 Open Journal of Genetics, 2013, 3, 33-37 OJGen http://dx.doi.org/10.4236/ojgen.2013.32A3005 Published Online August 2013 (http://www.scirp.org/journal/ojgen/) Cytogenetic abnormalities in 200 male infertile cases in the southern region of India* G. Sreenivasa1, Suttur S. Malini1, Prasanna Kumari2, Usha R. Dutta3# 1Molecular Reproductive and Human Genetics Laboratory, Department of Studies in Zoology, University of Mysore, Mysore, India 2Department of Pathology (Cytog enetics), Kidwai Memorial Institute of Oncology, Bangalore, India 3Diagnostics Division, Center for DNA Fingerprinting and Diagnostics (CDFD), Hyderabad, India Email: #ushadutta@hotmail.com, #usha@cdfd.org.in Received 21 June 2013; revised 2 July 2013; accepted 15 July 2013 Copyright © 2013 G. Sreenivasa et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Chromosomal abnormalities are one of the major causes of male infertility. But the exact mechanism by which chromosomal anomalies induces infertility is still not clear. Many studies have documented the chromosomal abnormalities ranging from 2.2% to 15.7% in infertile men. The present study has been carried out to document and find out the genetic cause of male infertility in the Southern region of In- dia. The cytogenetic analysis of 200 male infertile cases, referred due to primary infertility from 2009 to 2012, were analyzed by GTG banding and evaluated retrospectively. The semen analysis was also per- formed. A total of 15 cases (7.5%) showed chromo- somal aberrations. Four (2%) were 47, XXY and mo- saic 47,XXY; Two (1%) were structural autosomal abnormalities; Two (1%) were inversion Y; Seven (3.5%) cases were Y heterochromatin variants and 185 cases (92.5%) showed normal 46,XY karyotype. The chromosomal abnormalities in our study is also in agreement with the data from the literature. Also abnormal spermatogenesis is observed in these cases. Apart from chromosomal analysis further in depth molecular analysis and genetic counseling is sugges- tive in such cases, especially those interested in IVF technologies. Keywords: Male Infertility; Chromosomal Abnormalities; Semen Analysis 1. INTRODUCTION Male infertility is characterized by the in ability of a sex- ually active, non-contraceptive couple to achieve preg- nancy within one year [1]. It is a worldwide problem af- fecting people of all communities, though the cause and magnitude may vary with geographical location. The ex- act cause of male infertility is still unknown in more than 50% of cases [2]. Due to the advancement in the diagno- sis technologies in genetics, it is now becoming evident that a significant percentage of male infertility cases are due to genetic abnormalities [3]. Several studies have shown increased chromosomal aberrations [4,5] in 5% to 7% of patients with oligospermia, and 10% to 15% in patients with Azoospermia. Among several etiological factors, chromosomal abnormalities play a significant role in male infertility with 10% to 15% of aberrations [6,7] among which 5% of these are numerical or struc- tural abnormalities, 80% to 85% of cases are due to sex chromosome anomalies and about 2% are mosaics with autosomal abnormalities [8-11]. This value increases to about 15% in Azoospermia males, largely due to cases with 47,XXY aneuploidy. The most common type of abnormality is Klinefelter Syndrome and also Y chromo- some long arm micro deletion which is described as the most frequent non chromosomal alteration [12]. Recent studies demonstrated the association between rare ge- netic sperm defects with a diverse inheritance pattern in the family which may be transmissible to the male off- spring [13]. In our study we aimed to investigate the per- centage of chromosomal abnormalities associated among infertile males in the southern region of India. 2. MATERIALS AND METHODS The present study was conducted retrospectively from 2009 to 2012 in the Department of studies in Zoology of our University. Blood samples of 200 infertile subjects were collected from different IVF clinics and hospitals referred due to primary and secondary infertility. Ethical *Authors’ Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported. #Corresponding author. OPEN ACCESS 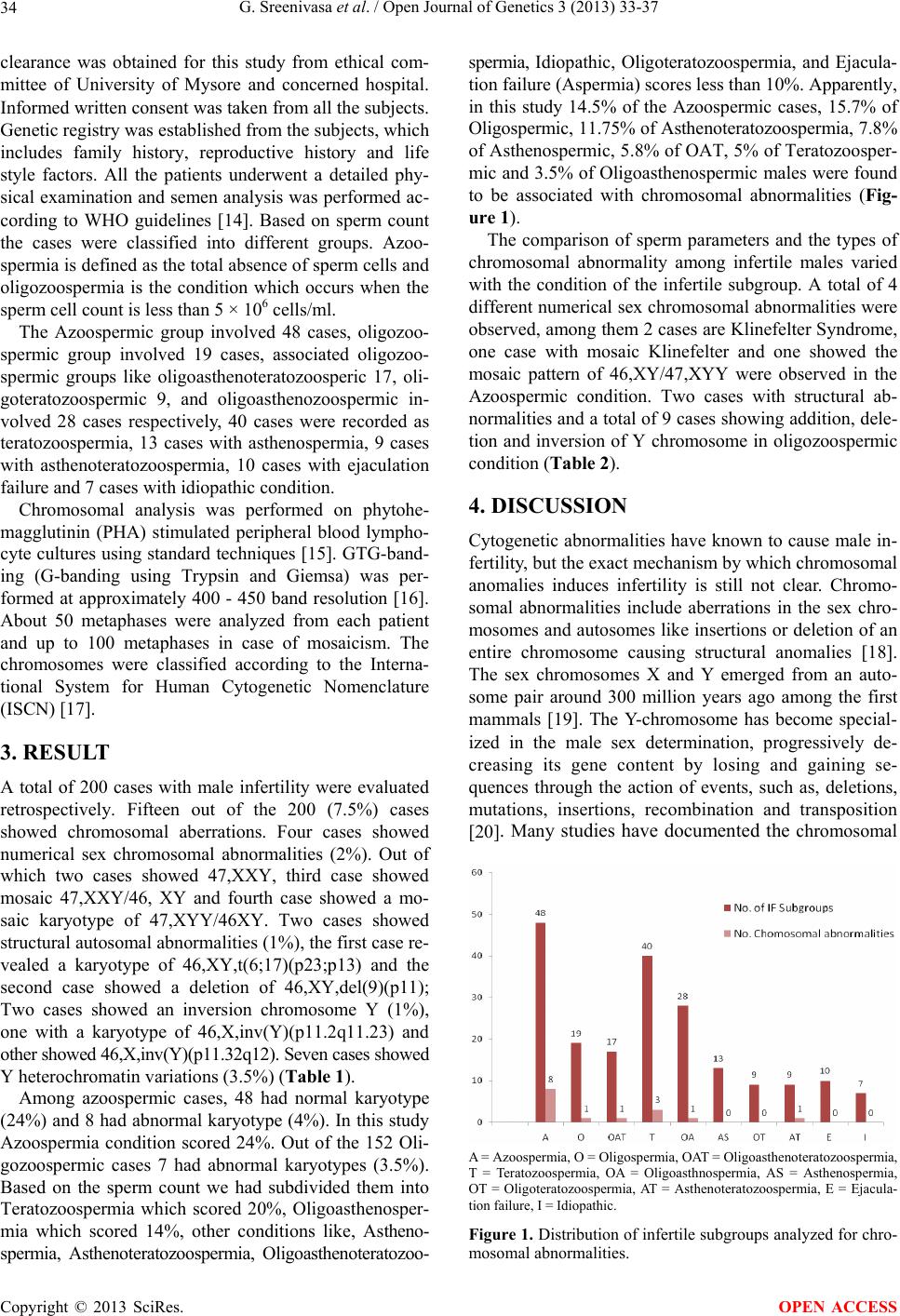 G. Sreenivasa et al. / Open Journal of Genetics 3 (2013) 33-37 34 clearance was obtained for this study from ethical com- mittee of University of Mysore and concerned hospital. Informed written consent was taken fro m all th e subj ects. Genetic registry was established from the subjects, which includes family history, reproductive history and life style factors. All the patients underwent a detailed phy- sical examination and semen analysis was performed ac- cording to WHO guidelines [14]. Based on sperm count the cases were classified into different groups. Azoo- spermia is defined as the total absence of sperm cells and oligozoospermia is the condition which occurs when the sperm cell count is less than 5 × 106 cells/ml. The Azoospermic group involved 48 cases, oligozoo- spermic group involved 19 cases, associated oligozoo- spermic groups like oligoasthenoteratozoosperic 17, oli- goteratozoospermic 9, and oligoasthenozoospermic in- volved 28 cases respectively, 40 cases were recorded as teratozoospermia, 13 cases with asthenospermia, 9 cases with asthenoteratozoospermia, 10 cases with ejaculation failure and 7 cases with idiopathic condition. Chromosomal analysis was performed on phytohe- magglutinin (PHA) stimulated peripheral blood lympho- cyte cultures using standard techniques [15]. GTG-band- ing (G-banding using Trypsin and Giemsa) was per- formed at approximately 400 - 450 band resolution [16]. About 50 metaphases were analyzed from each patient and up to 100 metaphases in case of mosaicism. The chromosomes were classified according to the Interna- tional System for Human Cytogenetic Nomenclature (ISCN) [17]. 3. RESULT A total of 200 cases with male infertility were evaluated retrospectively. Fifteen out of the 200 (7.5%) cases showed chromosomal aberrations. Four cases showed numerical sex chromosomal abnormalities (2%). Out of which two cases showed 47,XXY, third case showed mosaic 47,XXY/46, XY and fourth case showed a mo- saic karyotype of 47,XYY/46XY. Two cases showed structural autosomal abnormalities (1%), the first case re- vealed a karyotype of 46,XY,t(6;17)(p23;p13) and the second case showed a deletion of 46,XY,del(9)(p11); Two cases showed an inversion chromosome Y (1%), one with a karyotype of 46,X,inv(Y)(p11.2q11.23) and other showed 46,X,inv(Y)(p11.32q12). Seven cases showed Y heterochromatin variations (3.5%) (Table 1). Among azoospermic cases, 48 had normal karyotype (24%) and 8 had ab normal karyotype (4%). In this stud y Azoospermia condition scored 24%. Out of the 152 Oli- gozoospermic cases 7 had abnormal karyotypes (3.5%). Based on the sperm count we had subdivided them into Teratozoospermia which scored 20%, Oligoasthenosper- mia which scored 14%, other conditions like, Astheno- spermia, Asthenoteratozoospermia, Oligoasthenoteratozoo- spermia, Idiopathic, Oligoteratozoospermia, and Ejacula- tion failure (Aspermia) scores less than 10%. Apparently, in this study 14.5% of the Azoospermic cases, 15.7% of Oligospermic, 11.75% of Asthenoteratozoospermia, 7.8% of Asthenospermic, 5.8% of OAT, 5% of Teratozoosper- mic and 3.5% of Oligoasthenospermic males were found to be associated with chromosomal abnormalities (Fig- ure 1). The comparison of sperm parameters and the types of chromosomal abnormality among infertile males varied with the condition of the infertile subgroup. A total of 4 different numerical sex chro mosomal abnormalities were observed, among them 2 cases are Klinefelter Syndrome, one case with mosaic Klinefelter and one showed the mosaic pattern of 46,XY/47,XYY were observed in the Azoospermic condition. Two cases with structural ab- normalities and a total of 9 cases showing ad dition, dele- tion and inversion of Y chromosome in oligozoospermic condition (Table 2). 4. DISCUSSION Cytogenetic abnormalities have known to cause male in- fertility, b ut the ex act mechanism by which chromosomal anomalies induces infertility is still not clear. Chromo- somal abnormalities include aberrations in the sex chro- mosomes and autosomes like insertions or deletion of an entire chromosome causing structural anomalies [18]. The sex chromosomes X and Y emerged from an auto- some pair around 300 million years ago among the first mammals [19]. The Y-chromosome has become special- ized in the male sex determination, progressively de- creasing its gene content by losing and gaining se- quences through the action of events, such as, deletions, mutations, insertions, recombination and transposition [20]. Many studies have documented the chromosomal A = Azoospermia, O = Oligospermia, OAT = Oligoasthenoteratozoospermia, T = Teratozoospermia, OA = Oligoasthnospermia, AS = Asthenospermia, OT = Oligoteratozoospermia, AT = Asthenoteratozoospermia, E = Ejacula- tion failure, I = Idiopathic. Figure 1. Distribution of infertile subgroups analyzed for chro- mosomal abnormalities. Copyright © 2013 SciRes. OPEN ACCESS  G. Sreenivasa et al. / Open Journal of Genetics 3 (2013) 33-37 Copyright © 2013 SciRes. 35 OPEN ACCESS Table 1. Distribution of different types of chromosomal abnormalities among infertile males. Chromosomal abnormalities n = 200 % Numerical sex chromosomal abnormality 4 2 47,XXY 2 1 47,mosXXY/46,XY 1 0.5 47,mosXYY/46,XY 1 0.5 Struct ural autosomal chromosomal abnormality 2 1 46,XY,t(6;17)(p23;p13) 1 0.5 46,XY,del(9)(p11) 1 0.5 Struct ural Y chromosome abnormality 09 4.5 46,XYq12h 4 2.0 46,XYq12h- 3 1.5 46,X,inv(Y)(p11.2q11.23) 1 0.5 46,X,inv(Y)(p11.32q12) 1 0.5 Normal 46,XY 185 92.5 Table 2. Cytogenetic abnormalities with respect to the sperm parameters in different infertile subgroups, IF = Infertile. Karyotype (n = 15) IF Sperm count (millions/ml) Motility Morphology 47,XXY Azo 0 0% 0% 47,XXY Azo 0 0% 0% mos47,XYY/46,XY Azo 0 0% 0% mos47,XXY/46,XY Azo 0 0% 0% 46,XYq12h- Azo 0 0% 0% 46,XYq12h- OAT 10 5% 40% 46,XYq12h- Azo 2 15% 20% 46,XYq12h OA 5 0% 40% 46,XYq12h Azo 0 0% 0% 46,XYq12h T 0 0% 0% 46,XYq12h T 98 65% 15% 46,X,inv(Y)(p11.2q11.23) Azo 0 0% 0% 46,X,inv(Y)(p11.32q12) T 85 60% 15 46,XY,t(6;17)(p23;p13) AT 58 0% 10 46,XY,del(9)(p12) O 5 55% 21 abnormalities which ranges from 2.2% to 15.7% for in- fertile men [18] in our study we observed that 7.5% of the infertile males are associated with chromosomal ab- normality. Sex chromosome aberrations are the most frequently observed conditions in male infertility. Klinefelter syn- drome males can be identified only when they undergo fertility assessments. VanAssche et al. [21] reported 11% Azoospermic individuals with Klinefelter syndrome. In our study among 48 (24%) of the Azoospermia cases, 4% cases were true Klinefelter syndrome, 2% with mo- saic Klinefelter syndrome. The occurrence of Klinefelter syndrome and XYY is due to meiotic non disjunction of the X chromosome or anaphase lag of X chromosome from a normal 46,XY or XXY zygote. This abnormality is associated with severe spermatogenic failure causing a marked reduction in testicular size and resulting in Azo- ospermia [22]. Earlier studies have reported that the men with XYY syndrome are generally fertile but appear to have an increased likelihood of infertility compared to karyotypically normal 46,XY males. This type of chro- mosomal anomaly occurs in one for 1000 live male births in the general population, but more frequent in the infertile population (Martin , 2 008). We also h ad on e case with a frequency of 0.5% associated with Azoospermic condition having mosaic 46,XY/47,XYY karyotype. Few  G. Sreenivasa et al. / Open Journal of Genetics 3 (2013) 33-37 36 studies also reported the association of infertile patients with 47,XYY syndrome [23]. Among Klinefelter and 47,XYY syndrome males, there is a theoretical risk re- garding the m anifestat i on of sex chromosomal aneuploidy in at least 50% of their sperms [24]. Recent study reported the higher incidence of chro- mosomal abnormalities in azoospermic group than in the oligospermic groups and also increased chromosomal abnormalities with respect to decreased sperm count. Some researchers who had investigated chromosomal anomalies, specifically among patients with severe oli- gospermia and Azoospermia, have reported higher fig- ures such as 20.86% [11] and 21.1% [25] reported that the incidence of chromosomal abnormalities was 14.3% and 6.5% among Azoospermia and oligospermia respec- tively, also Ceylan et al. [26] reported that, chromosomal abnormality was 33.3% in the azoospermic and 13.3% in severe oligozoospermic group. Chromosomal abnormali- ties were detected in 17.4% of 86 azoospermic cases and in 6.8% of 73 oligozoospermic cases in a regional study in Turkey [27]. According to Samli et al. [28] chromo- somal abnormality were associated with 12% of azoo- spermic cases and in 4% of oligospermic patients. In our study the incidence of the chromosomal abnormalities in azoospermic and oligospermic males were found to be 14.5% and 5.2% respectively which accords with previ- ous studies. Additionally, the present study showed 2% of the as- sociated oligospermic cases, like Oligo Asthenospermia and oligoasthenoteratozoospermia condition showed Y chromosomal heterochromatin variation with a frequency of 1.2% have associated severe sperm defects. A rela- tionship between balanced autosomal translocation and infertility has been reported. In the present study one case with (0.5%) 46,XY,t(6;17)(p23;p13) and one case with deletion of 9p12 was observed. Many studies re- ported that, the association of chromosome 9 in inver- sion, translocation, and other chromosomal aberrations with the male infertility which may result in various cli- nical manifestations and also cause different kinds of sperm abnormalities [27,29]. In the majority of cases, carriers of balanced translocations are themselves pheno- typically normal, unless one of the translocation break- points interrupts an important gene or via position effect. 5. CONCLUSION Chromosomal abnormalities play a significant role in male infertility. In the present study it has been found that the infertile males associated with chromosomal ab- normalities involving structural as well as numerical al- terations might have affected the spermatogenesis. How- ever, further indepth Molecular analysis is needed to understand the addition al genetical factors of male infer- tility. 6. ACKNOWLEDGEMENTS GS would like to thank UGC-RFSMS for the financial assistance. REFERENCES [1] WHO (2000) WHO manual for the standardized investi- gation and diagnosis of the infertile couple. Cambridge University Press, Cambridge. [2] Dada, R. and Gupta, N.C. (2004) Yq microdeletions-Azo- ospermia factor candidate genes and spermatogenetic ar- rest. Journal of Biomolecular Techniques, 15, 176-183. [3] Maduro, M.R. and Lamb, D.J. (2002) Understanding new genetics of male i nfertility . Journal of Urology, 68, 2197- 2205. [4] Retief, A.E., Van Zyl, J.A., Menkveld, R., Fox, M.R., Kotze, G.M. and Brusnicky, J. (1984) Chromosome stud- ies in 496 infertile males with a sperm count below 10 million/ml. Human Genetics, 66, 162-164. doi:10.1007/BF00286592 [5] Ravel, C., Berthaut, I., Bresson, J.L. and Siffroi, J.P. (2006) Prevalence of chromosomal abnormalities in phe- notypically normal and fertile adult males: Large-scale survey of over 10000 sperm donor karyotypes. Human Reproduction, 21, 1484-1489. doi:10.1093/humrep/del024 [6] Penna, V.S., Araujo, H., Ballesta, F., Ballesca, J.L. and Vanrell, J.A. (2001) Chromosomal abnormalities and polymorphisms in infertile men. Archives of Andrology, 46, 205-210. doi:10.1080/01485010151096504 [7] Patsalis, P.C., Sismani, C., Quintana-Murci, L., Taleb-Bek- kouche, F., Krausz, C. and McElreavey, K. (2002) Effects of transmission of Y chromosome AZFc deletions. The Lancet, 360, 1222-1224. doi:10.1016/S0140-6736(02)11248-7 [8] Siffroi, J.P., Le Bourhis, C., Krausz, C., Barbaux, S., Quintana-Murci, L. and Kanafani, S. (2005) Sex chro- mosome mosacism in males carrying Y chromosome long arm deletions. Human Reproduction, 15, 2559-2562. doi:10.1093/humrep/15.12.2559 [9] Visootsak, J., Aylstock, M. and Graham, J.M. (2001) Klinefelter syndrome and its variants: An update and re- view for the primary pediatrician. Clinical Pediatrics, 40, 639-651. doi:10.1177/000992280104001201 [10] Huynh, T., Mollard, R. and Trounson, A. (2002) Selected genetic factors associated with male infertility. Human Reproduction, 8, 183-198. doi:10.1093/humupd/8.2.183 [11] Zhou, H., Zhu, J.W., Li, H.G. and Tang, Y.P. (2009) Genetic defect in Chinese azoospermic patients and their relationship with reproductive hormones. Chinese Jour- nal of Medical Genetics, 26, 427-430. [12] Yoshida, A., Miura, K. and Shirai, M. (1996) Chromo- some abnormalities and male infertility. Assisted Repro- duction Reviews, 6, 93-100. [13] Baccetti, B., Capitani, S., Collodel, G., Di Cairano, G., Gambera, L., Moretti, E. and Piomboni. P. (2001) Ge- netic sperm defects and consanguinity. Human Repro- duction, 16, 1365-1371. Copyright © 2013 SciRes. OPEN ACCESS  G. Sreenivasa et al. / Open Journal of Genetics 3 (2013) 33-37 Copyright © 2013 SciRes. 37 OPEN ACCESS [14] World Health Organization (1999) Laboratory manual for the examination of human semen and sperm-cervical mucus interaction. Cambridge University Press, New York. [15] Moorhead, P.S., Nowell, P.C. and Mellman, W.J. (1960) Chromosome preparations of leukocytes cultured from human peripheral blood. Experimental Cell Research, 20, 613-616. doi:10.1016/0014-4827(60)90138-5 [16] Seabright, M. (1971) A rapid banding technique for hu- man chromosomes. Lancet, 2, 971-972. doi:10.1016/S0140-6736(71)90287-X [17] ISCN (2009) An international system for human cyto- genetic nomenclature. [18] Azimi, C., Khaleghian, M. and Farzanfar, F. (2012) Cy- togenetic studies among Iranian infertile men: The first 20-year long-term report. African Journal of Biotechnol- ogy, 11, 8973-8978. [19] Lahn, B.T., Pearson, N.M. and Jegalian, K. (2001) The human Y chromosome, in the light of evolution. Nature Review s Genetics, 2, 207-216. doi:10.1038/35056058 [20] Charlesworth, D., Charlesworth, B. and Marais, G. (2005) Steps in the evolution of heteromorphic sex chromosomes. Heredity, 95, 118-128. doi:10.1038/sj.hdy.6800697 [21] VanAssche, E., Bonduelle. M., Tournaye, H., Joris, H., Verheyen, G., Devroey, P, Van Steirteghem, A. and Lie- baers, I. (1996) Cytogenetics of infertile men. Human Reproduction, 11, 1-26. doi:10.1093/humrep/11.suppl_4.1 [22] Pandiyan, N. and Jequier, A.M. (1996) Mitotic chromo- somal anomalies among 1210 infertile men. Human Re- production, 11, 2604-2608. doi:10.1093/oxfordjournals.humrep.a019178 [23] El-Dahtory, F. and Elsheikha, H.M. (2009) Male infertil- ity related to an aberrant karyotype, 47, XYY: Four case reports. Cases Journal, 2, 28. doi:10.1186/1757-1626-2-28 [24] Yoshida, A., Nakahori, Y., Kuroki, Y., Motoyama, M., Araki, Y., Miura, K., et al. (1997) Dicentric Y chromo- some in an azoospermic male. Molecular Human Repro- duction, 3, 709-712. doi:10.1093/molehr/3.8.709 [25] Paulina, P.Y., Mary, H.Y., Elizabeth, T., Lucy, K.L., Er- nest, H.Y., William, S.B. and Yeung, P.C. (2009) Chro- mosomal anomalies and Y-microdeletions among Chi- nese subfertile men in Hong Kong. Hong Kong Medical Journal, 15, 31-38. [26] Ceylan, G.G., Ceylan, C. and Elyas, H. (2009) Genetic anomalies in patients with severe oligozoospermia and azoospermia in eastern Turkey: A prospective study. Ge- netics and Molecular Research, 8, 915-922. doi:10.4238/vol8-3gmr616 [27] Akgul, M., Ozkinay, F., Ercal, D., Cogulu, O., Dogan, O., Altay, B., Tavmergen, E., Gunduz, C. and Ozkinay, C. (2009) Cytogenetic abnormalities in 179 cases with male infertility in Western Region of Turkey: Report and re- view. Journal of Assisted Reproduction and Genetics, 26, 119-122. doi:10.1007/s10815-009-9296-8 [28] Samli, H., Samli, M.M., Solak, M. and Imirzalioglu, N. (2006) Genetic anomalies detected in patients with non- obstructive azoospermia and oligozoospermia. Archives of Andrology, 52, 263-267. doi:10.1080/01485010600664032 [29] Nagvenkar, P., Desai, K., Hinduja, I. and Zaveri, K. (2005) Chromosomal studies in infertile men with oligo- zoospermia & non-obstructive azoospermia. Indian Jour- nal of Medical Research, 122, 34-42.
|