Paper Menu >>
Journal Menu >>
 Advances in Bioscience and Biotechnology, 2010, 1, 444-452 ABB doi:10.4236/abb.2010.15058 Published Online December 2010 (http://www.SciRP.org/journal/abb/). Published Online December 2010 in SciRes. http://www.scirp.org/journal/ABB Ubiquitous expression of Sry induces embryonic lethality related to suppression of Tie2/Tek expression Kazuhisa Yoshida1, Masanori Ito1,2, Kou Yokouchi1,3, Kiyoshi Kano1, Kunihiko Naito1, Hideaki Tojo1,4 1Laboratory of Applied Genetics, Graduate School of Agricultural and Life Sciences, The University of Tokyo, Yayoi, Bunkyo-ku, Tokyo, Japan; 2Department of Pharmacology, School of Medicine, Faculty of Medicine, Toho University, Omori-Nishi, Otaku, Tokyo, Japan; 3Shirakawa Institute of Animal Genetics, Odakura, Nishigo, Fukushima, Japan; 4Yamazaki College of Animal Health Technology, Minamioosawa, Hachioji, Tokyo, Japan. Email: itomasanori@med.toho-u.ac.jp Received 21 October 2010; revised 29 October 2010; accepted 29 October 2010. ABSTRACT Sry (sex-determining region on the Y chromosome) is a mammalian sex-determining gene on the Y chro- mosome. In mice, the transient expression of Sry in supporting cell precursor cells between 10.5 and 12.5 days post-coitus (dpc) triggers the differentiation of Sertoli cells from granulosa cells. The importance of the strict regulation of Sry expression remains un- known. Thus, we attempted to produce a Sry ubiqui- tous-expressing transgenic (Tg) mouse in which for- eign Sry is driven by the CAG (cytomegalovirus im- mediate-early enhancer, chicken beta-actin promoter, and the fusion intron of chicken beta-actin and rabbit beta-globin)-Sry gene for ubiquitous expressing Sry. A low rate (2/127) of Tg pups was observed, whereas the rate of early-stage transgenic embryos before birth was 19.2% (5/26). The Sry ubiquitous-express- ing embryos showed abnormal development. The results suggest that ubiquitous expression of Sry ex- erts a negative effect on embryonic development. One of the two adult Tg mice showed low levels of Sry ex- pression. The other Tg mouse showed high Sry transgene expression, but was mosaic for the trans- gene. Developmental analysis of transgenic F1 em- bryos produced from the mosaic Tg mouse revealed that ubiquitous expression of Sry had a lethal effect on embryonic development around 12.5 dpc. The histological data indicated that ubiquitous expression of Sry induced abnormal cardiovascular development, resulting in embryonic death. Enhanced expression of Sry suppressed endogenous Tie2/Tek (tyrosine kinase with Ig and EGF homology domains 2/tunica interna endothelial cell kinase) expression in Sry-transfected primary cultured cells from wild type embryonic hearts. The results indicate that the tissue-specific and stage-specific expression of Sry is essential for normal embryogenesis. Keywords: Sry; Ti e2/Tek; Transgenic Mice 1. INTRODUCTION Sry (Sex determining region on the Y chromosome) is a transcription factor with a DNA-binding domain referred to as the high mobility group (HMG), which triggers a gene expression cascade required for initiating male sex differentiation in the bipotential indifferent gonads of mammals [1]. Mouse Sry is expressed for a brief period between 10.5 and 12.5 days post-coitus (dpc) in the supporting cells of undifferentiated gonads that differen- tiate into Sertoli cells instead of granulosa cells [2-5]. It is well documented that Sry is a trigger and decisive gene for mammalian sex determination. Sry expression induces down- or up-regulation of the expression of various genes linked to the sex-determination cascade and subsequent testicular development. A large number of factors driving gonadal differentiation are encoded by autosomal genes. Testicular development after Sry ex- pression has been shown to be regulated by various genes such as SfI/Ad4bp (steroidogenic factor 1/Adrenal 4 binding protein) [6], Wt1 (Wilms’ tumor suppressor 1) [7,8], Amh/Mis (Anti-Mulerian hormone/Mulerian-in- hibiting substance) [9], and Sox9 (Sry-related high-mo- bility group box 9) [10]. In addition, the regions respon- sible for stage-specific and tissue-specific regulation of mouse Sry expression have also been investigated [11-14]. However, the gene responsible for gonadal dif- ferentiation, i.e., the direct target of Sry, remains to be identified. Previously, an XX-sex-reversal mouse line carrying the Sry transgene driven by a weak basal Hsp70.3 pro-  K. Yoshida et al. / Advances in Bioscience and Biotechnology 1 (2010) 444-452 Copyright © 2010 SciRes. ABB 445 moter (Hsp-Sry) was established. Comparison of Hsp- Sry/XY gonads with wild-type/XY and Hsp-Sry/XX go- nads has suggested that Sry mRNA expression alone is not likely to provide positional or timing information needed for male-specific Sox9 activation in developing gonads [15]. The ability of Sry to induce testis develop- ment is limited to approximately 11.0-11.25 dpc, a time window of only 6 hours after the normal onset of Sry expression in XY gonads [16]. It is generally expected that the tightly regulated spatiotemporal expression profile of Sry during embryogenesis is crucial for assuring the normal development not only of gonads, but also of other fetal organs; however, the precise biological significance of limited Sry expression has yet to be clarified. It is well established that the phenotype of transgenic (Tg) mice exhibiting ubiquitous expression of a gene of interest can provide evidence for speculations regarding natural biological functions [17-19]. Thus, to evaluate the role(s) played by Sry in development, we utilized transgenic mice. For our specific purposes, we con- structed a CAG (cytomegalovirus immediate-early en- hancer, chicken beta-actin promoter and fusion intron of chicken beta-actin and rabbit beta-globin)-Sry fusion gene construct that induces strong ubiquitous expression of a gene of interest [20], and we then attempted to gen- erate the corresponding Tg mice (Figure 1). Here, we describe the developmental effects of ubiquitous Sry expression in these Tg mice. 2. MATERIALS AND METHODS 2.1. Animals The following strains of mice were purchased from a commercial animal breeder (Sankyo Labo-Service Cor- poration, Inc., Tokyo, Japan): B6C3F1 (C57BL/6Nx C3H/HeN), C57BL/6J, and ICR. The mice were kept in an environment with regulated temperature (22-25℃), humidity (40-50%), and illumination cycles (14-h light, 10-h dark), and were provided with food and water ad libitum. The experiments were conducted according to guidelines for the care and use of laboratory animals at the College of Agriculture, the University of Tokyo. 2.2. Tg Mouse Generation Tg mice were generated by microinjecting DNA into the pronuclei of zygotes collected from the oviducts of su- perovulated B6C3F1 females that were mated with B6C3F1 males. All methods for generating the Tg mice used here have been described in the protocol reported by Hogan et al. [21]. The construction of pCX-Sry has been described previously [22]. The SalI/BamHI DNA fragment containing the CAG-Sry fusion gene was ex- cised from pCX-Sry and separated by electrophoresis Figure 1. Schematic representation of gonadal sex determina- tion and expression levels of endogenous Sry and CAG-Sry transgene. In mice, gonads are formed around 10.5 days post-coitus (dpc). Sry (Sex-determining region on the Y chro- mosome) triggers the testis developmental pathway in the bi- potential indifferent gonads. Sry is expressed in the mouse gonads during a narrow window of development, between 10.5 and 12.5 dpc. In this study we constructed a CAG (cytomega- lovirus immediate-early enhancer, chicken beta-actin promoter and fusion intron of chicken beta-actin and rabbit beta-glo- bin)-Sry fusion gene construct that induces strong ubiquitous expression of Sry and generate the transgenic (Tg) mice. through 1% agarose gel; the fragment was then purified by CsCl ultra-centrifugation. The purified DNA frag- ment was dissolved in a solution containing 10 mM Tris-HCl (pH 7.4) and 0.25 mM EDTA (pH 7.4) and was used for pronuclear microinjection. To identify Tg foun- der animals, genomic DNA was isolated from the tip of the tail, and the genomic DNA was screened by poly- merase chain reaction (PCR) amplification using the following primers: 5’-CTC-TGC-TAA-CCA-TGT-TCA- TGC-CTT-3’ and 5’-CCA-CTG-CAG-AAG-GTT-GTA- CAG-TTT-3’, which span the CAG promoter and Sry coding region (Figures 2(a) and (b)). The PCR amplify- cations were carried out using the following parameters: 35 cycles of 30 s at 94℃, 30 s at 58℃, and 1 min at 72℃. Sex chromosome karyotypes (XX or XY) were determined by PCR using the primers 5’-GCT-CGT- TAA-TTT-CTC-ACG-TTA-GTC-C-3’ and 5’-ACA-CTT- TAG-CCC-TCC-GAT-GAG-GCT-GA-3’, which span the Sry promoter and Sry coding region (Figures 2(c) and (d)). The PCR amplifications were carried out using the following parameters: 35 cycles of 30 s at 94℃, 30 s at 58℃, and 1 min at 72℃. 2.3. Preparation of Primary Cultured Cells and Transfection of Plasmids Primary cultured cells were prepared from the hearts of 12.0-dpc embryos according to methods described in our previous paper [12,14]. pCX-Sry and pCAGGS (mock) were transfected using Effectene Transfection Reagent (Qiagen, Valencia, CA) according to the manufacturer’s 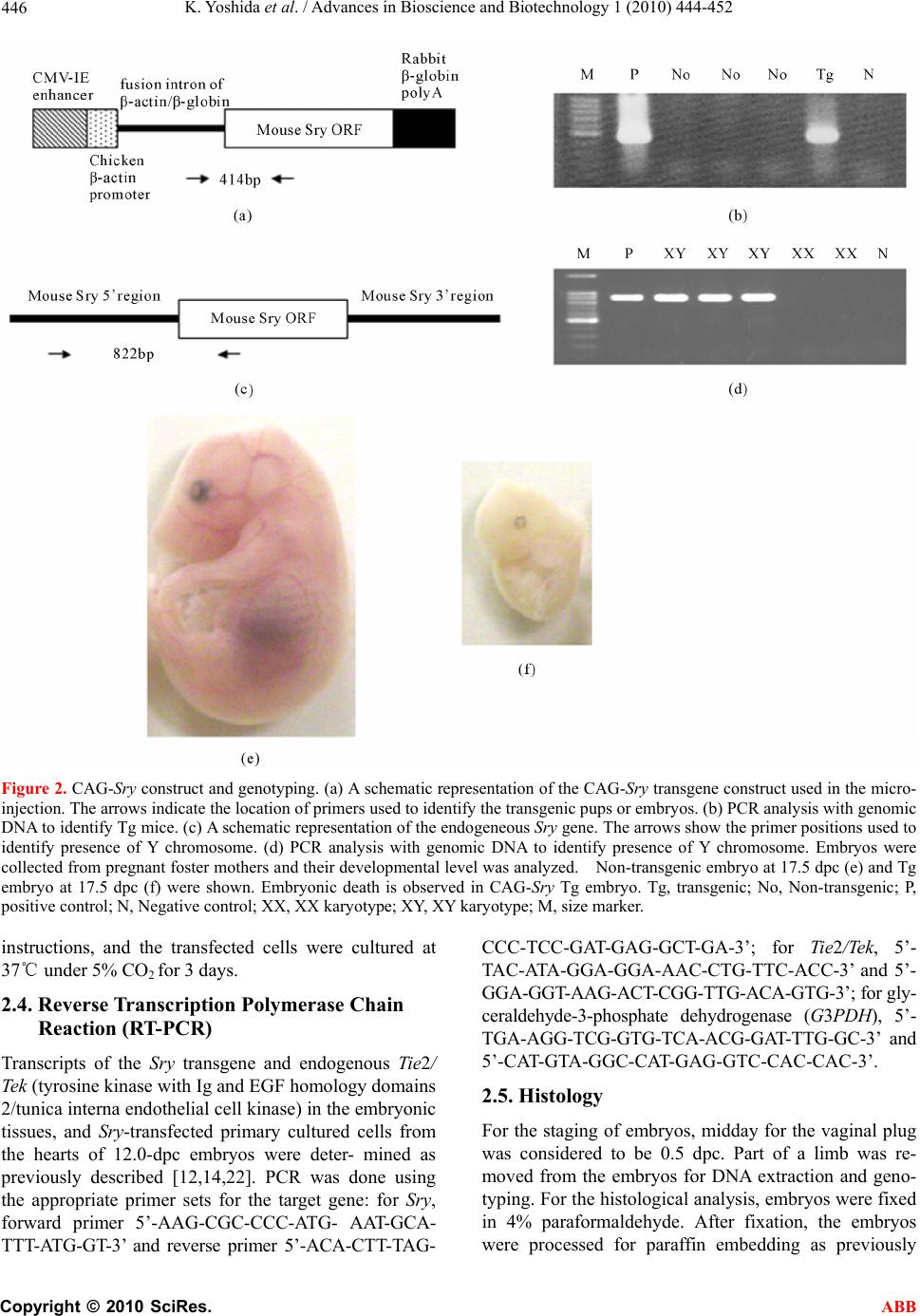 K. Yoshida et al. / Advances in Bioscience and Biotechnology 1 (2010) 444-452 Copyright © 2010 SciRes. ABB 446 Figure 2. CAG-Sry construct and genotyping. (a) A schematic representation of the CAG-Sry transgene construct used in the micro- injection. The arrows indicate the location of primers used to identify the transgenic pups or embryos. (b) PCR analysis with genomic DNA to identify Tg mice. (c) A schematic representation of the endogeneous Sry gene. The arrows show the primer positions used to identify presence of Y chromosome. (d) PCR analysis with genomic DNA to identify presence of Y chromosome. Embryos were collected from pregnant foster mothers and their developmental level was analyzed. Non-transgenic embryo at 17.5 dpc (e) and Tg embryo at 17.5 dpc (f) were shown. Embryonic death is observed in CAG-Sry Tg embryo. Tg, transgenic; No, Non-transgenic; P, positive control; N, Negative control; XX, XX karyotype; XY, XY karyotype; M, size marker. instructions, and the transfected cells were cultured at 37℃ under 5% CO2 for 3 days. 2.4. Reverse Transcription Polymerase Chain Reaction (RT-PCR) Transcripts of the Sry transgene and endogenous Tie2/ Tek (tyrosine kinase with Ig and EGF homology domains 2/tunica interna endothelial cell kinase) in the embryonic tissues, and Sry-transfected primary cultured cells from the hearts of 12.0-dpc embryos were deter- mined as previously described [12,14,22]. PCR was done using the appropriate primer sets for the target gene: for Sry, forward primer 5’-AAG-CGC-CCC-ATG- AAT-GCA- TTT-ATG-GT-3’ and reverse primer 5’-ACA-CTT-TAG- CCC-TCC-GAT-GAG-GCT-GA-3’; for Tie2/Tek, 5’- TAC-ATA-GGA-GGA-AA C-CTG-TTC-ACC-3’ and 5’- GGA-GGT-AAG-ACT-CGG-TTG-ACA-GTG-3’; for gly- ceraldehyde-3-phosphate dehydrogenase (G3PDH), 5’- TGA-AGG-TCG-GTG-TCA-ACG-GAT-TTG-GC-3’ and 5’-CAT-GTA-GGC-CAT-GAG-GTC-CAC-CAC-3’. 2.5. Histology For the staging of embryos, midday for the vaginal plug was considered to be 0.5 dpc. Part of a limb was re- moved from the embryos for DNA extraction and geno- typing. For the histological analysis, embryos were fixed in 4% paraformaldehyde. After fixation, the embryos were processed for paraffin embedding as previously  K. Yoshida et al. / Advances in Bioscience and Biotechnology 1 (2010) 444-452 Copyright © 2010 SciRes. ABB 447 described [5]. The embryos prepared in this manner were then sectioned at 6 μm, and the sections were used for hematoxylin and eosin staining. 2.6. Statistical Analysis The results are expressed as mean SEM. The signifi- cance of differences between groups was determined by Student’s t-test. 3. RESULTS 3.1. Low Production Rate of Sry Transgenic Mice The construct containing Sry under the control of the CAG promoter (CAG-Sry) was used for DNA microin- jection to produce Tg mice (Figure 2(a)). The CAG promoter was selected because it is a ubiquitously and strongly expressed promoter. In analyses of DNA pups born, the percentage of Tg animals was 1.5% (2/127). Two CAG-Sry Tg lines (female and male) were gener- ated. The female karyotype was XX, and that of the male was XY. Then, in the next experiment, pregnant mice that had undergone a transfer of CAG-Sry microinjected eggs were sacrificed between 11.5 dpc and 17.5 dpc, and the percentage of Tg embryo was investigated. The per- centage of Tg embryo taking into consideration both embryonic stages, was approximately 19.2% (5/26). Tg embryos sacrificed at 17.5 dpc showed histological ab- normalities (Figure 2(f)), suggesting that the survival rate of Tg embryos was reduced by the ubiquitous ex- pression of Sry. 3.2. Lethal Effect of Sry-Ubiquitous Expression during Embryonic Development Although the transgene was detected in F1 mice in the case of female CAG-Sry Tg mouse, low transcripts from the transgene were detected by RT-PCR, and female- to-male sex reversal did not occur with the Sry transgene (data not shown). The male Tg mouse was characterized as XY and fertile. No newborn pups resulting from breeding with the male Tg mouse were transgenic. This result suggested that ubiquitous and strong expression of Sry yields embryonic lethality; the mosaic integration of the transgene in the founder Tg mouse could explain why the founder Tg mouse was able to live to adulthood. To further investigate this lack of generation of trans- genic offspring, we performed a series analysis of litters at embryonic stages. Tg embryos showing high levels of Sry transgene expression were detected at a rate of 7% (12/175). The rate of occurrence of Tg embryos indi- cated the mosaic integration of the transgene. Next, we attempted to determine the stage of embryonic develop- ment at which the ubiquitous-expression of Sry could exert a negative impact. At 11.5 dpc, control and CAG-Sry Tg embryos displayed no morphological dif- ferences (Figures 3(a) and (b)). In contrast, edema and congestion were observed in CAG-Sry Tg embryos at 12.5 dpc (Figure 3(d)). The CAG-Sry Tg embryos were dead by 13.5 dpc (Figure 3(f)). Therefore, the present results indicate that the ubiquitous expression of Sry has a lethal effect on embryonic development at approxi- mately 12.5 dpc. Histological analysis of the genital ridge of CAG-Sry transgenic embryos (XY) at 12.5 dpc showed no testis cord formation (Figure 4(g)), and an enlargement of the diameter of the atrium was also ob- served (Figure 4(h)). The layer of smooth muscle cells of Tg embryos was thin, compared to that of wild-type embryos, in the dorsal aorta region (Figure 4(d) and (i)). Moreover, the endothelial cells of Tg embryos were ab- normally round (Figure 4(j)). 3.3. Suppression of Tie2/Tek Expression by Sry Expression We also examined the expression levels of Tie2/Tek in Sry-transgenic tissues, because Ti e2/Tek has been re- ported to be involved in cardiovascular development. In wild-type embryos, Tie2/Tek expression levels were highest in the hearts, compared with those of the other two tissue types examined, i.e., brains and gonads, at 12.0 dpc (Figure 5(a)). As regards the rates of expres- sion observed in these three tissues, Ti e2/Tek expression levels of Sry-transgenic tissues were lower than that of wild-type tissues (Figure 5(a)). Thus, in these embry- onic tissues, Tie2/Tek expression was suppressed by the ubiquitous expression of foreign Sry. This suppression of Tie2/Tek expression by Sry expression was also seen in Sry-transfected primary cultured cells from wild-type embryonic hearts (Figure 5(c)). The present findings sug- gest that ubiquitous Sry expression exerts a negative effect on cardiovascular development via changes in the expression of other genes, which ultimately results in embryonic lethality. The Tg male became unable to im- pregnate any females, which rendered it impossible to conduct further analyses of embryos from the Tg male. 4. DISCUSSION In this study, we used a Tg approach to characterize the biological function of Sry. We generated Tg mice that ubiquitously expressed Sry as a means of elucidating the biological functions of this transcription factor. The effi- ciency of transgenesis was remarkably low (1.5%) com- pared with the efficiency of our usual transgenic ex- speriments [23-26]. Furthermore, no newborn pups gen- erated by breeding of the CAG-Sry Tg founder (mosaic for the transgene) carried the CAG-Sry transgene. Time- series analyses of F1 CAG-Sry Tg embryos clearly re- 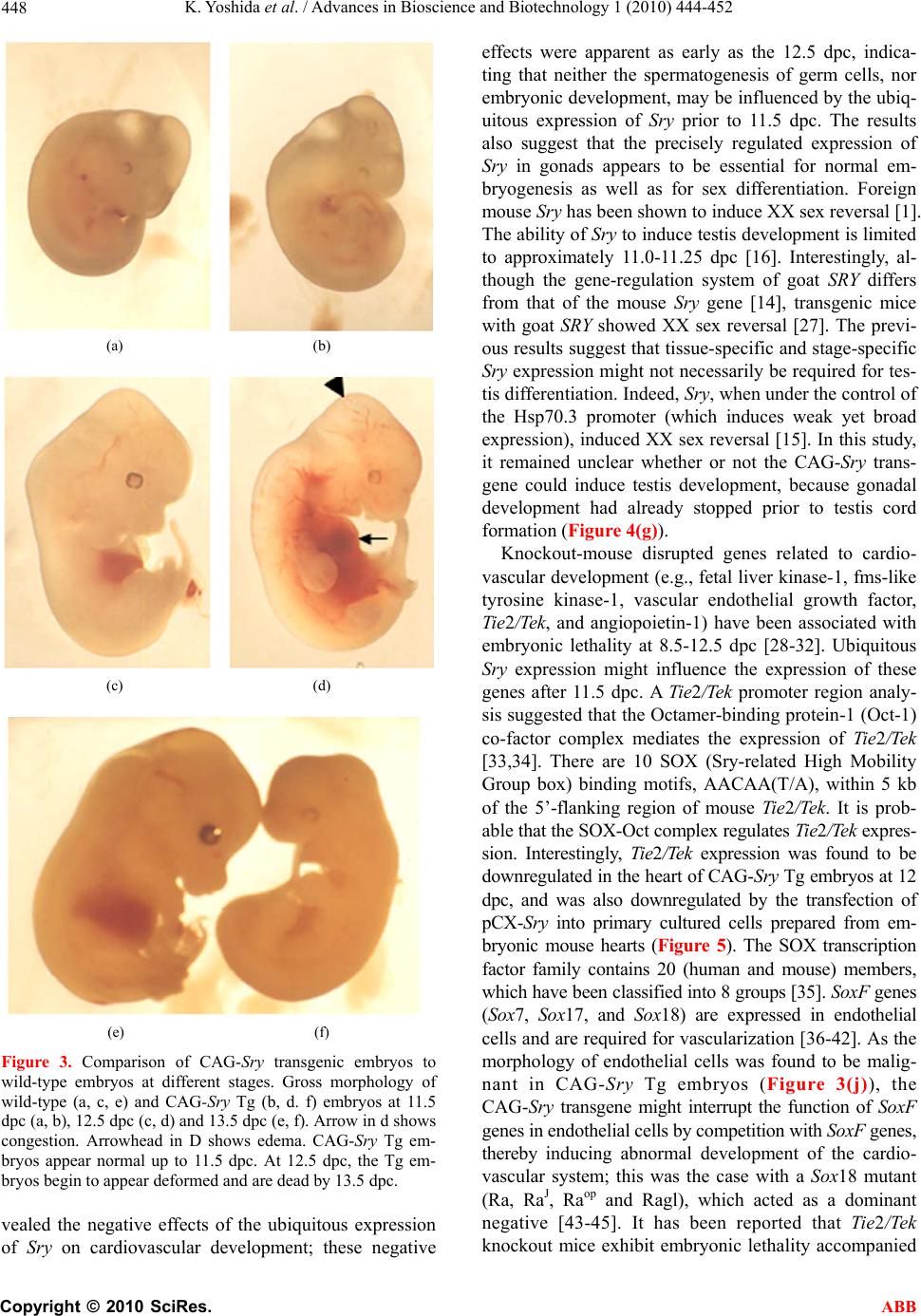 K. Yoshida et al. / Advances in Bioscience and Biotechnology 1 (2010) 444-452 Copyright © 2010 SciRes. ABB 448 (a) (b) (c) (d) (e) (f) Figure 3. Comparison of CAG-Sry transgenic embryos to wild-type embryos at different stages. Gross morphology of wild-type (a, c, e) and CAG-Sry Tg (b, d. f) embryos at 11.5 dpc (a, b), 12.5 dpc (c, d) and 13.5 dpc (e, f). Arrow in d shows congestion. Arrowhead in D shows edema. CAG-Sry Tg em- bryos appear normal up to 11.5 dpc. At 12.5 dpc, the Tg em- bryos begin to appear deformed and are dead by 13.5 dpc. vealed the negative effects of the ubiquitous expression of Sry on cardiovascular development; these negative effects were apparent as early as the 12.5 dpc, indica- ting that neither the spermatogenesis of germ cells, nor embryonic development, may be influenced by the ubiq- uitous expression of Sry prior to 11.5 dpc. The results also suggest that the precisely regulated expression of Sry in gonads appears to be essential for normal em- bryogenesis as well as for sex differentiation. Foreign mouse Sry has been shown to induce XX sex reversal [1]. The ability of Sry to induce testis development is limited to approximately 11.0-11.25 dpc [16]. Interestingly, al- though the gene-regulation system of goat SRY differs from that of the mouse Sry gene [14], transgenic mice with goat SRY showed XX sex reversal [27]. The previ- ous results suggest that tissue-specific and stage-specific Sry expression might not necessarily be required for tes- tis differentiation. Indeed, Sry, when under the control of the Hsp70.3 promoter (which induces weak yet broad expression), induced XX sex reversal [15]. In this study, it remained unclear whether or not the CAG-Sry trans- gene could induce testis development, because gonadal development had already stopped prior to testis cord formation (Figure 4(g)). Knockout-mouse disrupted genes related to cardio- vascular development (e.g., fetal liver kinase-1, fms-like tyrosine kinase-1, vascular endothelial growth factor, Tie2/Tek, and angiopoietin-1) have been associated with embryonic lethality at 8.5-12.5 dpc [28-32]. Ubiquitous Sry expression might influence the expression of these genes after 11.5 dpc. A Ti e2/Tek promoter region analy- sis suggested that the Octamer-binding protein-1 (Oct-1) co-factor complex mediates the expression of Tie2/Tek [33,34]. There are 10 SOX (Sry-related High Mobility Group box) binding motifs, AACAA(T/A), within 5 kb of the 5’-flanking region of mouse Tie2/Tek . It is prob- able that the SOX-Oct complex regulates Tie2/Tek expres- sion. Interestingly, Tie2/Tek expression was found to be downregulated in the heart of CAG-Sry Tg embryos at 12 dpc, and was also downregulated by the transfection of pCX-Sry into primary cultured cells prepared from em- bryonic mouse hearts (Figure 5). The SOX transcription factor family contains 20 (human and mouse) members, which have been classified into 8 groups [35]. SoxF genes (Sox7, Sox17, and Sox18) are expressed in endothelial cells and are required for vascularization [36-42]. As the morphology of endothelial cells was found to be malig- nant in CAG-Sry Tg embryos (Figure 3(j)), the CAG-Sry transgene might interrupt the function of SoxF genes in endothelial cells by competition with SoxF genes, thereby inducing abnormal development of the cardio- vascular system; this was the case with a Sox18 mutant (Ra, RaJ, Raop and Ragl), which acted as a dominant negative [43-45]. It has been reported that Tie2/Tek knockout mice exhibit embryonic lethality accompanied  K. Yoshida et al. / Advances in Bioscience and Biotechnology 1 (2010) 444-452 Copyright © 2010 SciRes. ABB 449 Figure 4. Histological analysis of CAG-Sry transgenic embryos and wild-type littermates. Hematoxylin and eosin staining of whole embryos (a, f), gonad regions (b, g), heart regions (c, h) and dorsal aortic regions (d, e, i, j) of wild-type XY embryos (a to e) and Tg XY embryos (f to j) from breeding of wild-type female mouse and male CAG-Sry Tg mouse are shown. Tg embryos show the accu- mulation of blood cells in the cardinal veins (f, arrow). In the sections of genital ridge region, the tubule structure (arrow) is observed in gonad region of wild-type XY embryo (b). There is no tubule structure in gonad region of Tg XY embryo (g). Arrow shows bleed- ing in abdominal cavity. Sections of heart region of wild-type (c) and Tg (h) embryos show enlarged atrium in Tg embryo (arrow). Thin layer of muscle cells of aortic region (indicated by two arrows) is observed in section of Tg embryo (i) compared with that of wild-type embryo (d). Normal endothelial cells of aorta show flat morphology (arrows in e) and abnormal round shape of endothelial cells are observed in sections of Tg embryo (arrows in j). bar, 50 μm. by abnormal cardiovascular development [31]. In the present study, it was revealed that Tie2/Tek expression was suppressed by enhanced Sry expression in both the Sry-transgenic heart and Sry-transfected primary cul- tured heart cells. As hypothesis of mutant Sox18 by Downes and Koopman [45], Sry proteins might act as dominant negative form by disruptive interaction with co-factor(s) of Sox18 (Figure 5). In conclusion, we demonstrated that the tissue-speci- fic and stage-specific expression of Sry is essential for normal embryogenesis, and in particular for cardiovas- cular development.  K. Yoshida et al. / Advances in Bioscience and Biotechnology 1 (2010) 444-452 Copyright © 2010 SciRes. ABB 450 Figure 5. Effects of Sry-transgene on endogenous Ti e2/Tek expression. (a) Ti e2/Tek expression levels relative to those of G3PDH in the CAG-Sry transgenic tissues at 12.0 dpc. Expression levels of Tie2/Tek in the Sry transgenic tissues were reduced (n = 1). (b) Determination of expression of Sry transgene in the primary cultured cells collected from embryonic hearts at 12.0 dpc. pCX-Sry: Sry-transfected cells, Mock: mock-transfected cells, G3PDH: a house-keeping gene used as a refer- ence, RT-: no reverse transcription. (c) Tie2/Tek expression levels relative to those of G3PDH. Tie2/Tek expression levels were reduced in the Sry-transfected primary cultured cells. *: P < 0.05, Vertical bars indicate the means SEM (n = 3). (d-f) The hypothesized effects of ubiquitous expres- sion of Sry on the function of Sox18. Sox18 binds to the upstream regulatory sequence of a Ti e2/Tek gene. The functional trans-activation domain is shown as opening that complement in shape its re- spective interacting co-factor (d). When affected by mutation, the domain is depicted as non-complementary opening (e). In the case of CAG-Sry Tg mice, Sox18 is replaced by Sry proteins which act as if mutant Sox18 (f). Successful interaction of Sox18 domain with its co-factor is indi- cated by an arrow, while disrupted interaction is indicated by a crossed arrow.  K. Yoshida et al. / Advances in Bioscience and Biotechnology 1 (2010) 444-452 Copyright © 2010 SciRes. ABB 451 5. ACKNOWLEDGEMENTS We would like to thank Dr. Toshiyasu Matsui (The University of Tokyo) for expert advice on histology. This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan (No.16380197). REFERENCES [1] Koopman, P., Gubbay, J., Vivian, N., Goodfellow, P. and Lovell-Badge, R. (1991) Male development of chromo- somally female mice transgenic for Sry. Nature, 351, 117-121. [2] Hacker, A., Capel, B., Goodfellow, P. and Lovell-Badge, R. (1995) Expression of Sry, the mouse sex determining gene. Development, 121, 1603-1614. [3] Bullejos, M. and Koopman, P. (2001) Spatially dynamic expression of Sry in mouse genital ridges. Developmen- tal Dynamics, 221, 201-205. [4] Albrecht, K.H. and Eicher, E.M. (2001) Evidence that Sry is expressed in pre-Sertoli cells and Sertoli and granulosa cells have a common precursor. Developmental Biology, 240, 92-107. [5] Ito, M., Yokouchi, K., Yoshida, K., Kano, K., Naito, K., Miyazaki, J. and Tojo, H. (2006) Investigation of the fate of Sry-expressing cells using an in vivo Cre/loxP system. Development Growth and Differentiation, 48, 41-47. [6] Shen, W.H., Moore, C.C., Ikeda, Y., Parker, K.L. and Ingraham, H.A. (1994) Nuclear receptor steroidogenic factor 1 regulates the mullerian inhibiting substance gene: A link to the sex determination cascade. Cell, 77, 651- 661. [7] Toyooka, Y., Tanaka, S.S., Hirota, O., Tanaka, S., Takagi, N., Yamanouchi, K., Tojo, H. and Tachi, C. (1998) Wilms’ tumor suppressor gene (WT1) as a target gene of SRY function in a mouse ES cell line transfected with SRY. International Journal of Developmental Biology, 42, 1143-1151. [8] Hsu, S.Y., Kubo, M., Chun, S.Y., Haluska, F.G., Hous- man, D.E. and Hsueh, A.J. (1995) Wilms’ tumor protein WT1 as an ovarian transcription factor: Decreases in ex- pression during follicle development and repression of inhibin-alpha gene promoter. Molecular Endocrinology, 9, 1356-1366. [9] Musterberg, A. and Lovell-Badge, R. (1991) Expression of the mouse anti-mulerian hormone gene suggests a role in both male and female sexual differentiation. Develop- ment, 11 3, 613-624. [10] De Santa Barbara, P., Bonneaud, N., Boizet, B., Des- clozeaux, M., Moniot, B., Sudbeck, P., Scherer, G., Pou- lat, F. and Berta, P. (1998) Direct interaction of SRY-related protein SOX9 and steroidogenic factor 1 regulates transcription of the human anti-mulerian hor- mone gene. Molecular and Cellular Biology, 18, 6653- 6665. [11] Dolci, S., Grimaldi, P., Geremia, R., Pesce, M. and Rossi, P. (1997) Identification of a promoter region generating Sry circular transcripts both in germ cells from male adult mice and in male mouse embryonal gonads. Biol- ogy of Reproduction, 57, 1128-1135. [12] Ito, M., Yokouchi, K., Naito, K., Endo, H., Hakamata, Y., Miyazaki, J. and Tojo, H. (2002) In vitro Cre/loxP system in cells from developing gonads: Investigation of the Sry promoter. Development Growth and Differentiation, 44, 549-557. [13] Yokouchi, K., Ito, M., Nishino, K., Yamanouchi, K., Naito, K., Suzawa, M., Kato, S., Hakamata, Y., Endo, H. and Tojo, H. (2003) Stage-specific regulatory element of mouse Sry gene. Molecular Reproduction and Develop- ment, 64, 389-396. [14] Ito, M., Yokouchi, K., Naito, K., Endo, H., Hakamata, Y., Miyazaki, J. and Tojo, H. (2005) Detection of elements responsible for stage- and tissue-specific expression of mouse Sry using an in vitro Cre/loxP system. Biochemi- cal and Biophysical Resea rch Communications, 337, 264- 270. [15] Kidokoro, T., Matoba, S., Hiramatsu, R., Fujisawa, M., Kanai-Azuma, M., Taya, C., Kurohmaru, M., Kawakami, H., Hayashi, Y., Kanai, Y. and Yonekawa, H. (2005) In- fluence on spatiotemporal patterns of a male-specific Sox9 activation by ectopic Sry expression during early phases of testis differentiation in mice. Developmental Biology, 278, 511-525. [16] Hiramatsu, R., Matoba, S., Kanai-Azuma, M., Tsune- kawa, N., Katoh-Fukui, Y., Kurohmaru, M., Morohashi, K., Wilhelm, D., Koopman, P. and Kanai, Y. (2009) A critical time window of Sry action in gonadal sex deter- mination in mice. Development, 136, 129-138. [17] Ikeda, K., Nukumi, N., Iwamori, T., Osawa, M., Naito, K. and Tojo, H. (2004) Inhibitory function of whey acidic protein in the cell-cycle progression of mouse mammary epithelial cells (EpH4/K6 cells). Journal of Reproduction and Development, 50, 87-96. [18] Nukumi, N., Ikeda, K., Osawa, M., Iwamori, T., Naito, K. and Tojo, H. (2004) Regulatory function of whey acidic protein in the proliferation of mouse mammary epithelial cells in vivo and in vitro. Developmental Biology, 274, 31-44. [19] Iwamori, T., Oosawa, M., Nukumi, N., Kano, K., Sudo, K., Naito, K. and Tojo, H. (2005) Aberrant development of mammary glands, but precocious expression of beta-casein in transgenic females ubiquitously expressing whey acidic protein transgene. Journal of Reproduction and Development, 51, 579-592. [20] Niwa, H., Yamamura, K. and Miyazaki, J. (1991) Effi- cient selection for high-expression transfectants with a novel eukaryotic vector. Gene, 108, 193-199. [21] Hogan, B., Constantini, F. and Lacy, E. (1994) Manipu- lating the mouse embryo. 2nd Edition, Cold Spring Har- bor Laboratory Press, New York. [22] Ito, M., Miyagishi, M., Murata, C., Kawasaki, H., Baba, T., Tachi, C. and Taira, K. (2006) Down-regulation of endogenous Wt1 expression by Sry transgene in the murine embryonic mesonephros-derived M15 cell line. Journal of Reproduction and Development, 52, 415-427. [23] Kato, M., Yamanouchi, K., Ikawa, M., Okabe, M., Naito, K. and Tojo, H. (1999) Efficient selection of transgenic mouse embryos using EGFP as a marker gene. Molecular Reproduction and Development, 54, 43-48. [24] Seo, B.B., Kim, C.H., Yamanouchi, K., Takahashi, M., Sawasaki, T., Tachi, C. and Tojo, H. (2000) Co-injection of restriction enzyme with foreign DNA into the pro-  K. Yoshida et al. / Advances in Bioscience and Biotechnology 1 (2010) 444-452 Copyright © 2010 SciRes. ABB 452 nucleus for elevating production efficiencies of trans- genic animals. Animal Reproduction Science, 63, 113- 122. [25] Kubo, J., Yamanouchi, K., Naito, K. and Tojo, H. (2002) Expression of the gene of interest fused to the EGFP- expressing gene in transgenic mice derived from selected transgenic embryos. Journal of Experimental Zoology, 293, 712-718. [26] Ito, M., Yamanouchi, K., Naito, K. Calos, M.P. and Tojo, H. Site-specific integration of transgene targeting an endogenous lox-like site in early mouse embryos. Jour- nal of Applied Genetics, in press. doi:10.1007/s13353-010-0011-3 [27] Pannetier, M., Tilly, G., Kocer, A., Hudrisier, M., Renault, L., Chesnais, N., Costa, J., Le Provost, F., Vaiman, D., Vilotte, J.L. and Pailhoux, E. (2006) Goat SRY induces testis development in XX transgenic mice. FEBS Letters, 580, 3715-3720. [28] Fong, G.H., Rossant, J., Gertsenstein, M. and Breitman, M.L. (1995) Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature, 376, 66-70. [29] Shalaby, F., Rossant, J., Yamaguchi, T.P., Gertsenstein, M., Wu, X.F., Breitman, M.L. and Schuh, A.C. (1995) Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature, 376, 62-66. [30] Ferrara, N., Carver-Moore, K., Chen, H., Dowd, M., Lu, L., O'Shea, K.S., Powell-Braxton, L., Hillan, K.J. and Moore, M.W. (1996) Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Na- ture, 380, 439-442. [31] Dumont, D.J., Gradwohl, G., Fong, G.H., Puri, M.C., Gertsenstein, M., Auerbach, A. and Breitman, M.L. (1994) Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes and Development, 8, 1897-1909. [32] Suri, C., Jones, P.F., Patan, S., Bartunkova, S., Maisonpi- erre, P.C., Davis, S., Sato, T.N., Yancopoulos, G.D. (1996) Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell, 87, 1171-1180. [33] Fadel, B.M., Boutet, S.C. and Quertermous, T. (1998) Functional analysis of the endothelial cell-specific Tie2/Tek promoter identifies unique protein-binding elements. Biochemical Journal, 330, 335-343. [34] Fadel, B.M., Boutet, S.C. and Quertermous, T. (1999) Octamer-dependent in vivo expression of the endothelial cell-specific TIE2 gene. Journal of Biological Chemistry, 274, 20376-20383. [35] Bowles, J., Schepers, G. and Koopman, P. (2000) Phy- logeny of the SOX family of developmental transcription factors based on sequence and structural indicators. De- velopmental Biology, 227, 239-255. [36] Zhang, C., Basta, T. and Klymkowsky, M.W. (2005) SOX7 and SOX18 are essential for cardiogenesis in Xenopus. Developmental. Dynamics, 234, 878-891. [37] Young, N., Hahn, C.N., Poh, A., Dong, C., Wilhelm, D., Olsson, J., Muscat, G.E., Parsons, P., Gamble, J.R. and Koopman, P. (2006) Effect of disrupted SOX18 tran- scription factor function on tumor growth, vasculariza- tion, and endothelial development. Journal of National Cancer Institute, 98, 1060-1067. [38] Matsui, T., Kanai-Azuma, M., Hara, K., Matoba, S., Hiramatsu, R., Kawakami, H., Kurohmaru, M., Koopman, P. and Kanai, Y. (2006) Redundant roles of Sox17 and Sox18 in postnatal angiogenesis in mice. Journal of Cel- lular Science, 11 9, 3513-3526. [39] Sakamoto, Y., Hara, K., Kanai-Azuma, M., Matsui, T., Miura, Y., Tsunekawa, N., Kurohmaru, M., Saijoh, Y., Koopman, P. and Kanai, Y. (2007) Redundant roles of Sox17 and Sox18 in early cardiovascular development of mouse embryos. Biochemical Biophysical Research Communications, 360, 539-544. [40] Liu, Y., Asakura, M., Inoue, H., Nakamura, T., Sano, M., Niu, Z., Chen, M., Schwartz, R.J. and Schneider, M.D. (2007) Sox17 is essential for the specification of cardiac mesoderm in embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America, 104, 3859-3864. [41] Francois, M., Caprini, A., Hosking, B., Orsenigo, F., Wilhelm, D., Browne, C., Paavonen, K., Karnezis, T., Shayan, R., Downes, M., Davidson, T., Tutt, D., Cheah, K.S., Stacker, S.A., Muscat, G.E., Achen, M.G., Dejana, E. and Koopman, P. (2008) Sox18 induces development of the lymphatic vasculature in mice. Nature, 456, 643- 647. [42] Hosking, B., Francois, M., Wilhelm, D., Orsenigo, F., Caprini, A., Svingen, T., Tutt, D., Davidson, T., Browne, C., Dejana, E. and Koopman, P. (2009) Sox7 and Sox17 are strain-specific modifiers of the lymphangiogenic de- fects caused by Sox18 dysfunction in mice. Development, 136, 2385-2391. [43] Pennisi, D., Gardner, J., Chambers, D., Hosking, B., Peters, J., Muscat, G., Abbott, C. and Koopman, P. (2000) Mutations in Sox18 underlie cardiovascular and hair fol- licle defects in ragged mice. Nature Genetics, 24, 434- 437. [44] James, K., Hosking, B., Gardner, J., Muscat, G.E. and Koopman, P. (2003) Sox18 mutations in the ragged mouse alleles ragged-like and opossum. Genesis, 36, 1-6. [45] Downes, M. and Koopman, P. (2001) SOX18 and the transcriptional regulation of blood vessel development. Trends in Cardiovascular Medicine, 11 , 318-324. |

