposure to VOCs at the Gantry Gasoline terminal,” Environmental Science and Engineering, Vol. 48, No. 3, 2006, pp. 175-182. [22] N. Hopf, J. Kirkeleit, M. Bratveit, P. Succop, G. Talaska and B. E. Moen, “Evaluation of Exposure Biomarkers in Offshore Workers Exposed to Low Benzene and Toluene Concentrations,” International Archives of Occupational and Environmental Health, Vol. 85, No. 3, 2011, pp. 261- 271. [23] Y. Lui, E. B. Gold, B. L. Lasley and W. O. Johnson, “Factors Affecting Menstrual Cycle Characteristics,” American Journal of Epidemiology, Vol. 160, No. 2, 2004, pp. 131-140. doi:10.1093/aje/kwh188 Copyright © 2013 SciRes. JEP  Effects of Gasoline Inhalation on Menstrual Characteristics and the Hormonal Profile of Female Petrol Pump Workers Copyright © 2013 SciRes. JEP 73 [24] G. Toft, A. Axmon, C. Lindh, A. Giwereman and J. P. Bonde, “Menstrual Cycle Characteristics in European and Inuit Women Exposed to Persistent Organochlorine Pol- lutants,” Human Reproduction, Vol. 23, No. 1, 2008, pp. 193-200. doi:10.1093/humrep/dem349 [25] World Health Organization (WHO), “Obesity: Preventing and Managing the Global Epidemic: Environmental and Societal Influences,” WHO, Geneva, 2000, pp. 118-122. [26] M. J. Faddy, R. G. Gasden, A. Gougeon, S. J. Richardson and J. F. Nelson, “Accelerated Disappearance of Ovarian Follicles in Mid-Life: Implication for Forecasting Meno- pause,” Human Reproduction, Vol. 7, No. 10, 1992, pp. 1342-1346. [27] V. Sirotkin, A. Kâdas, A. Balâžá, Z. Baková, Z. Halim, A. Harrath, A. V. Makarevich, A. Kolesárova, P. Chrenek, J. Kotwica and T. Tόth, “Influence of Petrochemical Indus- try Environment Contaminants on Animal Ovarian Cells,” Journal of Microbiology, Biotechnology and Food Sci- ences, Vol. 32, No. 2, 2012, pp. 517-525. [28] R. R. Maronpot, “Ovarian Toxicity and Carcinogenicity in Eight Recent National Toxicology Program Studies,” Environmental Health Perspectives, Vol. 73, 1987, pp. 125-130. doi:10.1289/ehp.8773125 [29] J. H. Hannigan and E. S. Bowen, “Reproductive Toxi- cology and Teratology of Abused Toluene,” Systems Bi- ology in Reproductive Medicine, Vol. 56, No. 2, 2010, pp. 184-200. [30] A. Sato and T. Nkajima, “Pharmacokinetics of Organic Solvent Vapors in Relation to Their Toxicity,” Scandina- vian Journal of Work, Environment and Health, Vol. 13, No. 2, 1987, pp. 81-93. [31] D. M. Robertson, G. E. Hale, D. Jolley, I. S. Fraser, C. L. Hughes and H. G. Burger, “Interrelationships between Ovarian and Pituitary Hormones in Ovulatory Menstrual Cycles across Reproductive Age,” Journal of Clinical Endocrinology and Metabolism, Vol. 91, No. 1, 2009, pp. 138-144. [32] M. Mukhametova and Vozovaya, “Reproductive Power and the Incidence of Gynecological Disorders in Female Workers Exposed to the Combined Effect of Benzene and Chlorinated Hydrocarbons,” Gig Tr Prof Zabol, Vol. 16, 1972, pp. 6-9. (in Russian) [33] X. Y. Huang, “Influence on Benzene and Toluene to Re- productive Functions of Female Workers in Leather Shoe Making Industry,” Chinese Journal of Preventive Medi- cine, Vol. 25, No. 2, 1991, pp. 89-91. [34] K. Sexton, S. H. Linder, D. Marko, H. Bethal and P. J. Lupo, “Comparative Assessment of Air Pollution-Related Health Risks in Houston,” Environmental Health Per- spectives, Vol. 115, No. 10, 2007, pp. 1388-1393. [35] R. M. Guisti, K. Iwamoto and E. E. Hatch, “Diethylstil- bestrol Revisited: A Review of the Long-Term Health Effects,” Annals of Internal Medicine, Vol. 122, No. 10, 1995, 778-788. doi:10.7326/0003-4819-122-10-199505150-00008 [36] B. M. Sherman and S. G. Koreman, “Hormonal Charac- teristics of Human Menstrual Cycle throughout Repro- ductive Life,” The Journal of Clinical Investigation, Vol. 55, No. 4, 1975, pp. 699-706. doi:10.1172/JCI107979
|