 World Journal of Cardiovascular Diseases, 2013, 3, 361-370 WJCD http://dx.doi.org/10.4236/wjcd.2013.35056 Published Online August 2013 (http://www.scirp.org/journal/wjcd/) Opiate exposure increases arterial stiffness, advances vascular age and is an independent cardiovascular risk factor in females: A cross-sectional clinical study* Albert Stuart Reece#, Gary Kenneth Hulse School of Psychiatry and Clinical Neurosciences, University of Western Australia, Crawley, Australia Email: #sreece@bigpond.net.au Received 11 May 2013; revised 11 June 2013; accepted 15 July 2013 Copyright © 2013 Albert Stuart Reece, Gary Kenneth Hulse. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Background: Whilst several studies have demonstra- ted poor cardiovascular health in opiate dependence, its role as a cardiovascular risk factor has not been considered. Methods: Pulse wave analysis was under- taken by radial arterial tonometry (SphygmoCor) in female control and opiate-dependent patients and compared to lifetime opiate use. Results: 222 opiate dependent women were compared to 175 controls. Opiate dependent patients were receiving treatment with buprenorphine (83.3%), methadone (13.5%), or naltrexone (3.2%). Non log transformed chronologic age (CA) for the two groups was 33.58 ± 0.57 (opiate) vs. 32.62 ± 0.96 (controls) years (mean ± S.E.M.; P = 0.39). Vascular Reference Age (RA) 39.30 ± 1.28, vs. 35.03 ± 1.41 the RA-CA difference (5.73 ± 1.02 vs. 2.41 ± 0.91) and the RA/CA ratio (1.16 ± 0.03 vs. 1.07 ± 0.02; all P < 0.02), and all measurements of central arterial stiffness (P < 0.02) were significantly worse for opiates compared to controls. When adjusted for CA, RA and central augmentation pressure and index were all worse by themselves and in interaction with CA (all P < 0.005). At 60 years the modelled RA’s were 83.79 and 67.52 years respectively. The opiate dose-duration interaction showed a dose-response effect with RA (P = 0.0033). After full adjustment for established cardiovascular risk factors, the dose-du- ration interaction remained significant (P = 10−6), was included in 10 other terms, and dose or duration was included in 15 other interactions. Conclusion: These data show that lifetime opiate use is significantly as- sociated with increased arterial stiffness and vascular age and suggest a dose-response relationship. This relationship is robust and persists after full multi- variate adjustment. These findings carry far-reaching implications for opiate-induced generalized accelera- tion of organismal ageing. Keywords: Arterial Stiffness; Heroin; Opiates; Vascular Ageing; Dependence; Human Ageing 1. INTRODUCTION Opiate dependence which can arise from illegal drug use, inappropriate use of medically derived opiates, or iatro- genically as in the course of chronic pain management, is a major public health issue in modern western nations. Chronic pain is experienced by 90 million Americans over a lifetime, and is responsible for $100 billion in annual healthcare costs [1]. In 2004, it was estimated that 235 million scripts for opiates were prescribed. Indeed, it has been noted that in the USA in recent years overdoses with legal opiates outnumber those from heroin and co- caine combined [1]. Data from the Drug Abuse Warning Network suggested that from 1998-2009 non-heroin non- methadone opiate overdoses presenting to US Emer- gency Rooms rose from 2.2% of all ER presentations to 11.5% [2]. Whilst cardiovascular complications of amphetamine and cocaine addiction are well recognized, a small but increasing amount of empirical data exist linking long term opiate dependence with heart and vascular disease [3-5]. 17% of heroin dependent patients of 44 or more years of age in one study had coronary stenoses of more than 75% [6]. High rates of myocardial fibrosis, ven- tricular hypertrophy, interstitial fibrosis, perivascular fi- brosis and severe coronary disease were identified in *Conflict of Interest: Nil declared. Sources of Funding: Nil. Statement of Authorship: This work is the work of the authors in its entirety. #Corresponding author. OPEN ACCESS  A. S. Reece, G. K. Hulse / World Journal of Cardiovascular Diseases 3 (2013) 361-370 362 another study; all worse in those patients treated with methadone [4]. In Australia, large state-wide autopsy population based study reviewing in excess of 20 years of opiate maintenance treatments from Sydney, showed a relative risk of cardiovascular disease of 2.2 (95% C.I. 1.8 - 2.7, Appendix 6) [7]. A careful angiographic study of 2405 patients showed that opium use was significantly related to coronary disease with odds ratio 1.8 (C.I. 1.8 - 4.7, P = 0.01), including a dose response effect with dis- ease severity (P = 0.002) [8]. This relationship persisted when only non-smokers were considered (P < 0.001). These workers further showed that the coronary disease presents four years earlier in opium users [9]. Most of this literature however does not report on gender specific outcomes. The results of various genome wide association studies (GWAS) in coronary disease [10-13] have identified a “hotspot” on the senescence locus at chromosome 9p21.3, a site which is adjacent to genes CDKN2A and CDKN2B which code for P16INK4A, P15INK4B and P19ARF, and actually maps to the long non-protein cod- ing senescence-associated RNA called ANRIL [14]. ANRIL has been shown to interact in cis with the pro- moter for CDKN2A (coding for P16), and to act via γ-interferon [15,16]. P16 activation is known to be asso- ciated with senescence induction in many tissues [17,18]. Such tissues secrete various factors including pro-in- flammatory cytokines which further maintain and induce the senescent state [19]. It is of great interest therefore to note that opiates have long been known to interfere with tissue growth [20,21] by an effect which has been shown to be mediated by P16 and P21 [22,23]. Indeed, it has been shown that most tissues are under a normal tonic endorphin/enkephalin mediated negative growth sup- pression, which can be unmasked pharmacologically by opiate antagonists, or mechanistically with appropriate targeted siRNA’s directed against the perinuclear recep- tor which mediates these activities called the opiate growth factor receptor [24]. Pulse Wave Analysis (PWA) by radial arterial tono- metry is a technique which has been widely used in re- cent years to ascertain central arterial function, arterial stiffness and vascular compliance. It is able to differenti- ate the forward pressure wave originating from the heart from the backward projected pressure wave originating from peripheral resistance sites. The speed and amplitude of the reflected wave are a function of the stiffness of the arterial system, which in turn has been related to age in large population based studies. The SphygmoCor system automatically generates a vascular reference age (VA or RA) from the data output. The technique has several ad- vantages in research into vascular health in opiate de- pendent persons, particularly, that it becomes sensitive to changes early in life in the third and fourth decade, prior to the time when many other vascular function tests be- come discriminative and is the age of most of our drug dependent cohort. PWA is also rapid, and can readily be repeated on subjects who re-present at a later time. Importantly, cardiovascular ageing has been found to account for more than half the effect of aging in western populations [25]. For this reason, the importance of such studies extends well beyond its implications for cardio- vascular medicine, and suggests that a demonstration of advanced vascular age is actually a surrogate marker for generalized organismal ageing. As this clinic sees both general medical and opiate dependent patients and has experience with the PWA technique, we were ideally placed to formally test whe- ther long term opiate dependence is associated with in- creased vascular stiffness and central arterial ageing. Data in males, in longitudinal studies and by pharma- cological treatment types is presented in companion pa- pers. 2. METHODS Patient Selection and Treatment. Control females (N = 175) were recruited from patients having insurance or employment medical examinations, patients with minor physical health problems, such as ear blockages or minor psychological presentations (N = xx), university students (N = xx) and community volunteers (N = xx). 222 heroin dependent females maintained on methadone (13.5%), buprenorphine (83.3%), or implant naltrexone (3.2%) were recruited and sampled opportunistically at the time of their presentation to the clinic. Pharmacotherapy treatment was in accordance with established clinical practices either at this clinic or by their usual treating physicians. Naltrexone implants were not performed as part of this study, but patients who had them previously inserted for management of heroin dependence were studied on the occasion of their visit to the clinic. Naltrexone implants are not a registered therapeutic good in Australia, but are available to patients on a compass- sionate access scheme within the Special Access scheme of the Therapeutic Goods Administration. These techni- ques have been previously described [26]. Implants were obtained from Go Medical Industries in Perth, Western Australia, were of 1.7 g, and designed to last about five months. PWA Measurements. Patients were not permitted to talk or sleep during the performance of PWA studies. Access to food, drink, and tobacco was not restricted. If patients identified use of alcohol prior to the testing, the studies from that day were discarded. PWA was per- formed with the Miller microtonometer, the Sphygmo- Cor software and the AtCor preamplifier and hardware, obtained from AtCor in Sydney. Studies were performed over the right radial artery unless it was not available. The brachial blood pressure was taken from the contra- Copyright © 2013 SciRes. OPEN ACCESS  A. S. Reece, G. K. Hulse / World Journal of Cardiovascular Diseases 3 (2013) 361-370 363 lateral arm to the study side using an Omron HEM 907 oscillometric device. Studies were done in quintuplicate wherever possible. A history of recent and lifetime drug use was taken from the patient at the time of study, and entered into the software’s database. If patients’ main opiate of abuse was not heroin, it was converted into morphine equivalents, and then into heroin equivalents, at a conversion rate of 500 mg of morphine per gram of street heroin (REF). The heroin dose was the dose usu- ally taken at the time of study. Length of heroin use was the total period of opiate use from the time of first use to the present. Cardiovascular parameters were generated automatically by the software and outputted from it. Major indices calculated from this technique included the Vascular or Reference Age (VA, RA), Central Sys- tolic Pressure (C_SP), Central Diastolic Pressure (C_DP), the Chronologic Age (CA), the Central Augmentation Pressure at Heart Rate 75 (C_AP_HR75), the Central Augmentation Pressure/Pulse Height Ratio at Heart Rate 75 (C_AGPH_HR75) also known as the Augmentation Index, Peripheral-Central Pulse Pressure Amplification Ratio (PPAmpRatio), Central Pulse Height (C_PH), Central Mean Pressure (C_MEANP), Central End Sys- tolic Pressure (C_ESP), the Central Diastolic Time Index (C_DTI), the Central Tension Time index (C_TTI), the Central Diastolic Duration (C_DD), and an index of sub- endocardial perfusion known variously as the Central Stroke Volume Index (C_SVI), the Subendocardial Per- fusion Ratio (SEVR) or the Buckberg ratio, which is defined as the C_TTI/C_DTI. Statistics. Data are presented as mean ± S.E.M. Epi- Info 7.0.8.3 from CDC Atlanta, Georgia was used to per- form Chi Squared tests to compare categorical variables. “Statistica” 7.1 from Statsoft, Oklahoma was used to compare continuous variables using student’s t-tests. Se- parate variances were employed were Levene’s test was significant. Data was log transformed as indicated by the Shapiro test in the interests of normality assumptions with the sole exception of CRP. CRP was transformed by the arcsinh transformation which is similar to log trans- formation, but it also accepts negative and zero argu- ments. Model appropriateness was determined by the outcome of Anova tests and Akiane Information Criteria (A.I.C.). Multiple-regression was performed in “R” 2.13.1 obtained from the Central “R” Archive Network mirror at the University of Melbourne. Graphs were drawn with the aid of Ggplot 2 software. P < 0.05 was considered significant. Ethical Approval. Informed consent was obtained prior to the performance of the study in all patients. Pa- tients undergoing naltrexone implants also gave infor- med written consent. This study was approved by the Human Research Ethics Committee (HREC) of South- city Medical Centre, a recognized HREC by the National Health and Medical Research Council. All procedures complied with the Declaration of Helsinki. Relevant re- gulatory requirements were met throughout. 3. RESULTS As shown in Table 1 there were 175 control and 222 opiate dependent female patients. The chronological mean age (CA) of the two groups was 32.62 ± 0.96 and 33.58 ± 0.57 years (mean ± S.E.M.), respectively, was not statistically different (t = 0.85, dF = 292.37, P = 0.39). Significant differences between substance exposure and some laboratory values were also documented and have been previously reported [27]. 83.33% of the opiate de- pendent patients were treated with buprenorphine, 13.51% were treated with methadone, and 3.15% were treated with naltrexone. The mean dose of buprenorphine used was 6.83 ± 0.36 mg, and the mean dose of metha- done used by these patients was 55.80 ± 6.04 mg. Table 2 presents the results of the direct comparison of the two groups for central and peripheral cardiovascu- lar parameters. The quality index (Operator Index) in the two groups was uniformly and similarly high. The vas- cular age, the difference between the vascular and chro- nologic ages, and the RA/CA ratio were all significantly elevated in the opiate dependent group. All five cited measures of arterial stiffness were elevated amongst ad- dicted patients, except the pulse pressure amplification ratio, where depression is associated with age related stiffening. Figures 1-4 present various plots of age, arterial stiff- ness, pressure and timing indices respectively against CA. Judged by the AIC, the best way to model age related changes is the semi-log model. Using this technique, when patients achieve a CA of 60 years, the controls (intercept = 2.4868, slope = 0.0287) have a predicted modelled age of 67.52 years, and the opiate dependent patients (intercept = 2.4267, slope = 0.00333) of 83.79 years. This represents an elevation in the modelled age of 16.27 years or 24.10%. When the (log) RA is regressed against the CA and addictive status, the addictive status is significantly pre- dictive both as a factor in an additive model, and in in- teraction with CA. Details of these results and other re- sults for a similar age dependent analysis of major cen- tral cardiovascular parameters are given in Table 3. Possible dose-response relationship with lifetime opi- ate exposure in opiate exposed individuals was examined in an interactive model of the log of the RA/CA ratio against both heroin dose and duration. In the final model (Adj. R2 = 0.193, F = 8.73, dF = 1391, P = 0.0033) the only remaining significant variable was the dose-dura- tion interaction (est. = 0.0057 ± 0.0019, t = 2.954, P = 0.0033). Persistence of a dose-response effect after adjustment Copyright © 2013 SciRes. OPEN ACCESS 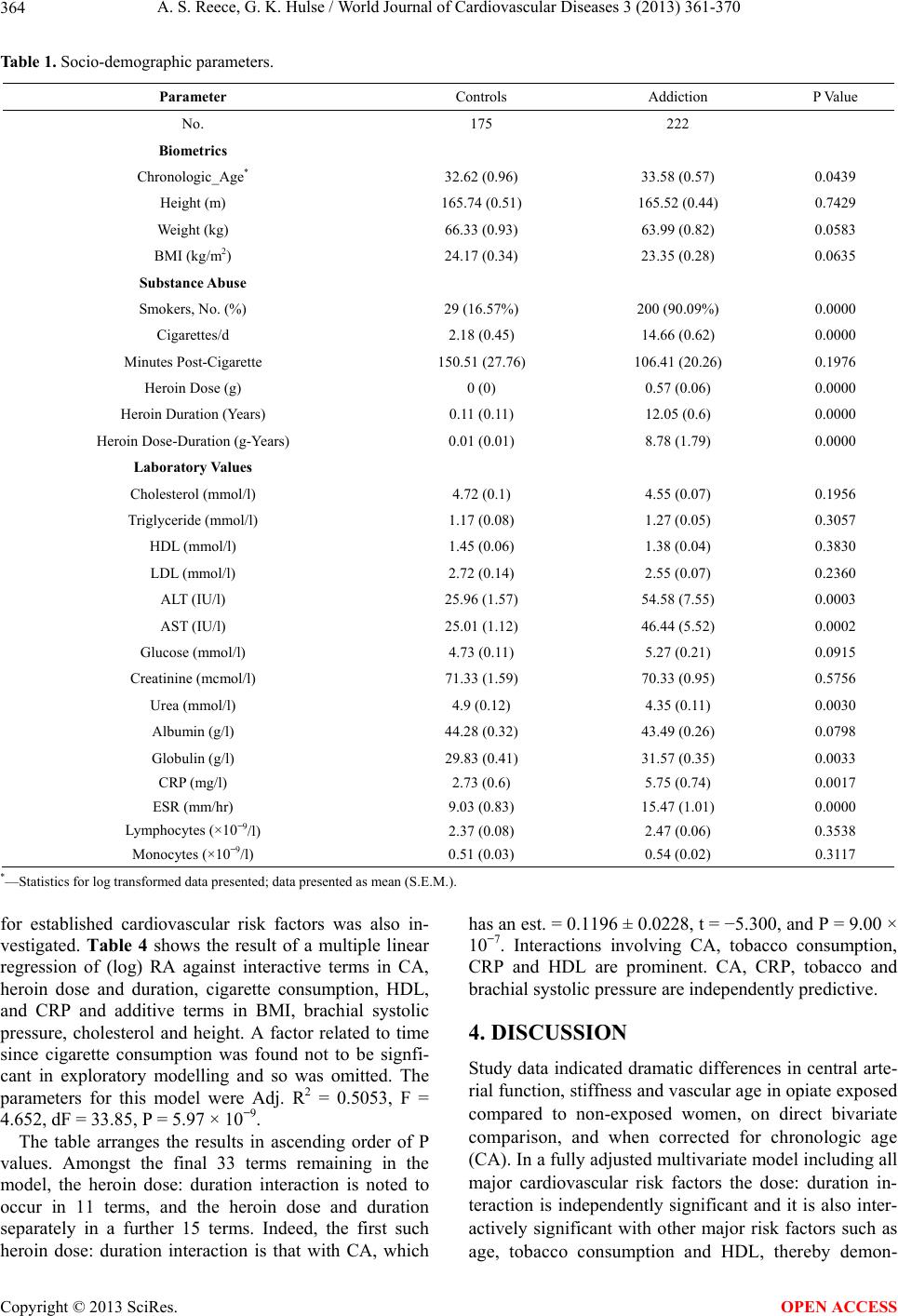 A. S. Reece, G. K. Hulse / World Journal of Cardiovascular Diseases 3 (2013) 361-370 Copyright © 2013 SciRes. 364 OPEN ACCESS Table 1. Socio-demographic parameters. Parameter Controls Addiction P Value No. 175 222 Biometrics Chronologic_Age* 32.62 (0.96) 33.58 (0.57) 0.0439 Height (m) 165.74 (0.51) 165.52 (0.44) 0.7429 Weight (kg) 66.33 (0.93) 63.99 (0.82) 0.0583 BMI (kg/m2) 24.17 (0.34) 23.35 (0.28) 0.0635 Substance Abuse Smokers, No. (%) 29 (16.57%) 200 (90.09%) 0.0000 Cigarettes/d 2.18 (0.45) 14.66 (0.62) 0.0000 Minutes Post-Cigarette 150.51 (27.76) 106.41 (20.26) 0.1976 Heroin Dose (g) 0 (0) 0.57 (0.06) 0.0000 Heroin Duration (Years) 0.11 (0.11) 12.05 (0.6) 0.0000 Heroin Dose-Duration (g-Years) 0.01 (0.01) 8.78 (1.79) 0.0000 Laboratory Values Cholesterol (mmol/l) 4.72 (0.1) 4.55 (0.07) 0.1956 Triglyceride (mmol/l) 1.17 (0.08) 1.27 (0.05) 0.3057 HDL (mmol/l) 1.45 (0.06) 1.38 (0.04) 0.3830 LDL (mmol/l) 2.72 (0.14) 2.55 (0.07) 0.2360 ALT (IU/l) 25.96 (1.57) 54.58 (7.55) 0.0003 AST (IU/l) 25.01 (1.12) 46.44 (5.52) 0.0002 Glucose (mmol/l) 4.73 (0.11) 5.27 (0.21) 0.0915 Creatinine (mcmol/l) 71.33 (1.59) 70.33 (0.95) 0.5756 Urea (mmol/l) 4.9 (0.12) 4.35 (0.11) 0.0030 Albumin (g/l) 44.28 (0.32) 43.49 (0.26) 0.0798 Globulin (g/l) 29.83 (0.41) 31.57 (0.35) 0.0033 CRP (mg/l) 2.73 (0.6) 5.75 (0.74) 0.0017 ESR (mm/hr) 9.03 (0.83) 15.47 (1.01) 0.0000 Lymphocytes (×10−9/l) 2.37 (0.08) 2.47 (0.06) 0.3538 Monocytes (×10−9/l) 0.51 (0.03) 0.54 (0.02) 0.3117 *—Statistics for log transformed data presented; data presented as mean (S.E.M.). for established cardiovascular risk factors was also in- vestigated. Table 4 shows the result of a multiple linear regression of (log) RA against interactive terms in CA, heroin dose and duration, cigarette consumption, HDL, and CRP and additive terms in BMI, brachial systolic pressure, cholesterol and height. A factor related to time since cigarette consumption was found not to be signfi- cant in exploratory modelling and so was omitted. The parameters for this model were Adj. R2 = 0.5053, F = 4.652, dF = 33.85, P = 5.97 × 10−9. The table arranges the results in ascending order of P values. Amongst the final 33 terms remaining in the model, the heroin dose: duration interaction is noted to occur in 11 terms, and the heroin dose and duration separately in a further 15 terms. Indeed, the first such heroin dose: duration interaction is that with CA, which has an est. = 0.1196 ± 0.0228, t = −5.300, and P = 9.00 × 10−7. Interactions involving CA, tobacco consumption, CRP and HDL are prominent. CA, CRP, tobacco and brachial systolic pressure are independently predictive. 4. DISCUSSION Study data indicated dramatic differences in central arte- rial function, stiffness and vascular age in opiate exposed compared to non-exposed women, on direct bivariate comparison, and when corrected for chronologic age (CA). In a fully adjusted multivariate model including all major cardiovascular risk factors the dose: duration in- teraction is independently significant and it is also inter- actively significant with other major risk factors such as age, tobacco consumption reby demon- and HDL, the  A. S. Reece, G. K. Hulse / World Journal of Cardiovascular Diseases 3 (2013) 361-370 365 Table 2. Selected cardiovascular parameters. Parameter Controls Addiction P Value Operator Index 86.23 (0.53) 87.1 (0.45) 0.2138 Ages Vascular_Age* 35.03 (1.41) 39.30 (1.28) 0.0083 Difference 2.41 (0.91) 5.73 (1.02) 0.0156 RA/CA 1.07 (0.02) 1.16 (0.03) 0.0197 Log (RA/CA) 0.02 (0.02) 0.08 (0.02) 0.0732 Arterial Stiffness C_AP_HR75 4.29 (0.4) 5.96 (0.34) 0.0016 C_AGPH_HR75 11.66 (1.07) 15.81 (0.83) 0.0023 C_PH (mmHg) 33.49 (0.59) 35.8 (0.5) 0.0029 PPAmpRatio 149.62 (1.59) 144.32 (1.38) 0.0120 P_AI 64.35 (1.48) 69.7 (1.29) 0.0066 Timing HR (bpm) 69.42 (0.84) 70.63 (0.71) 0.2681 Ejection_Duration (msec) 331.06 (1.51) 326.27 (1.34) 0.0179 C_SVI 141.15 (2.21) 138.86 (1.71) 0.4066 C_DTI 2976.29 (29.29) 2971.69 (27.11) 0.9089 C_TTI 2175.61 (30.12) 2191.48 (25.16) 0.6841 C_Diastolic Duration (msec) 558.05 (10.1) 544.46 (8.02) 0.2866 Pressures SP 118.11 (0.9) 118.68 (0.77) 0.6320 DP 69.03 (0.7) 68 (0.69) 0.3029 Central_SP 103.82 (0.93) 105.21 (0.81) 0.2575 Central_DP 70.35 (0.72) 69.5 (0.69) 0.3967 Central_ESP 92.86 (0.89) 94.12 (0.8) 0.2929 Central_MEANP 85.93 (0.76) 86.17 (0.7) 0.8161 *—Statistics for log transformed data presented; abbreviations as in methods; data presented as mean (S.E.M.). Table 3. Age dependent multiple regression of central CVS parameters. Parameter Variable Estimate (Std. Error)t ValuePr (> |t|)Adjusted R2F DF1, DF2 Model P RA Opiate. Status 0.095 (0.034) 2.82000.0050 0.4912 192.10 2394 <2.0E−16 RA CA: Opiate. Status 0.003 (0.001) 3.21400.0014 0.4942 194.40 2394 <2.0E−16 C_AP_HR75 Opiate. Status 1.3344 (0.3697) 3.610 0.0003 0.5158 211.90 2394 <2.0E−16 C_AP_HR75 CA: Opiate. Status 0.041 (0.0106) 3.869 0.0001 0.5181 213.80 2394 <2.0E−16 C_AGPH_HR75 Opiate. Status 3.3319 (0.9692) 3.438 0.0006 0.4820 185.30 2394 <2.0E−16 C_AGPH_HR75 CA: Opiate. Status 0.0999 (0.0278) 3.588 0.0004 0.4834 186.30 2394 <2.0E−16 C_SVI Opiate. Status −28.371 (9.274) −3.0590.0024 0.0159 3.13 3393 0.0255 C_SVI CA: Opiate. Status 0.4766 (0.2121) 2.247 0.0252 0.0159 3.13 3393 0.0255 C_SP/CA Opiate. Status −0.0513 (0.027) −1.9000.0582 0.0065 3.61 1395 0.0582 Abbreviations as in Methods. strating a positive dose-response. Opiate dose or duration exposure featured in 26 of the terms in the final regres- sion model, which accounted for 50.5% of the variance in (log) RA. The most powerful interaction we demon- strated was that between the dose-duration interaction and age, which had a P value < 10−6 (Table 4). Based on the regression models established, at a CA of 60 years, controls would have a mean vascular age of 67.52 years Copyright © 2013 SciRes. OPEN ACCESS  A. S. Reece, G. K. Hulse / World Journal of Cardiovascular Diseases 3 (2013) 361-370 366 Table 4. Final model of CVS risk factors. Variable Parameter Estimates Estimate_(S. E.) t Value Pr (> |t|) CA 2.244 (0.3621) 6.196 0.000000 Cigarettes: CRP 0.0548 (0.0102) 5.359 0.000001 CA: H. Dose: H. Dura. n −0.1196 (0.0226) −5.3 0.000001 H. Dura. n: Cigarettes: CRP −0.0046 (0.0009) −5.091 0.000002 CA: H. Dose: Cigarettes: HDL: CRP −0.1412 (0.0278) −5.076 0.000002 CA: H. Dose: HDL: CRP 3.056 (0.6055) 5.047 0.000003 CA: H. Dose: Cigarettes: HDL 1.685 (0.3351) 5.028 0.000003 CRP −1.111 (0.2237) −4.965 0.000004 CA: H. Dose: H. Dura. n: HDL: CRP −0.2134 (0.0449) −4.758 0.000008 CA: H. Dose: H. Dura. n: Cigarettes 0.0057 (0.0012) 4.755 0.000008 H. Dose: Cigarettes: HDL −5 (1.055) −4.74 0.000009 CA: H. Dose: HDL −29.4 (6.708) −4.383 0.000033 CA: H. Dose: H. Dura. n: HDL 2.533 (0.5862) 4.321 0.000042 H. Dose: HDL 84.36 (20.76) 4.063 0.000107 CA: H. Dose: H. Dura. n: Cigarettes: HDL: CRP 0.0086 (0.0022) 3.989 0.000140 H. Dose: H. Dura. n: HDL −7.555 (1.969) −3.837 0.000239 CA: H. Dose: H. Dura. n: Cigarettes: HDL −0.1146 (0.0304) −3.772 0.000298 CA: H. Dose: CRP −1.309 (0.3512) −3.727 0.000349 H. Dose 2.164 (0.5981) 3.617 0.000504 H. Dose: CRP 4.644 (1.297) 3.58 0.000572 CA: H. Dose: H. Dura. n: CRP 0.1494 (0.0419) 3.571 0.000589 CA: Cigarettes: HDL −0.2205 (0.0638) −3.454 0.000864 H. Dura. n: Cigarettes: HDL: CRP 0.0061 (0.0018) 3.395 0.001046 Cigarettes: HDL 0.7377 (0.2177) 3.388 0.001069 H. Dose: H. Dura. n: Cigarettes: HDL 0.341 (0.1024) 3.332 0.001279 Cigarettes −0.0447 (0.0134) −3.328 0.001296 CA: H. Dose: Cigarettes −0.0297 (0.0091) −3.277 0.001520 H. Dose: H. Dura. n: CRP −0.4326 (0.146) −2.962 0.003964 H. Dura. n: CRP 0.1944 (0.0669) 2.905 0.004681 CA: H. Dose: H. Dura. n: Cigarettes: CRP −0.0009 (0.0003) −2.673 0.009015 SP 0.9822 (0.3713) 2.645 0.009717 CA: H. Dura. n: HDL: CRP −0.014 (0.0054) −2.595 0.011135 CA: H. Dura. n: CRP −0.0361 (0.0171) −2.115 0.037373 CA—Chronologic Age; H. Dose—Usual Heroin Dose; H. Dura. n—Lifetime Duration of Heroin Use. and opiate dependent patients a mean vascular age of 83.79 years, an elevation of 16.27 years or 24.1%. These results demonstrate that long term opiate dependence is an independent and interactive cardiovascular risk factor in females. Figures 1, 2, and 4 show a very clear separation be- tween the age, arterial stiffness and timing data for the opiate exposed and control groups. Even when the mean CA of the two groups was assessed as significantly dif- ferent using log-transformed data, the magnitude of this is less than one year, which is much less than the demon- strated differences in the various measures of vascular age which was 4.2 years. The changes in vascular age were paralleled by significant changes in measures of central arterial stiffness, subendocardial perfusion and systolic pressure (Table 3). It should also be noted that although this study has considered all the opiate dependent patients together, in fact this group is heterogeneous when judged by treat- ment type (methadone (13.5%), buprenorphine (83.3%), Copyright © 2013 SciRes. OPEN ACCESS  A. S. Reece, G. K. Hulse / World Journal of Cardiovascular Diseases 3 (2013) 361-370 367 Figure 1. Ageing indices by chronologic age by opiate de- pendency status. Figure 2. Arterial stiffness by chronologic age by opiate de- pendency status. or implant naltrexone (3.2%)). Unpublished data from this project (manuscript submitted) shows that the type of pharmacotherapy used to treat opiate dependence has a very material impact on the central cardiovascular out- comes. Other studies in the literature are consistent with this view [4,8]. 83.3% of our patients were treated with buprenorphine with a mean dose of 6.83 ± 0.36 mg which is an unusually low dose judged by literature stan- dards. As a partial µ-agonist buprenorphine is a rela- tively mild intoxicant compared to other treatments. For this reason we consider that the results reported herein represent a best case scenario for opiate dependence and likely a lower bound of their cardiovascular toxicological effect. The strength of association and the uniformity of the Figure 3. Central pressures by chronologic age by opiate de- pendency status. Figure 4. Central timing indices by chronologic age by opiate dependency status. results presented raise the question as to possible mecha- nisms of action by which opiates might be impacting the cardiovasculature. At the outset it is important to note that opiate use has been associated with exacerbation of most of the major cardiovascular risk factors including smoking [8], cholesterol [28], poor dietary habits, weight gain [29], hypertension [30,31], and hyperglycaemia and diabetes [32]. Higher levels of immune activity have also been shown [27]. Indeed the present study also demon- strated elevated levels of ESR, CRP and globulins in our patients (Table 1). The pro-senescence activities of opiates have been noted in the Introduction. This effect is likely com- pounded by the affects of opiates to stimulate or prime apoptosis [33] and to induce immune responses both Copyright © 2013 SciRes. OPEN ACCESS  A. S. Reece, G. K. Hulse / World Journal of Cardiovascular Diseases 3 (2013) 361-370 368 through toll-like receptor 4 [34] and chemokines [35]. Senescent tissues have also been shown to secrete vari- ous substances including interleukins -6 and -8 [19] which are highly toxic to most stem cells niches [36] and perpetuate the senescent phenotype. From a mechanistic point it is possible that in opiate dependent patients, there is a pro-senescence stimulus mediated via ANRIL and P16, compounded by apoptotic activities, further im- pacted by heightened immune activity, which is further exacerbated by the interactive effect of immune stimula- tion on stem cell vulnerability to produce the observed phenotype of accelerated ageing in all organ beds exam- ined. The relative hypercalcaemia likely exacerbates these changes and contributes to the increased vascular stiffness. The importance of cardiovascular age as a surrogate marker for generalized organismal ageing was also noted in the introduction [25]. The present findings are there- fore consistent with other data which shows acceleration of age dependent changes in bone, hair, teeth and stem cells [37-39], and with the hypothesis that opiate de- pendent patients are ageing in a more accelerated manner across all tissue beds [4,6,30] (see also Appendix 6 [7]). This study had a number of limitations, primarily re- lated to its observational design. Design of a randomised study however has ethical concerns with the randomisa- tion of opiate or non-opiate dependent persons to treat- ment or non treatment by an opiate pharmacotherapy. Additionally when log as opposed to non-log trans- formed data was used there was a significant difference between the control and drug dependent group. Further, a systematic drug history was not collected in a format which facilitated easy statistical analysis: a key feature that would be required in future prospective work. Future iterations of this study would also need to give careful consideration to the treatment make-up of the opiate de- pendent condition. Ideally the numbers in the treatment groups would be broadly comparable, and treatment as- signment might be randomized between the various con- ditions. Further studies might also consider investigating the mechanism of these changes with prospectively col- lected immune, stem cell and senescence related pa- rameters of circulating cells able to be quantified and studied. In summary, this study provides evidence suggesting an increased vascular age including central arterial stiff- ness in opiate dependent patients, and demonstrated a dose-response relationship between these features and lifetime opiate exposure. This relationship is robust and persists after adjustment for other cardiovascular risk factors. At age 60, this is equivalent to a 24.1% ad- vancement in cardiovascular age above controls. These findings are consistent with the observations of other workers, and have the advantage that by studying a sub- clinical endophenotype, they are performed on living patients. These results are also consistent with an emerg- ing body of evidence showing accelerated ageing in all body systems in opiate dependent patients. Whilst the present study has not presented evidence for likely mechanisms of these changes, they are consistent with the known pro-senescence, pro-apoptotic, immunostimu- latory activities of opiates, and the interactions of these effects. Opiates are also known to exacerbate classical cardiovascular risk factors. Various suggestions for fur- ther research are made. 5. ACKNOWLEDGEMENTS The authors would like to thank Dr Mervyn Thomas of Emphron for assistance with the statistics and graphical design. REFERENCES [1] Jamison, R.N., Serraillier, J. and Michna, E. (2011) As- sessment and treatment of abuse risk in opioid prescribe- ing for chronic pain. Pain Research and Treatment, 941808. [2] Drug Abuse Warning Network (DAWN). (2009) Treat- ment Episode Data Set—Admissions (Teds-A)—Concate- nated, 1992 to 2009 (Computer file). Prepared by Synec- tics for Management Decisions, Incorporated. ICP- SR25221-v4. [3] Darke, S., Degenhardt, L. and Mattick, R. (2007) Mortal- ity amongst illicit drug users: Epidemiology, causes and intervention. Cambridge University Press, Sydney, 2007. [4] Darke, S., Duflou, J. and Torok, M. (2010) The compara- tive toxicology and major organ pathology of fatal meth- adone and heroin toxicity cases. Drug and Alcohol De- pendence, 106, 1-6. doi:10.1016/j.drugalcdep.2009.07.014 [5] Oviedo-Joekes, E., Brissette, S., Marsh, D.C., Lauzon, P., Guh, D., Anis, A. and Schechter, M.T. (2009) Diacetyl- morphine versus methadone for the treatment of opioid addiction. The New England Journal of Medicine, 361, 777-786. doi:10.1056/NEJMoa0810635 [6] Darke, S., Kaye, S. and Duflou, J. (2006) Systemic dis- ease among cases of fatal opioid toxicity. Addiction, 101, 1299-1305. doi:10.1111/j.1360-0443.2006.01495.x [7] Degenhardt, L., Randall, D., Hall, W., Law, M., Butler, T. and Burns, L. (2009) Mortality among clients of a state- wide opioid pharmacotherapy program over 20 years: Risk factors and lives saved. Drug and Alcohol Depend- ence, 105, 9-15. doi:10.1016/j.drugalcdep.2009.05.021 [8] Sadeghian, S., Darvish, S., Davoodi, G., Salarifar, M., Mahmoodian, M., Fallah, N. and Karimi, A.A. (2007) The association of opium with coronary artery disease. European Journal of Cardiovascular Prevention & Re- habilitation, 14, 715-717. doi:10.1097/HJR.0b013e328045c4e9 [9] Sadeghian, S., Dowlatshahi, S., Karimi, A. and Tazik, M. (2011) Epidemiology of opium use in 4398 patients ad- Copyright © 2013 SciRes. OPEN ACCESS  A. S. Reece, G. K. Hulse / World Journal of Cardiovascular Diseases 3 (2013) 361-370 369 mitted for coronary artery bypass graft in Tehran Heart Center. Journal of Cardiothoracic Surgery (Torino), 52, 140-141. [10] Feero, W.G., Guttmacher, A.E. and McCarthy, M.I. (2010) Genomics, type 2 diabetes, and obesity. New England Journal of Medicine, 363, 2339-2350. doi:10.1056/NEJMra0906948 [11] O’Donnell, C.J. and Nabel, E.G. (2011) Genomics of Cardiovascular Disease. New England Journal of Medi- cine, 365, 2098-2109. doi:10.1056/NEJMra1105239 [12] Helgadottir, A., Thorleifsson, G., Manolescu, A., Gre- tarsdottir, S., Blondal, T., Jonasdottir, A., Sigurdsson, A., Baker, A., Palsson, A., Masson, G., et al. (2007) A com- mon variant on chromosome 9p21 affects the risk of my- ocardial infarction. Science, 316, 1491-1493. doi:10.1126/science.1142842 [13] McPherson, R., Pertsemlidis, A., Kavaslar, N., Stewart, A., Roberts, R., Cox, D.R., Hinds, D.A., Pennacchio, L.A., Tybjaerg-Hansen, A., Folsom, A.R., et al. (2007) A common allele on chromosome 9 associated with coro- nary heart disease. Science, 316, 1488-1491. doi:10.1126/science.1142447 [14] Pasmant, E., Sabbagh, A., Vidaud, M. and Bieche, I. (2010) ANRIL, a long, noncoding RNA, is an unexpected major hotspot in GWAS. The FASEB Journal, 25, 444-448. doi:10.1096/fj.10-172452 [15] Visel, A., Zhu, Y., May, D., Afzal, V., Gong, E., Attana- sio, C., Blow, M.J., Cohen, J.C., Rubin, E.M. and Pen- nacchio, L.A. (2010) Targeted deletion of the 9p21 non-coding coronary artery disease risk interval in mice. Nature, 464, 409-412. doi:10.1038/nature08801 [16] Harismendy, O., Notani, D., Song, X., Rahim, N.G., Tanasa, B., Heintzman, N., Ren, B., Fu, X.D., Topol, E.J., Rosenfeld, M.G. and Frazer, K.A. (2011) 9p21 DNA variants associated with coronary artery disease impair interferon-gamma signalling response. Nature, 470, 264- 268. doi:10.1038/nature09753 [17] Bazarov, A.V., Van Sluis, M., Hines, W.C., Bassett, E., Beliveau, A., Campeau, E., Mukhopadhyay, R., Lee, W.J., Melodyev, S., Zaslavsky, Y., et al. (2010) p16INK4a -me- diated suppression of telomerase in normal and malignant human breast cells. Aging Cell, 9, 736-746. doi:10.1111/j.1474-9726.2010.00599.x [18] Coppe, J.P., Rodier, F., Patil, C.K., Freund, A., Desprez, P.Y. and Campisi, J. (2011) Tumor suppressor and aging biomarker p16INK4 a induces cellular senescence without the associated inflammatory secretory phenotype. The Journal of Biological Chemistry, 286, 36396-36403. doi:10.1074/jbc.M111.257071 [19] Bhaumik, D., Scott, G.K., Schokrpur, S., Patil, C.K., Orjalo, A.V., Rodier, F., Lithgow, G.J. and Campisi, J. (2009) MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8. Aging (Albany NY), 1, 402-411. [20] Zagon, I.S. and McLaughlin, P.J. (1977) Morphine and brain growth retardation in the rat. Pharmacology, 15, 276-282. doi:10.1159/000136699 [21] McLaughlin, P.J., Zagon, I.S. and White, W.J. (1978) Perinatal methadone exposure in rats. Effects on body and organ development. Biology of the Neonat, 34, 48-54. doi:10.1159/000241104 [22] Cheng, F., Zagon, I.S., Verderame, M.F. and McLaughlin, P.J. (2007) The opioid growth factor (OGF)-OGF recep- tor axis uses the p16 pathway to inhibit head and neck cancer. Cancer Research, 67, 10511-10518. doi:10.1158/0008-5472.CAN-07-1922 [23] Cheng, F., McLaughlin, P.J., Verderame, M.F. and Zagon, I.S. (2009) The OGF-OGFr axis utilizes the p16INK4a and p21WAF1/CIP1 pathways to restrict normal cell pro- liferation. Molecular Biology of the Cell, 20, 319-327. doi:10.1091/mbc.E08-07-0681 [24] Zagon, I.S., Verderame, M.F. and McLaughlin, P.J. (2002) The biology of the opioid growth factor receptor (OGFr). Brain Research Reviews, 38, 351-376. doi:10.1016/S0165-0173(01)00160-6 [25] Chien, K.R. and Karsenty, G. (2005) Longevity and line- ages: Toward the integrative biology of degenerative dis- eases in heart, muscle, and bone. Cell, 120, 533-544. doi:10.1016/j.cell.2005.02.006 [26] Reece, A.S. (2007) Psychosocial and treatment correlates of opiate free success in a clinical review of a naltrexone implant program. Substance Abuse Treatment, Prevention, and Policy, 2, 35-49. doi:10.1186/1747-597X-2-35 [27] Reece, A.S. (2007) Evidence of Accelerated Ageing in Clinical Drug Addiction from Immune, Hepatic and Me- tabolic Biomarkers. Immunity & Ageing, 4, 6-15. doi:10.1186/1742-4933-4-6 [28] Cooper, O.B., Brown, T.T. and Dobs, A.S. (2003) Opiate drug use: A potential contributor to the endocrine and metabolic complications in human immunodeficiency vi- rus disease. Clinical Infectious Diseases, 37, S132-S136. doi:10.1086/375879 [29] Kolarzyk, E., Pach, D., Wojtowicz, B., Szpanowska-Wo- hn, A. and Szurkowska, M. (2005) Nutritional status of the opiate dependent persons after 4 years of methadone maintenance treatment. Przegl Lek, 62, 373-377. [30] Rosen, D., Smith, M.L. and Reynolds, C.F. (2008) The prevalence of mental and physical health disorders among older methadone patients. The American Journal of Geri- atric Psychiatry, 16, 488-497. doi:10.1097/JGP.0b013e31816ff35a [31] Hser, Y.I., Gelberg, L., Hoffman, V., Grella, C.E., Mc- Carthy, W. and Anglin, M.D. (2004) Health conditions among aging narcotics addicts: Medical examination re- sults. Journal of Behavioral Medicine, 27, 607-622. doi:10.1007/s10865-004-0005-x [32] Ceriello, A., Giugliano, D., Passariello, N., Quatraro, A., Dello Russo, P., Torella, R. and D’Onofrio, F. (1987) Impaired glucose metabolism in heroin and methadone users. Hormone and Metabolic Research, 19, 430-433. doi:10.1055/s-2007-1011844 [33] Cunha-Oliveira, T., Rego, A.C., Garrido, J., Borges, F., Macedo, T. and Oliveira, C.R. (2007) Street heroin in- duces mitochondrial dysfunction and apoptosis in rat cor- tical neurons. Journal of Neurochemistry, 101, 543-554. doi:10.1111/j.1471-4159.2006.04406.x [34] Hutchinson, M.R., Zhang, Y., Shridhar, M., Evans, J.H., Copyright © 2013 SciRes. OPEN ACCESS  A. S. Reece, G. K. Hulse / World Journal of Cardiovascular Diseases 3 (2013) 361-370 Copyright © 2013 SciRes. 370 OPEN ACCESS Buchanan, M.M., Zhao, T.X., Slivka, P.F., Coats, B.D., Rezvani, N., Wieseler, J., et al. (2010) Evidence that opi- oids may have toll-like receptor 4 and MD-2 effects. Brain, Behavior, and Immunity, 24, 83-95. doi:10.1016/j.bbi.2009.08.004 [35] Adler, M.W., Geller, E.B., Chen, X. and Rogers, T.J. (2005) Viewing chemokines as a third major system of communication in the brain. AAPS Journals, 7, E865- E870. doi:10.1208/aapsj070484 [36] Anderson, J.E. (2000) A role for nitric oxide in muscle repair: Nitric oxide-mediated activation of muscle satel- lite cells. Molecular Biology of the Cell, 11, 1859-1874. doi:10.1091/mbc.11.5.1859 [37] Reece, A.S. (2011) Differing age related trajectories of dysfunction in several organ systems in opiate depend- ence. Aging Clinical and Experimental Research, 24, 85- 96. [38] Kim, T.W., Alford, D.P., Malabanan, A., Holick, M.F., Samet, J.H. (2006) Low bone density in patients receiv- ing methadone maintenance treatment. Drug and Alcohol Dependence, 85, 258-262. doi:10.1016/j.drugalcdep.2006.05.027 [39] Reece, A.S. and Davidson, P. (2007) Deficit of circulat- ing stem—Progenitor cells in opiate addiction: A pilot study. Substance Abuse Treatment, Prevention, and Poli- cy, 2, 19-28. doi:10.1186/1747-597X-2-19
|