Paper Menu >>
Journal Menu >>
 Open Journal of Physical Chemistry, 2013, 3, 115-118 http://dx.doi.org/10.4236/ojpc.2013.33014 Published Online August 2013 (http://www.scirp.org/journal/ojpc) Sorption of Gadolinium (III) Ions by a Natural Clinoptilolite-Containing Tuff Nina M. Kozhevnikova, Ekaterina N. Styazhkina Baikal Institute of Nature Management, Siberian Branch, Russian Academy of Sciences, Ulan-Ude, Russia Email: nicas@binm.bscnet.ru Received July 3, 2013; revised July 31, 2013; accepted August 7, 2013 Copyright © 2013 Nina M. Kozhevnikova, Ekaterina N. Styazhkina. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT The equilibrium and kinetics of sorption of gadolinium (III) ions from sulfate solutions by a natural clinoptilolite con- taining tuff are studied. It is shown that gadolinium is completely extracted from dilute solutions (<0.002 M). The ki- netic parameters of the sorption process are determined. Keywords: Natural Zeolite; Gadolinium Ions; Sulfate Solutions; Sorption Isotherms; Kinetic Laws 1. Introduction Natural zeolites are used as sorbents in chemical engi- neering, hydrometallurgy, industrial ecology, agriculture, and medicine [1]. Natural zeolites modified with ions of rare earth elements (REEs) are promising for the prepa- ration of effective stimulants for regener ative therapy and biologically active agents [1,2]. REEs are used in the treatment of tuberculosis, tumors, and skin diseases. Lanthanum sulfate exhibits anticoagulant action. Lan- thanum, cerium, praseodymium, gadolinium introduced into the zeolite matrix have a neuroprotective effect [3]. It is considered effective to use calcium blockers for re- ducing the ischemic brain damage. In biological systems, ions of lanthanum, cerium, praseodymium, gadolinium replace calcium ions, block their entry into cells, and have an inhibitory effect on the development of a cal- cium induced cascade of pathological reactions in cere- bral ischemia [3]. Sorption technology makes it possible to enhance the biological activity of natural zeolites, which play the role of a prolonging carrier of REE ions. Our insufficient knowledge of the ion exchange prop- erties of natural zeolites with respect to REE ions limits the possibilities of their use. The ion exchange properties of natural zeolites with respect to REE ions are inade- quately understood. Earlier, we studied the sorption of lanthanum, cerium, neodymium, and samarium ions by mordenite and clinoptilolite tuff [1,3]. There are no data on the sorption of gadolinium ions by natural zeolites, and such data are needed to estimate the effect of the nature of REEs on the equilibrium and kinetics of sorp- tion, which require deeper analysis. We therefore studied the sorption of gadolinium (III) ions from sulfate solu- tions by a clinoptilolite tuff as function of solution con- centration, sorbent grain size, and solid to liquid weight ratio. 2. Experimental 2.1. The Compounds Clinoptilolite Tuff In this work, we used clinoptilolite tuff of the Kholinsk deposit with the following content of components (wt%): 68.11—SiO2, 12.84—Al2O3, 1.08—Fe2O3, 0.35—FeO, 0.08—Mn, 0.05—P2O5, 0.58—TiO2, 4.17—CaO, 2.65— MgO, 2.47—K2O, 2.87—Na2O, 0.003—CuO, and 0.002— F; Si/Al = 5.25. The content of the zeolite in the rock of ~60% - 62% was determined via X ray diffraction analy- sis using a PTsL-2 instrument [4]. Grain fractions with sizes of 0.25 - 0.5 and 1 - 2 mm were selected for analy- sis by the sieving method. The sorption equilibrium was studied under static conditions from gadolinium sulfate solutions in the concentration range of the ion to be de- termined of 0.0003 to 0.025 M at the ratio of solid(s) and liquid (l) phases of 1:10 and 1:0. 2.2. The Kinetic Parameters The kinetic parameters were calculated from Q—τ curves (where Q is the sorbed amount of gadolinium ions, mmol/g; τ is the time, s) using the technique described for sorption on zeolites in [5]. For the initial period of time, when sorption occurs on the surface of the sorbent C opyright © 2013 SciRes. OJPC 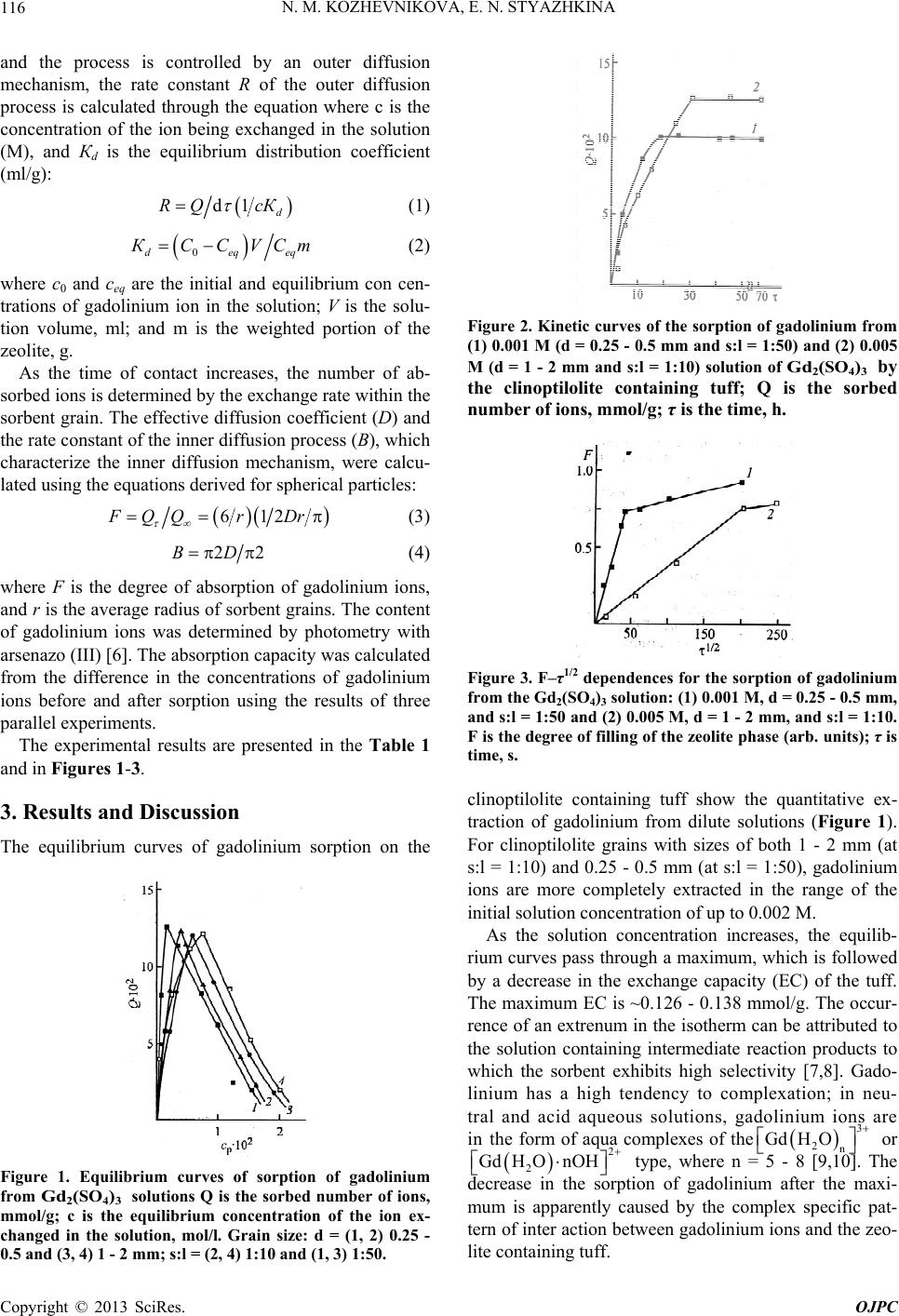 N. M. KOZHEVNIKOVA, E. N. STYAZHKINA 116 and the process is controlled by an outer diffusion mechanism, the rate constant R of the outer diffusion process is calculated through the equation where c is the concentration of the ion being exchanged in the solution (M), and Кd is the equilibrium distribution coefficient (ml/g): d1 d RQ cК (1) 0deq КCCVСm eq (2) where c0 and ceq are the initial and equilibrium con cen- trations of gadolinium ion in the solution; V is the solu- tion volume, ml; and m is the weighted portion of the zeolite, g. As the time of contact increases, the number of ab- sorbed ions is determined by the exchange rate within the sorbent grain. The effective diffusion coefficient (D) and the rate constant of the inner diffusion process (В), which characterize the inner diffusion mechanism, were calcu- lated using the equations derived for spherical particles: 612 F QQr Dr (3) 2BD 2 (4) where F is the degree of absorption of gadolinium ions, and r is the average radius of sorbent grains. The content of gadolinium ions was determined by photometry with arsenazo (III) [6]. The absorption capacity was calculated from the difference in the concentrations of gadolinium ions before and after sorption using the results of three parallel experiments. The experimental results are presented in the Table 1 and in Figures 1-3. 3. Results and Discussion The equilibrium curves of gadolinium sorption on the Figure 1. Equilibrium curves of sorption of gadolinium from Gd 2 ( S O 4 ) 3 solutions Q is the sorbed number of ions, mmol/g; c is the equilibrium concentration of the ion ex- changed in the solution, mol/l. Grain size: d = (1, 2) 0.25 - 0.5 and (3, 4) 1 - 2 mm; s:l = (2, 4) 1:10 and (1, 3) 1:50. Figure 2. Kinetic curves of the sorption of gadolinium from (1) 0.001 M (d = 0.25 - 0.5 mm and s:l = 1:50) and (2) 0.005 M (d = 1 - 2 mm and s:l = 1:10) solution of Gd 2 ( S O 4 ) 3 by the clinoptilolite containing tuff; Q is the sorbed number of ions, mmol/g; τ is the time, h. Figure 3. F–τ1/2 dependences for the sorption of gadolinium from the Gd2(SO4)3 solution: (1) 0.001 M, d = 0.25 - 0.5 mm, and s:l = 1:50 and (2) 0.005 M, d = 1 - 2 mm, and s:l = 1:10. F is the degree of filling of the zeolite phase (arb. units); τ is time, s. clinoptilolite containing tuff show the quantitative ex- traction of gadolinium from dilute solutions (Figure 1). For clinoptilolite grains with sizes of both 1 - 2 mm (at s:l = 1:10) and 0.25 - 0.5 mm (at s:l = 1:50), gadolinium ions are more completely extracted in the range of the initial solution concentration of up to 0.002 M. As the solution concentration increases, the equilib- rium curves pass through a maximum, which is followed by a decrease in the exchange capacity (EC) of the tuff. The maximum EC is ~0.126 - 0.138 mmol/g. The occur- rence of an extrenum in the isotherm can be attributed to the solution containing intermediate reaction products to which the sorbent exhibits high selectivity [7,8]. Gado- linium has a high tendency to complexation; in neu- tral and acid aqueous solutions, gadolinium ions are in the form of aqua complexes of the 3 2n GdH O or 2 2 GdH OnOH type, where n = 5 - 8 [9,10]. The decrease in the sorption of gadolinium after the maxi- mum is apparently caused by the complex specific pat- tern of inter action between gadolinium ions and the zeo- lite containing tuff. Copyright © 2013 SciRes. OJPC  N. M. KOZHEVNIKOVA, E. N. STYAZHKINA Copyright © 2013 SciRes. OJPC 117 Table 1. K i n e t i c p a r a m e t e r s o f t h e s orp t i o n o f gadolinium b y n a t u r a l c l i n op t i l o l i t e ‚ c o n t a i n i n g t u ff f ro m Cd 2 ( S O 4 ) 3 s o l u t i o n s. c , M d , mm s : l τ ∞ , m i n ddQ × 10 5 , mmo l / ( g · m i n) К d , m l / g R × 10 4 , s −1 В × 10 4 , s −1 D ’ × 10 8 , c m 2 / s 0 . 001 0 . 25 - 0 . 5 1:10 1555 3 .8 54 . 8 23 . 3 9 . 7 4 . 6 0 . 005 0 . 25 - 0 . 5 1:50 1578 3 . 3 47 . 2 21 . 4 10 . 5 5 . 2 0 . 005 1 - 2 1:10 2063 1 . 6 28 .1 11 . 6 6 . 9 3 . 4 The formation of complexes with the composition n , where n = 1 - 3, was also observed in gadolinium sulfate solutions. At low concentrations of sulfate ions (<0.02 mol/l), cations dominate [9], although anionic complexes can also be formed un- der these conditions [10-12]. The sorption of aqua and hydroxo complexes of gadolinium apparently takes place before the extremum point, since the high selectivity of zeolite to gadolinium ions in the initial portion of the equilibrium curve, when the ions are nearly completely absorbed, is due to the high charge of the ion. The me- chanism of sorption of lanthanum aqua complexes on a synthetic zeolite is described in [12]; the assumed me- chanism is evidenced by the reversal of the selectivity of the sorbent to gadolinium ions after their certain concen- tration in the solution is achieved. This can be attributed to the manifestation of electroselectivity, because (ac- cording to theory [7,8,10]) the equilibrium in the solu- tion-sorbent system shifts in the direction of an increase in the sorption of an ion with a higher charge upon the dilution of the solution; when it is concentrated, a com- plete reversal of selectivity is observed. 32n 4 Gd SO 4 GdSO The absorption of gadolinium ions is accompanied by a decline in the solution pH by 0.6 - 0.9 units. The pH value is 6.3 for a 0.005 M gadolinium sulfate solution and 6.1 for 0.001 M. The grain size and the solid to liq- uid weight ratio affect the position of the maximum in the isotherms (Figure 1). The maximum of EC on grains with d = 0.25 - 0.5 mm and s:l = 1:50 (Figure 1, curve 1) is shifted to the range of lower equilibrium concentra- tions; with an increase in the grain size (d = 1 - 2 mm) at s:l = 1:10, the maximum is the range of higher concen- trations (Figure 1, curve 4). Low solution concentrations are thus sufficient for achieving a maximum EC on fine grains; this is important in choosing the mode for satu- rating the zeolite with gadolinium ions to prepare a modi- fied form of the clinoptilolite containing tuff. It was found that a nearly equilibrium state is achieved within 28 h on grains with a size of 0.25 - 0.5 mm and within 35 h on grains with a size of 1 - 2 mm; after that, a slight desorption within 1.5% - 2.0% is observed (Figure 2). An analysis of the kinetic parameters (Table 1 ) shows that the sorption of gadolinium is controlled by a mixed diffusion mechanism, since the rate constants of outer (R) and inner diffusion processes (В) are on approximately the same order of magnitude. The behavior of the de- pendence of the degree of filling of the zeolite phase F on τ1/2 (where τ is the time in seconds), the initial por- tions of which are linear up to high values of F (Figure 3), is indicative of a considerable contribution from the inner diffusion mechanism. An increase in the rate of sorption of gadolinium ddQ from a dilute solution on grains with a size of 0.25 - 0.5 mm by the outer diffusion mechanism and an increase in the equilibrium distribution coefficient К are observed. The acceleration of the process is attributed to an increase in the degree of dispersion of the tuff grains. The values of the effective diffusion coefficient D are in agreement with those from [5]. The relatively high values of D, relative to those derived for mono and divalent metals, are explained by the Helfferich principle [12], according to which more mobile ions decelerate and less mobile ions accelerate during the exchange of a mixture of ions. 4. Conclusion When natural zeolite tuff comes into contact with aque- ous sulfate solutions of gadolinium, the sorbent has the property of extracting gadolinium ions. At low concen- trations (<0.002 M), a high selectivity of sorption of gadolinium ions is observed; as the concentration in- creases, the sorption capacity of the clinoptilolite con- taining tuff decreases. The sorption isotherms exhibit a maximum attributed to a complex pattern of interaction in the sorbent-solution system. The kinetics of sorption of gadolinium (III) ions by natural clinoptilolite contain- ing tuff is controlled by a mixed diffusion sorption mechanism; it is found that the contribution of the inner diffusion sorption mechanism is substantial. It is estab- lished that the rate of absorption of gadolinium ions from a dilute solution on grains with sizes of 0.25 - 0.5 mm by the outer diffusion mechanism and the equilibrium dis- tribution coefficient increase with decreasing solution concentration. 5. Acknowledgements This work was carried out with the financial support of  N. M. KOZHEVNIKOVA, E. N. STYAZHKINA 118 the Russian Foundation for Basic Research, grant num- ber 12-05-00020a. REFERENCES [1] T. E. Aleksandrova, V. S. Maksarov, V. S. Ubasheev and N. M. Kozhevnikova, “Experimental Evaluation of Phar- maceutics Properties of Lanthanum Sulfate,” Siberian Medical Zhurnal, No. 4, 2001, p. 65. [2] O. A. Verkhova and V. R. Soroka, “The Biological Role of the Lanthanide,” Biology Bulletin Reviews, No. 3, 1980, p. 365. [3] S. M. Gulyaev, I. O. Ubasheev and N. M. Kozhevnikova, “Neuroprotective Effect of Lanthanum Acetate in Patients with Chronic Cerebral Ischemia in Rats,” Vestnik Buryat State University, Ulan-Ude, No. 2, 2007, p. 86. [4] I. A. Belitskii, I. V. Drobot and G. P. Valueva, “Experi ence in Rapid Determination of Zeolite Content in Rock, Using PTsL 1 and PTsL 2 Portable Zeolite Laboratories,” Nauka, Novosibirsk, 1979, p. 80. [5] Yu. A. Kokotov, P. P. Zolotarev and G. E. El’kin, “The oretic Bases of Ion Exchange,” Khimiya, Leningrad, 1986, p. 280. [6] N. N. Tunitskii, V. A. Kaminskii and S. F. Timashev, “Methods of Physicochemical Kinetics” Khimiya, Mos- cow City, 1972, p. 246. [7] P. Geoffrey, “Chemical Methods of Rock Analysis,” Mir, Moscow City, 1973, p. 360. [8] N. F. Chelishchev, V. F. Volodin and V. L. Kryukov, “Ion Exchange Properties of Natural High Silicon Zeo lites,” Nauka, Moscow City, 1988, p. 128. [9] L. N. Komissarova, V. M. Shatskii, G. Ya. Pushkina, et al., “Compounds of Rare Earth Elements. Carbonates, Oxalates, Sulfates, Titanates,” Nauka, Moscow City, 1986, p. 366. [10] K. B. Yatsemirskii, N. A. Kostromina, Z. A. Sheka, et al., “Chemistry of Complex Rare Earth Compounds,” Naukova Dumka, Kiev, 1966, p. 494. [11] F. H. Spedding and S. Jappe, ““Conductances, Solubili- ties and Ionization Constants of Some Rare-Earth Sulfates in Aqueous Solutions at 25˚,” Journal of the American Chemical Society, Vol. 76, No. 5, 1964, p. 882. [12] F. Helfferich, “Ion Exchange,” Foreign Literature Pub- lishing House, Moscow City, 1962, p. 460. Copyright © 2013 SciRes. OJPC |

