 Open Journal of Genetics, 2013, 3, 59-66 OJGen http://dx.doi.org/10.4236/ojgen.2013.32A3009 Published Online August 2013 (http://www.scirp.org/journal/ojgen/) The I550V polymorphism in the renal human sodium/dicarboxylate cotransporter 1 (hNaDC-1) gene is associated with the risk for urolithiasis in adults from Southeastern, Mexico Martha Medina-Escobedo1*, Diana Franco-Bocanegra1, Salha Villanueva-Jorge1, Lizbeth González-Herrera2 1Departamento de Investigación en Enfermedades Renales, Hospital General O’Horán, Secretaria de Salud de Yucatán, Mérida, México 2Laboratorio de Genética, Centro de Investigaciones Regionales, Universidad Autónoma de Yucatán, Mérida, México Email: *marthamedinaescobedo@hotmail.com Received 10 July 2013; revised 31 July 2013; accepted 5 August 2013 Copyright © 2013 Martha Medina-Escobedo et al. This is an open access article distributed under the Creative Commons Attribu- tion License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Urolithiasis (UL) is an endemic disease in Southeast- ern, Mexico. In order to evaluate the association of I550V polymorphism in the hNaDC-1 gene with risk for hypocitraturia and/or for UL; 139 adults with UL and 132 adults without UL, were included under a case-control association study. Citrate levels in 24-h urine were quantified (citraturia). The polymorphism I550V-hNaDC-1 was determined by PCR-RFLP. Sta- tistical analysis was performed using the STATA10.2 software. Comparison of genotype a nd allele frequen- cies between subjects with and without UL showed significant differences for genotype bb (OR = 2.34, CI: 1.19 - 4.59, p = 0.01) and for allele b (OR = 1.62, CI: 1.15 - 2.28, p = 0.005), suggesting an association with the risk for UL. Comparison of genotype and allele frequencies between subjects with hypocitraturia and subjects with normocitraturia, did not show any sig- nificant difference (p > 0.05), suggesting that this polymorphism is not associated with the risk of hypo- citraturia. Interestingly, the risk for UL was in- creased due to an additive effect of hypocitraturia with the genotype bb (OR = 6.6, CI: 2.38 - 18.28, p = 0.0002) or with the allele b (OR: 4.2, CI = 2.52 - 6.97, p < 0.0001) in the studied population. Keywords: Hypocitraturia; hNaDC-1; Urolithiasis; Mexico; Yucatan 1. INTRODUCTION The population of Yucatan, Mexico, is characterized by a high prevalence of urolithiasis (UL) [1]. Hypocitraturia, which is considered as a urine citrate excretion below 320 mg in 24 hours [2], represents the most common me- tabolic abnormality in adults with UL [3]. Hypocitraturia is known to be a major risk factor for the development of lithiasis [4-7], because of citrate acts as a calcium che- lating agent, which reduces the formation of oxalate and calcium phosphate, thus inhibiting the generation of uri- nary calculus [8]. In nephrons, urine citrate is reabsorbed from the glo- merular filtrate in the apical membrane of kidney proxi- mal tubule cells by means of the transmembrane protein known as human sodium/dicarboxylate cotransporter 1 (NaDC-1), through a sodium-dependent mechanism [9]. In these cells, NaDC-1 is responsible for the reabsorption of other di- and tri-carboxylic acids most of which are citric acid cycle intermediates, such as fumarate, succi- nate and α-ketoglutarate, which are compounds involved in numerous important physiological processes in the body [10]. NaDC-1 is a protein with 593 amino acids, which con- tains 11 transmembrane domains. The gene hNaDC-1 belongs to the family of genes encoding the SLC13 sol- ute carriers [11] and; it is located on locus 17p13.2. The coding portion of the hNaDC-1 gene contains 1953 base pairs in 12 exons [9]. Certain single nucleotide poly- morphisms (SNPs) in the hNaDC-1 gene, have been de- scribed to produce differences in the rate of gene expres- *Corresponding author. OPEN ACCESS  M. Medina-Escobedo et al. / Open Journal of Genetics 3 (2013) 59-66 60 sion as well as, significant variation in the affinity to so- dium and to various substrates in NaDC-1 protein; such conditions would alter the rate of tubular reabsorption of these compounds [12]. One of these SNPs, I550V, consists of a substitution of adenine by guanine at position 1716, in exon 12, which produces a change in amino acid 550 of NaDC-1 protein from isoleucine to valine. This SNP has been associated with the risk of hypocitraturia in recurrent renal stone formers [13]. However, those results have not yet been reproduced in any other population. Also, such study found no direct association between the I550V SNP and UL [13], nor the influence of the SNP on either the rate of gene expression or the NaD C-1 transport activity have been proven through in vitro studies [12]. Based on the above and in order to provide evidence to help determine the role performed by this SNP in the genetic risk of UL, this study focuses on evaluating the association of I550V SNP in hNaDC-1 gene with the genetic risk for develop hypocitraturia and/or urolithiasis in a population of adult subjects from Southeastern Mexico, where UL is highly prevalent and considered endemic. 2. MATERIALS AND METHODS 2.1. Population We performed a case-control association study. The sam- ple consisted of 271 subjects, 95 men and 176 women, above 17 years old. The case group included 139 subjects with urolithiasis and 132 subjects without urolithiasis were used as controls. UL subjects were 51 (36.7%) men and 88 (63.3%) women, with mean age of 34 ± 10.5 years; whereas control subjects were 44 (33.3%) men and 88 (66.7%) women, with mean age of 36.2 ± 10.6 years. The presence or absence of UL in each subject was confirmed by ultrasound or X-ray. Subjects with urinary tract obstruction diseases, diabetes mellitus, hypertension, asthma, bronchitis, kidney failure, metabolic acidosis and those with prolonged medical therapy for any disease; were excluded. Likewise, the control group did not in- clude subjects with a history of chronic disease or of diu- retic drug intake. It should be noted that during the study the subjects continued their usual diet without modifica- tions. All selected subjects were born and had lived for at least three generations in Yucatan, Mexico. We also used anthropologic and demographic parameters such as lan- guage, birth place, surnames, genealogy, and history of lifestyle, among others, to match ethnically control with case subjects belonging to the same ethnic group of Mes- tizos, defined as individuals born in the country having a Spanish-derived last name, and with a family history of Mexican ancestors back at least to the third generation. In addition, we previously determined the absence of substructure or population stratification within the popu- lation of Yucatan by using 16 autosomal STR markers [14], which may represent a confounder. Informed consent was obtained from cases and con- trols according to the recommendations of the Helsinki Declaration. This study was approved by the Bioethics Committee of Hospital General Agustin O’Horan de Me- rida, Yucatan, México. 2.2. Determination of Urine Citrate Levels (Citraturia) To determine citrate excretion, a 24-h urine sample was collected. The concentration of citrate in urine was deter- mined using a commercial citrate determination kit (Boe- hringer-Mannheim Cat # 10139076035) and citraturia was expressed in terms of mg/day measured in 24-hour urine samples. Additionally, urea and creatinine serum levels were measured to verify proper kidney function [15,16]. 2.3. Genotyping Leukocyte DNA extraction with anticoagulant (EDTA) from peripheral blood was carried out by a conventional phenol-chloroform method [17]. The polymerase chain reaction technique (PCR) was used to amplify a 520 bp fragment of exon 12 at the hNaDC1 gene, wherein the I550V SNP is located. Each 25 μl PCR reaction con- tained 18.3 μl of sterile H2O, 2.5 μl of 5X Green reaction buffer (PromegaCat#M5005), 1.5 mM MgCl2, 0.4 μM dNTPs, 0.3 μM of each primer; and 0.65u of Taq poly- merase enzyme (Promega). The sequences of the primers used were 5’-ACGGGAGGACTTCCCAGAGA-3’ for the forward primer and 5’-CAGGCGCACACATATC- GCA-3’ for the reverse primer, previously described by Okamoto et al. 2007 [13]. The prepared samples were placed in an Applied Biosystems Veriti thermocycler with the following PCR conditions: initial denaturation for 1 min at 94˚C, 30 cycles of denaturation for 1 min at 94˚C, annealing for 1 min at 59˚C and extension for 1 min at 72˚C and finally 10 min final extension at 72˚C, followed by cooling for 5 min at 4˚C. The amplified DNA was incubated at 50˚C overnight in the presence of the restriction endonuclease Bcl I (Bacillus caldolyticus I) [13]. Each restriction reaction contained 10 μl of ampli- fied DNA, 7.62 μl of sterile H2O, 2 μl of buffer C, 0.2 BSA and 1.8u of Bcl I restriction enzyme Promega Cat # R4654). The Bcl I enzyme cuts only those fragments which have adenine (B allele) instead of guanine (b al- lele). When cut, the 520 bp fragment produces two frag- ments, one with 354 bp and one with 166 bp. After in- cubation, the samples were again subject to polyacryla- mide gel electrophoresis, from which genotypes were identified, according to Okamoto et al. [13]. Copyright © 2013 SciRes. OPEN ACCESS  M. Medina-Escobedo et al. / Open Journal of Genetics 3 (2013) 59-66 Copyright © 2013 SciRes. 61 OPEN ACCESS 2.4. Statistical Analysis Mean levels of citrate excretion between cases and con- trols, as well as among genotypes were compared with Student t test. A value of p < 0.05 was considered sig- nificant. Hardy-Weinberg equilibrium deviations were determined in the studied population by means of Chi square test. Genotype and allele frequencies of I550V SNP were also evaluated and compared between cases and controls in order to determine significant associa- tions. To determine whether the studied genotypes or al- leles conferred a significant increase to the risk of hypo- citraturia and/or UL, we calculated the odds ratio (OR) with a confidence interval (CI) of 95%, using the Woolf approximation. Significance was considered when p < 0.05. All statistical tests were performed using the STATA version 10.2 software. 3. RESULTS Genotype and allele frequencies of the I550V polymer- phism in hNaDC gene in 139 subjects with urolithiasis and 132 subjects without urolithiasis are shown in Table 1. Genotype frequencies for the I550V polymorphism in hNaDC-1 gene were distributed according to Hardy- Weinberg equilibrium expectations (p > 0.05). The three I550V-hNaDC-1 SNP genotypes were observed in the studied population, being the heterozygous genotype Bb the most frequent in control subjects, whereas the ho- mozygous bb genotype was the most frequent in subjects with UL. The frequency of the allele b in the control population was 47.73%. Comparison of genotype and allele frequencies between subjects with and without UL, showed significant differences for the homozygous genotype bb (p = 0.017); and for the allele b (p = 0.005); suggesting that the I550V polymorphism in hNaDC gene is associated with the genetic risk for UL in the studied population. Mean levels of citrate excretion obtained in subjects with UL were 309 ± 223 mg/24h, which were signifi- cantly lower than mean levels of citrate excretion in con- trol subjects of 408 ± 187 mg/24h (p = 0.0001). This finding remarked the hipocitraturic condition en cases of UL. Moreover, hypocitraturia (<320 mg/24h) was sig- nificantly more frequent in cases of UL (61.9%, N = 86), than in control subjects (38.1%, N = 53), suggesting that hypocitraturia might be an associated risk factor for UL in the studied population (OR = 2.5, CI: 1.49 - 4.20, p < 0.001). According to the levels of citrate excretion, studied subjects were stratified in hypocitraturia and normocit- raturia in order to evaluate its association with the I550V polymorphism in hNaDC-1 gene, independently of the UL. We identified an overall of 138 subjects with hypo- citraturia and 132 subjects with normocitraturia in our population. Genotype and allele frequencies of the I550V polymorphism in hNaDC-1 gene in subjects with hypo- citraturia and subjects with normocitraturia, as well as the comparison of frequencies on an association analysis are listed in Table 2. Genotype frequencies for the I550V polymorphism in hNaDC-1 gene was distributed accord- ing to Hardy-Weinberg equilibrium expectations (p > 0.05). The heterozygous genotype Bb was the most fre- quent in both subjects with hypocitraturia (50.72%) and normocitraturia (40.60%). The comparison of genotype and allele frequencies of I550V polymorphism in hNaDC- 1 gene between subjects with hypocitraturia and subjects with a normal level of citrate excretion (normocitraturia) did not show significant difference for any genotype or allele (p > 0.05), suggesting that the I550V polymer- phism in hNaDC-1 gene is not associated with the risk for low levels of citrate excretion or with the risk for hy- pocitraturia within the studied population. Mean levels of citrate excretion according to genotype for I550V-NaDC-1 polymorphism, are shown in Table 3. Mean levels of citrate excretion for each genotype were compared within genotypes, showing no significant dif- Table 1. Genotype and allele frequencies of the I550V polymorphism in hNaDC-1 gene in subjects with urolithiasis (UL) and subjects without lithiasis, Hardy-Weinberg expectations; and association analysis (OR, IC, p), N (%). Cases vs. control Genotypes and Alleles of the SNP I550V in hNaDC-1 gene Subjects with urolithiasisSubjects without urolithiasis OR 95% CI p N = 139 N = 132 2N = 278 2N = 264 BB 29 (20.86) 34 (25.76) Reference Bb 54 (38.85) 70 (53.03) 0.90 0.49 - 1.66 0.757 bb 56 (40.29) 28 (21.21) 2.34 1.19 - 4.59 0.017** Allele B 112 (40.29) 138 (52.27) Reference Allele b 166 (59.71) 126 (47.73) 1.62 1.15 - 2.28 0.005** Hardy-Weinberg (p) (0.291) (0.905) **significant. 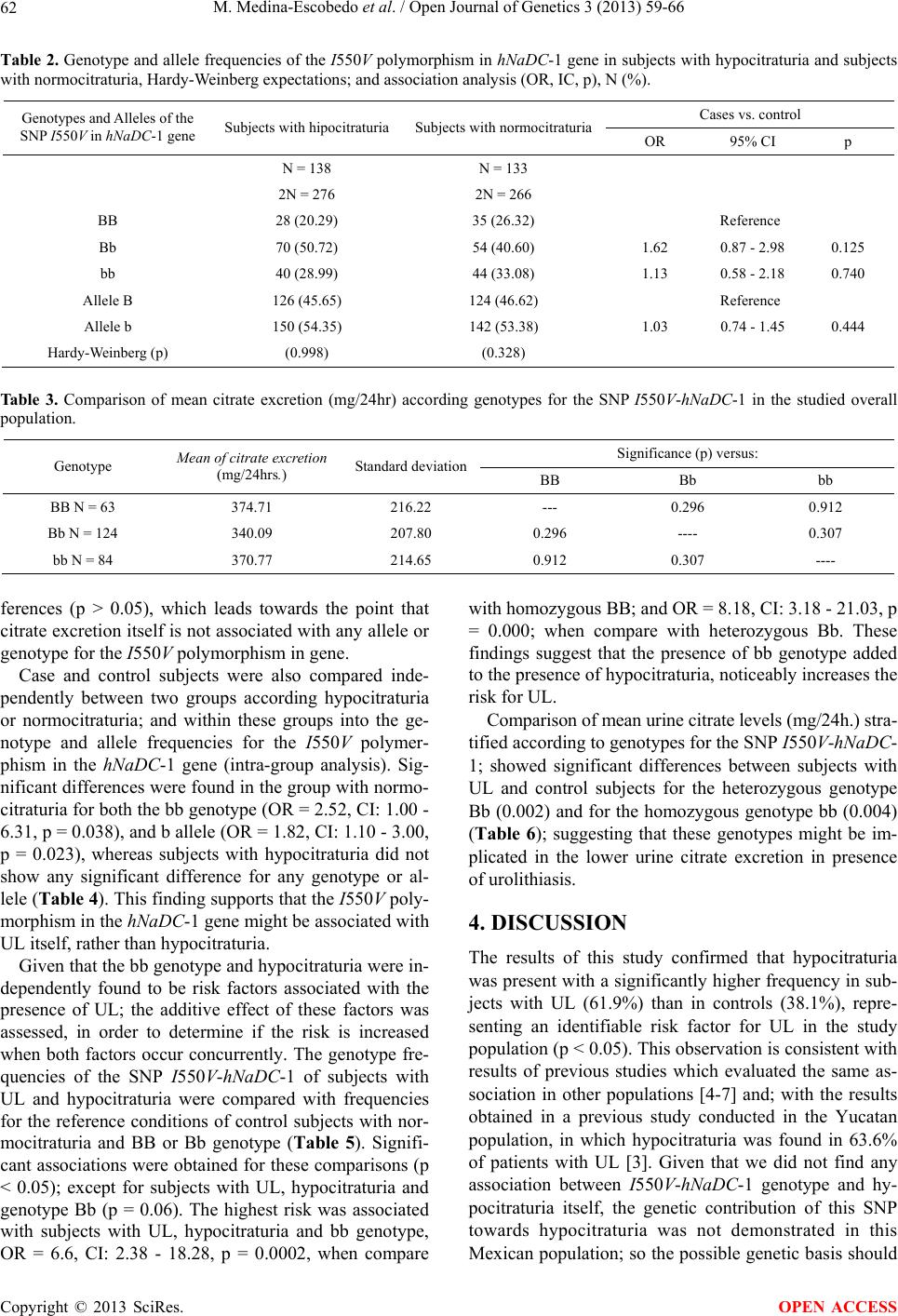 M. Medina-Escobedo et al. / Open Journal of Genetics 3 (2013) 59-66 62 Table 2. Genotype and allele frequencies of the I550V polymorphism in hNaDC-1 gene in subjects with hypocitraturia and subjects with normocitraturia, Hardy-Weinberg expectations; and association analysis (OR, IC, p), N (%). Cases vs. control Genotypes and Alleles of the SNP I550V in hNaDC-1 gene Subjects with hipocitraturiaSubjects with normocitraturia OR 95% CI p N = 138 N = 133 2N = 276 2N = 266 BB 28 (20.29) 35 (26.32) Reference Bb 70 (50.72) 54 (40.60) 1.62 0.87 - 2.98 0.125 bb 40 (28.99) 44 (33.08) 1.13 0.58 - 2.18 0.740 Allele B 126 (45.65) 124 (46.62) Reference Allele b 150 (54.35) 142 (53.38) 1.03 0.74 - 1.45 0.444 Hardy-Weinberg (p) (0.998) (0.328) Ta b l e 3 . Comparison of mean citrate excretion (mg/24hr) according genotypes for the SNP I550V-hNaDC-1 in the studied overall population. Significance (p) versus: Genotype Mean of citrate excretion (mg/24hrs.) Standard deviation BB Bb bb BB N = 63 374.71 216.22 --- 0.296 0.912 Bb N = 124 340.09 207.80 0.296 ---- 0.307 bb N = 84 370.77 214.65 0.912 0.307 ---- ferences (p > 0.05), which leads towards the point that citrate excretion itself is not associated with any allele or genotype for the I550V polymorphism in gene. Case and control subjects were also compared inde- pendently between two groups according hypocitraturia or normocitraturia; and within these groups into the ge- notype and allele frequencies for the I550V polymer- phism in the hNaDC-1 gene (intra-group analysis). Sig- nificant differences were found in the group with normo- citraturia for both the bb genotype (OR = 2.52, CI: 1.00 - 6.31, p = 0.038), and b allele (OR = 1.82, CI: 1.10 - 3.00, p = 0.023), whereas subjects with hypocitraturia did not show any significant difference for any genotype or al- lele (Table 4). This finding supports that the I550V poly- morphism in the hNaDC-1 gene might be associated with UL itself, rather than hypocitraturia. Given that the bb genotype and hypocitraturia were in- dependently found to be risk factors associated with the presence of UL; the additive effect of these factors was assessed, in order to determine if the risk is increased when both factors occur concurrently. The genotype fre- quencies of the SNP I550V-hNaDC-1 of subjects with UL and hypocitraturia were compared with frequencies for the reference conditions of control subjects with nor- mocitraturia and BB or Bb genotype (Table 5). Signifi- cant associations were obtained for these comparisons (p < 0.05); except for subjects with UL, hypocitraturia and genotype Bb (p = 0.06). The highest risk was associated with subjects with UL, hypocitraturia and bb genotype, OR = 6.6, CI: 2.38 - 18.28, p = 0.0002, when compare with homozygous BB; and OR = 8.18, CI: 3.18 - 21.03, p = 0.000; when compare with heterozygous Bb. These findings suggest that the presence of bb genotype added to the presence of hypocitraturia, noticeably increases the risk for UL. Comparison of mean urine citrate levels (mg/24h.) stra- tified according to genotypes for the SNP I550V-hNaDC- 1; showed significant differences between subjects with UL and control subjects for the heterozygous genotype Bb (0.002) and for the homozygous genotype bb (0.004) (Table 6); suggesting that these genotypes might be im- plicated in the lower urine citrate excretion in presence of urolithiasis. 4. DISCUSSION The results of this study confirmed that hypocitraturia was present with a significantly higher frequency in sub- jects with UL (61.9%) than in controls (38.1%), repre- senting an identifiable risk factor for UL in the study population (p < 0.05). This observation is consistent with results of previous studies which evaluated the same as- sociation in other populations [4-7] and; with the results obtained in a previous study conducted in the Yucatan population, in which hypocitraturia was found in 63.6% of patients with UL [3]. Given that we did not find any association between I550V-hNaDC-1 genotype and hy- pocitraturia itself, the genetic contribution of this SNP towards hypocitraturia was not demonstrated in this Mexican population; so the possible genetic basis should Copyright © 2013 SciRes. OPEN ACCESS 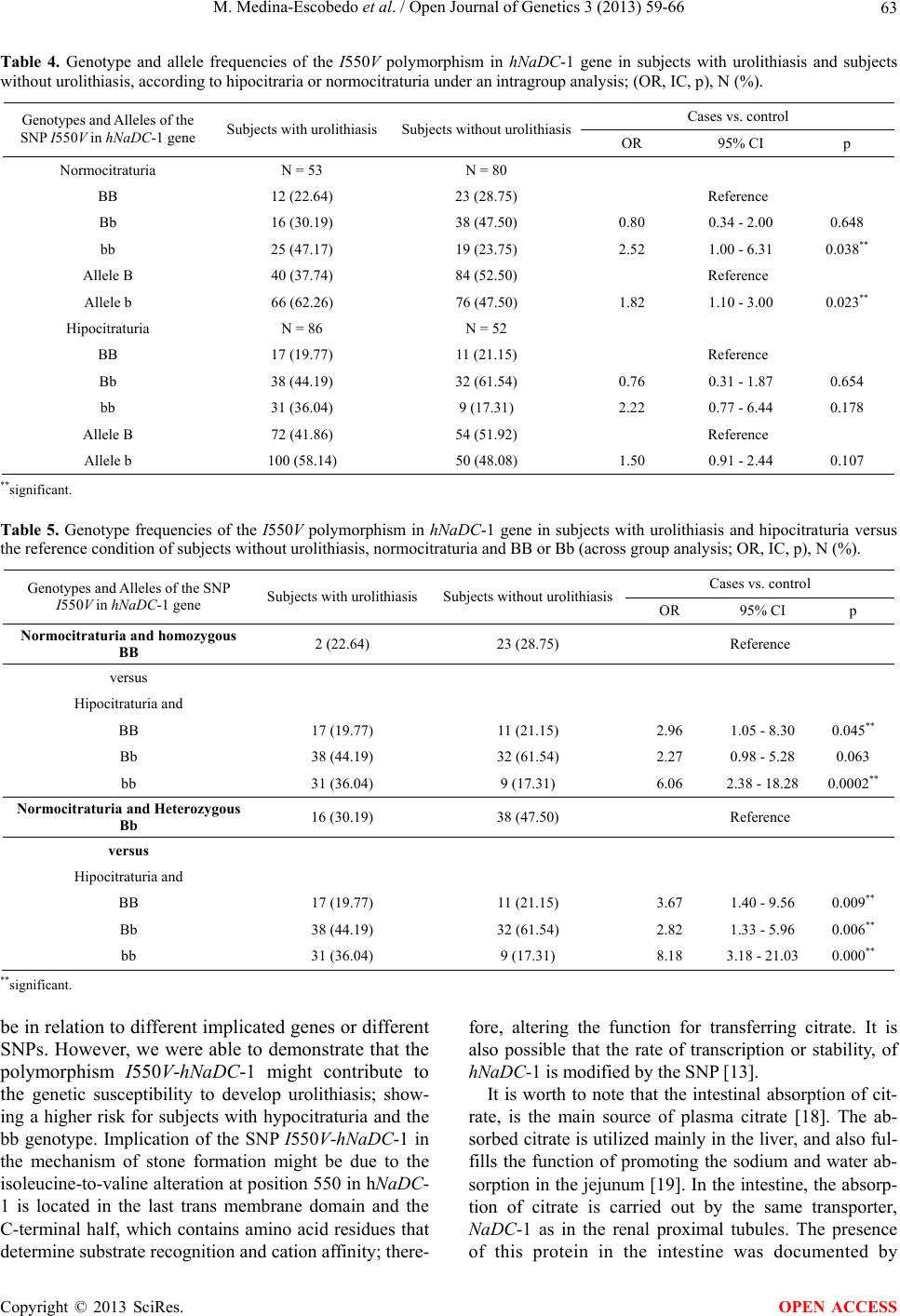 M. Medina-Escobedo et al. / Open Journal of Genetics 3 (2013) 59-66 63 Table 4. Genotype and allele frequencies of the I550V polymorphism in hNaDC-1 gene in subjects with urolithiasis and subjects without urolithiasis, according to hipocitraria or normocitraturia under an intragroup analysis; (OR, IC, p), N (%). Cases vs. control Genotypes and Alleles of the SNP I550V in hNaDC-1 gene Subjects with urolithiasisSubjects without urolithiasis OR 95% CI p Normocitraturia N = 53 N = 80 BB 12 (22.64) 23 (28.75) Reference Bb 16 (30.19) 38 (47.50) 0.80 0.34 - 2.00 0.648 bb 25 (47.17) 19 (23.75) 2.52 1.00 - 6.31 0.038** Allele B 40 (37.74) 84 (52.50) Reference Allele b 66 (62.26) 76 (47.50) 1.82 1.10 - 3.00 0.023** Hipocitraturia N = 86 N = 52 BB 17 (19.77) 11 (21.15) Reference Bb 38 (44.19) 32 (61.54) 0.76 0.31 - 1.87 0.654 bb 31 (36.04) 9 (17.31) 2.22 0.77 - 6.44 0.178 Allele B 72 (41.86) 54 (51.92) Reference Allele b 100 (58.14) 50 (48.08) 1.50 0.91 - 2.44 0.107 **significant. Table 5. Genotype frequencies of the I550V polymorphism in hNaDC-1 gene in subjects with urolithiasis and hipocitraturia versus the reference condition of subjects without urolithiasis, normocitraturia and BB or Bb (across group analysis; OR, IC, p), N (%). Cases vs. control Genotypes and Alleles of the SNP I550V in hNaDC-1 gene Subjects with urolithiasisSubjects without urolithiasis OR 95% CI p Normocitraturia and homozygous BB 2 (22.64) 23 (28.75) Reference versus Hipocitraturia and BB 17 (19.77) 11 (21.15) 2.96 1.05 - 8.30 0.045** Bb 38 (44.19) 32 (61.54) 2.27 0.98 - 5.28 0.063 bb 31 (36.04) 9 (17.31) 6.06 2.38 - 18.28 0.0002** Normocitraturia and Heterozygous Bb 16 (30.19) 38 (47.50) Reference versus Hipocitraturia and BB 17 (19.77) 11 (21.15) 3.67 1.40 - 9.56 0.009** Bb 38 (44.19) 32 (61.54) 2.82 1.33 - 5.96 0.006** bb 31 (36.04) 9 (17.31) 8.18 3.18 - 21.03 0.000** **significant. be in relation to different implicated genes or different SNPs. However, we were able to demonstrate that the polymorphism I550V-hNaDC-1 might contribute to the genetic susceptibility to develop urolithiasis; show- ing a higher risk for subjects with hypocitraturia and the bb genotype. Implication of the SNP I550V-hNaDC-1 in the mechanism of stone formation might be due to the isoleucine-to-valine alteration at position 550 in hNaDC- 1 is located in the last trans membrane domain and the C-terminal half, which contains amino acid residues that determine substrate recognition and cation affinity; there- fore, altering the function for transferring citrate. It is also possible that the rate of transcription or stability, of hNaDC-1 is modified by the SNP [13]. It is worth to note that the intestinal absorption of cit- rate, is the main source of plasma citrate [18]. The ab- sorbed citrate is utilized mainly in the liver, and also ful- fills the function of promoting the sodium and water ab- sorption in the jejunum [19]. In the intestine, the absorp- tion of citrate is carried out by the same transporter, NaDC-1 as in the renal proximal tubules. The presence of this protein in the intestine was documented by Copyright © 2013 SciRes. OPEN ACCESS  M. Medina-Escobedo et al. / Open Journal of Genetics 3 (2013) 59-66 64 Table 6. Comparison of mean citrate excretion (mg/24h.) ac- cording to genotypes for the SNP I550V-hNaDC-1, between cases and control. Genotype Subjects with Urolithiasis N = 139 Subjects without Urolithiasis N = 132 Significance p BB 339.89 ± 216.22 N = 29 395.75 ± 164.11 N = 34 0.259 Bb 277.54 ± 222.19 N = 54 391.63 ± 182.82 N = 70 0.002** bb 324.87 ± 202.11 N = 56 470.62 ± 212.53 N = 28 0.004** **significant. Northern blot analysis, in which, mRNA of NaDC-1 was detected in intestinal tissue [9]. The fact that the same protein regulates citrate absorption in both the intestine as in the renal proximal tubules is interesting in the sense that one SNP affecting the recognition of the NaDC-1 substrate would result in a poor citrate absorption at the intestine level, which could be reflected with the onset of hypocitraturia [20]. Poor intestinal citrate absorption should not be dismissed as a possible cause for the high frequency of hypocitraturia in the Yucatan population. Compared with other UL associated SNPs, I550V SNP has been scarcely studied. The only study regarding this SNP, analyzed in a human population before the present work was carried on, was in Japan [13]. The results ob- tained in the present study markedly contrast those re- ported in the Japan study [13], in which, when comparing the genotype and allele frequencies of this SNP between subjects with hypocitraturia and subjects with normocit- raturia, significant differences were revealed for both genotype (p = 0.0007), and allele (p = 0.00005), exhibit- ing a higher frequency of the BB genotype and B allele in subjects with hypocitraturia, pointing to these factors as associated to the presence of hypocitraturia. Those data contrast with findings in our study in which no sig- nificant differences neither in genotype nor in allele fre- quencies between subjects with hypocitraturia and nor- mocitraturia, were shown. Okamoto et al., 2007 [13] explain the results suggest- ing that the b allele of the I550V SNP causes a change in the structure of the transporter, which would result in a decrease of its affinity to the substrate. With this, the rate of reabsorption of the filtered citrate would be reduced and, therefore, citrate excretion increased, whereby the b allele would be conferring a protection factor against hy- pocitraturia, and hence against UL. Nevertheless, these results have not been reproduced in any other population in the world, and the reported association between geno- type I550V and citraturia has not been confirmed through in vitro studies. A site-directed mutagenesis technique study in COS-7 cells has reported no significant differ- ences in the rate of gene expression or in the transport activity of NaDC-1 among the different genotypes of I550V SNP [12]. Lack of association between I550V SNP and citraturia obtained in this study, might be explained because of the NaDC-1 is capable of carrying other di- and tri-carbox- ylic acids, by having greater affinity with these than with citrate, as are fumarate and succinate as well as methyl-, dimethyl succinate, which can be present in the luminal liquid [21]. It has been reported from in vitro studies that fumarate and succinate significantly inhibit citrate uptake by NaDC-1 in brush border membrane vesicles through a competitive inhibition mechanism [19]. Likewise, it is known that sulfate may inhibit citrate transport; this in- hibition, unlike that of succinate and fumarate is non- competitive, since sulfate hinders the binding of other substrates to the transporter, but without being carried in to the tubular cell interior [22]. It is possible that the transporter saturation by these luminal components causes an inhibition of citrate transport, which may explain the observed lack of association among I550V genotype and citraturia in this study. Studies of the urinary profile, tak- ing into account the determination of the concentration of succinate and fumarate in plasma and urine, are needed to shed some light on this point. Another explanation is that other SNPs, either by them- selves, or through an interaction with I550V SNP; might have effects on the amount of transporters existing in the membrane, which could influence substrate concentra- tion at which the transporter reaches saturation. In the Mexican population analyzed for this study, sig- nificant differences were found with respect to I550V- hNaDC-1 genotype and allele frequencies between cases and controls, suggesting an association between the b allele and the presence of UL, opposite to what was ob- tained in the Japanese population studied by Okamoto et al., 2007, [13] who did not evidence such association. Given that in the present study, we did not find any asso- ciation of the bb genotype with citraturia, whereas a pos- sitive association was found with the presence of UL, the outcome suggests the existence of another mechanism, unrelated to urinary citrate excretion, whereby the trans- porter is involved in the lithogenic process. As mentioned above, in addition to I550V, other poly- morphisms that have implications on the activity and functioning of hNaDC-1 have been identified [12]. It is possible that some alleles of other polymorphisms are associated with the b allele in I550V SNP through link- age disequilibrium [23] and; that this would explain the association found between b allele, bb genotype and UL. The association among these polymorphisms and the pre- sence of UL has not been evaluated whatsoever. The complete lack of association between genotype and citraturia was confirmed once again by dividing the Copyright © 2013 SciRes. OPEN ACCESS  M. Medina-Escobedo et al. / Open Journal of Genetics 3 (2013) 59-66 65 study population into subjects with hypocitraturia and normocitraturia and comparing the genotype and allele frequencies between cases and controls, within each group. In this case, as mentioned before, frequency dif- ferences were obtained, pointing at the bb genotype and the b allele as risk factors for UL, only in the group of subjects with normocitraturia. The above confirms that the citraturia is not associated with genotype in this po- pulation, since the association between genotype and UL was present in subjects with normal levels of urinary citrate. The fact that the same association was not found in the group of subjects with hypocitraturia may be due to cit- raturia itself representing a heavier risk factor than the genotype (which is consistent with the higher value of OR obtained for hypocitraturia than that for genotype), which implies that subjects with hypocitraturia develop UL more often, even in the absence of the risk genotype. Another important aspect to consider, especially when dealing with multicausal origin conditions is the fact that gene expression is a mechanism capable of changing ac- cording to outer environmental stimuli, performing what is known as gene-environment interactions. UL is known to be a multifactorial pathology resulting from the inter- action between environmental influences and genetic as well as hormonal factors [24,25]. The differences be- tween the results obtained in the Japanese population [13] and; those reported in this paper can also owe to the many variations that exist in the environmental condi- tions to which the subjects in both populations are ex- posed, same which, through repression or induction me- chanisms could produce differences in hNaDC-1 gene ex- pression, conversely, the combination of a certain geno- type with diverse environmental variables could produce a difference in the effect of this genotype on various phenotypic features With regard to the increased risk for UL due to the in- teraction of both risk factors observed in this study: hy- pocitraturia and the bb genotype or b allele, might be explained because UL is a disorder with a multifactorial etiology, the presence of only one of the risk factors is not sufficient to develop the disease, however, these risk factors show additive effect [26], in this study we showed that the presence of two risk factors significantly in- creases the risk of acquiring the disease compared with the presence of each of these factors independently. In order to find the answer to complete the new ques- tions arisen from this study, the dynamics of di- and tri- carboxylic acid transport must be analyzed further; as well as the determination of plasma citrate; and other intermediates of the citric acid cycle in urine as part of the UL patient’s metabolic profile. In conclusion, our study demonstrated that the poly- morphism I550V in the hNaDC-1 gene is associated with the risk for urolithiasis. Moreover, the risk for UL might increase due to an additive effect of hypocitraturia with the genotype bb or with the allele b in the population of Southeastern, Mexico. 5. ACKNOWLEDGEMENTS We thank Fundación Mexicana para la Salud Capítulo Peninsular A.C. and CONACYT Sectoral Funds, Registration No.2008-1-86740 for the necessary funding to carry out the present work. REFERENCES [1] Medina-Escobedo, M.M., Zaidi, M., Real-de-León, E. and Orozco-Rivadeneyra, S. (2002) Prevalencia y fac- tores de riesgo en Yucatán, México, para litiasis urinaria. Salud Pública de México, 44, 541-545. doi:10.1590/S0036-36342002000600006 [2] Arrabal, M., Fernández, A., Arrabal, M.A., Ruiz, M.J. and Zuluaga, A. (2006) Estudio de factores físico-quími- cos en pacientes con litiasis renal. Archivos Españoles de Urología, 59, 583-594. [3] Villanueva-Jorge, S., Medina-Escobedo, M.M. and Arcos, A. (2007) Excreción de oxalatos y citratos en adultos con litiasis urinaria. Bioquimia, 32, 134-140. [4] Akçay, T., Konukoğlu, D. and Celik, C. (1996) Hypocit- raturia in patients with urolithiasis. Archives of Disease in Childhood, 74, 350-351. doi:10.1136/adc.74.4.350 [5] Gambaro, G., Fabris, A., Puliatta, D. and Lupo, A. (2005) Lithiasis in cystic kidney disease and malformations of the urinary tract. Urological Research, 34, 102-107. doi:10.1007/s00240-005-0019-z [6] Spivacow, F.R., Valle, E.E. and Zanchetta, J.R. (2006) Litiasis renal. Modificaciones bioquímicas durante el seguimiento. Medicina, 66, 201-205. [7] Deshmukh, S.R. and Kahn, Z.H. (2006) Evaluation of urinary abnormalities in nephrolithiasis patients of Mara- thwada region. Indian Journal of Clinical Biochemistry, 21, 177-180. doi:10.1007/BF02913091 [8] Fan, J., Schwille, P.O., Schmiedl, A., Fink, E. and Mano- haran, M. (2001) Calcium oxalate crystallization in un- dilluted postprandial urine of healthy male volunteers as influenced by citrate. Arzneimittel-Forschung, 51, 848-857. [9] Pajor, A.M. (1996) Molecular cloning and functional ex- pression of a sodium-dicarboxylate cotransporter from hu- man kidney. American Journal of Physiology, 270, F642- F648. [10] Pajor, A.M. and Sun, N.N. (2000) Molecular cloning, chromosomal organization and functional characteriza- tion of a sodium-dicarboxylatecotransporter from mouse kidney. Renal Physiology: American Journal of Physiol- ogy, 279, F482-F490. [11] Pajor, A.M. (2006) Molecular properties of the SLC13 family of dicarboxylate and sulfate transporters. Pflügers Archiv, 451, 597-605. doi:10.1007/s00424-005-1487-2 [12] Pajor, A.M. and Sun, N.N. (2010) Single nucleotide poly- morphisms in the human Na+-dicarboxylate co-trans- porter affect transport activity and protein expression. Copyright © 2013 SciRes. OPEN ACCESS  M. Medina-Escobedo et al. / Open Journal of Genetics 3 (2013) 59-66 Copyright © 2013 SciRes. 66 OPEN ACCESS Renal Physiology: American Journal of Physiology, 299, F704-F711. doi:10.1152/ajprenal.00213.2010 [13] Okamoto, N., Aruga, S., Matsuzaki, S., Takahashi, S., Matsushita, K. and Kitamura, T. (2007) Associations be- tween renal sodium-citrate cotransporter (hNaDC-1) gene polymorphism and urinary citrate excretion in recurrent renal calcium stone formers and normal controls. Interna- tional Journal of Urology, 14, 344-349. doi:10.1111/j.1442-2042.2007.01554.x [14] González-Herrera, L., Vega-Navarrete, L., Roche-Canto, C., Canto-Herrera, J., Virgen-Ponce, D., Moscoso-Caloca, G., et al. (2010) Forensic parameters and genetic varia- tion of 15 autosomal STR Loci in Mexican Mestizo po- pulations from the States of Yucatan and Nayarit. Open Forensic Science Journal, 3, 57-63. [15] Gotsman, I., Zwas, D., Planer, D., Admon, D., Lotan, C. and Keren, A. (2010) The significance of serum urea and renal function in patients with heart failure. Medicine, 89, 197-203. doi:10.1097/MD.0b013e3181e893ee [16] Perrone, R.D., Madias, N.E. and Levey, A.S. (1992) Se- rum creatinine as an index of renal function: New insights into old concepts. Clinical Chemistry, 38, 1933-1953. [17] Blin, N. and Stafford, D.W. (1976) A general method for isolation of high molecular weight DNA. Nucleic Acids Research, 3, 2303-2308. doi:10.1093/nar/3.9.2303 [18] Hamm, L.L. (1990) Renal handling of citrate. Kidney In- ternational, 38, 728-735. doi:10.1038/ki.1990.265 [19] Rolston, D.D., Moriarty, K.J., Farthing, M.J., Kelly, M.J., Clark, M.L. and Dawspm, A.M. (1986) Acetate and cit- rate stimulate water and sodium absorption in the human jejunum. Digestion, 34, 101-104. doi:10.1159/000199317 [20] Rudman, D., Dedonis, J.L., Fountain, M.T., Chandler, J.B., Gerron, G.G., Fleming, G.A., et al. (1980) Hypocit- raturia in patients with gastrointestinal malabsorption. New England Journal of Medicine, 303, 657-661. doi:10.1056/NEJM198009183031201 [21] Yao, X. and Pajor, A.M. (2000) The transport properties of the human renal Na+-dicarboxylate cotransporter un- der voltage-clamp conditions. Renal Physiology: Ameri- can Journal of Physiology, 279, 54-64. [22] Aruga, S., Pajor, A.M., Nakamura, K., Liu, L., Moe, O.W., Preisig, P.A., et al. (2004) OKP cells express the Na-dicarboxylate cotransporter NaDC-1. Cell Physiology: American Journal of Physiology, 287, C64-C72. doi:10.1152/ajpcell.00061.2003 [23] Reich, D.E., Cargill, M., Bolk, S., Ireland, J., Sabeti, P.C., Richter, D.J., et al. (2001) Linkage disequilibrium in the human genome. Nature, 411, 199-204. doi:10.1038/35075590 [24] van Vliet, J., Oates, N.A. and Whitelaw, E. (2007) Epi- genetic mechanisms in the context of complex diseases. Cellular and Molecular Life Sciences, 64, 1531-1538. doi:10.1007/s00018-007-6526-z [25] Healy, K.A. and Ogan, K. (2005) Nonsurgical manage- ment of urolithiasis: An overview of expulsive therapy. Journal of Endourology, 19, 759-767. doi:10.1089/end.2005.19.759 [26] Clayton, D. and McKeigue, P.M, (2001) Epidemiological methods for studying genes and environmental factors in complex diseases. The Lancet, 358, 1356-1360. doi:10.1016/S0140-6736(01)06418-2
|