Q. LI ET AL. 25
positive cells of NF-κb and MCP-1.
3. Results
3.1. Morphological Observation
Surfaces of lung are smooth and no nodule formation in
control group. Canous nodules distribute sporadically on
the surface and cutting surface in silicotic model group.
Compared with silicotic mode group, the numbers of
canous nodules decreased on the surface and cutting sur-
face in the groups with AcSDKP treatment.
3.2. Expression of NF-κb and MCP-1 in Lung of
Rat with Silicosis
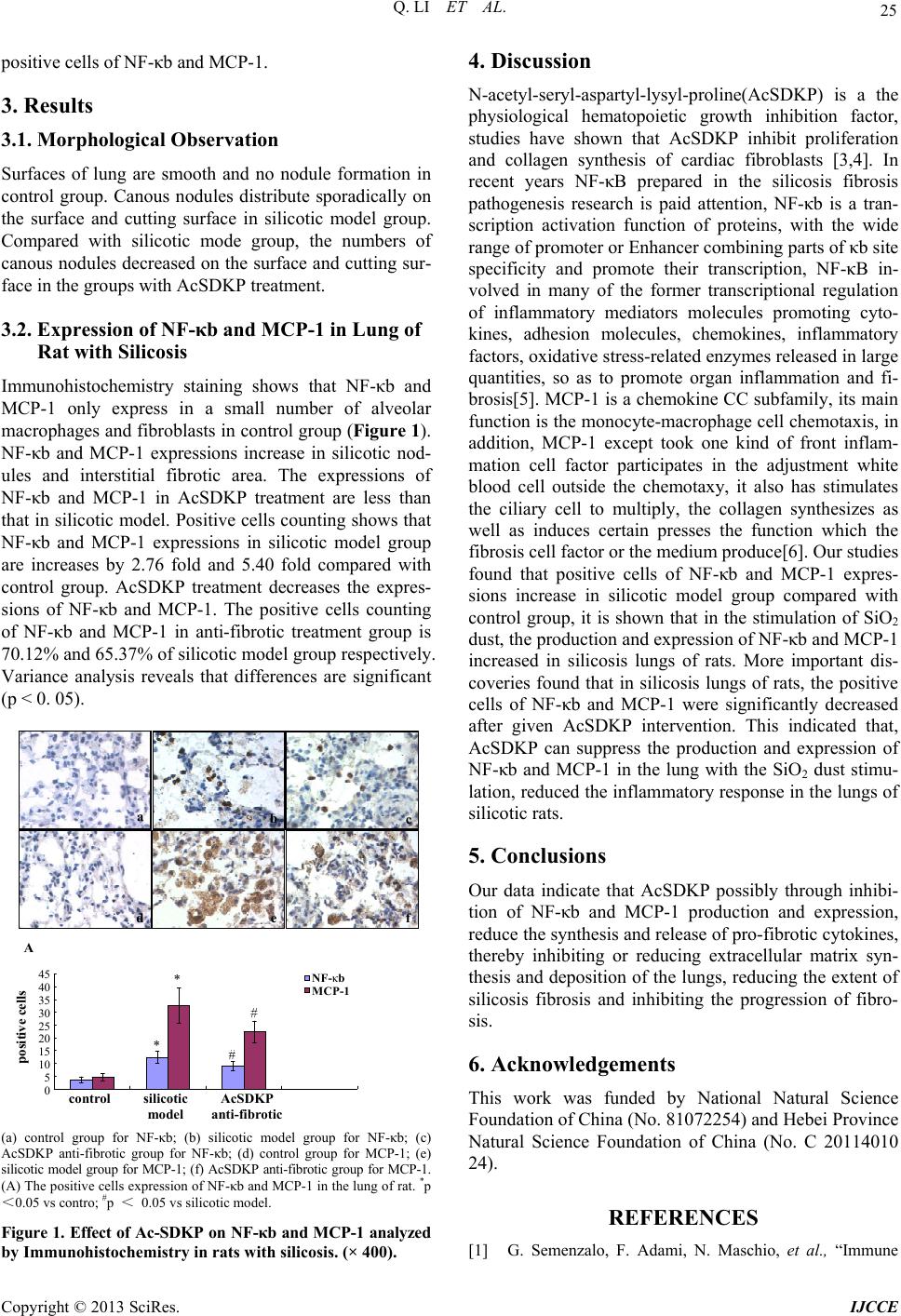
Immunohistochemistry staining shows that NF-κb and
MCP-1 only express in a small number of alveolar
macrophages and fibroblasts in control group (Figure 1).
NF-κb and MCP-1 expressions increase in silicotic nod-
ules and interstitial fibrotic area. The expressions of
NF-κb and MCP-1 in AcSDKP treatment are less than
that in silicotic model. Positive cells counting shows that
NF-κb and MCP-1 expressions in silicotic model group
are increases by 2.76 fold and 5.40 fold compared with
control group. AcSDKP treatment decreases the expres-
sions of NF-κb and MCP-1. The positive cells counting
of NF-κb and MCP-1 in anti-fibrotic treatment group is
70.12% and 65.37% of silicotic model group respectively.
Variance analysis reveals that differences are significant
(p < 0. 05).
a b c
d e f
*
*
#
#
A
45
40
35
30
25
20
15
10
5
0
control silicotic
model
AcSDKP
anti-fibrotic
ositive cells
NF-κb
MCP-1
(a) control group for NF-κb; (b) silicotic model group for NF-κb; (c)
AcSDKP anti-fibrotic group for NF-κb; (d) control group for MCP-1; (e)
silicotic model group for MCP-1; (f) AcSDKP anti-fibrotic group for MCP-1.
(A) The positive cells expression of NF-κb and MCP-1 in the lung of rat. *p
<0.05 vs contro; #p < 0.05 vs silicotic model.
Figure 1. Effect of Ac-SDKP on NF-κb and MCP-1 analyzed
by Immunohistochemistry in rats with silicosis. (× 400).
4. Discussion
N-acetyl-seryl-aspartyl-lysyl-proline(AcSDKP) is a the
physiological hematopoietic growth inhibition factor,
studies have shown that AcSDKP inhibit proliferation
and collagen synthesis of cardiac fibroblasts [3,4]. In
recent years NF-κB prepared in the silicosis fibrosis
pathogenesis research is paid attention, NF-κb is a tran-
scription activation function of proteins, with the wide
range of promoter or Enhancer combining parts of κb site
specificity and promote their transcription, NF-κB in-
volved in many of the former transcriptional regulation
of inflammatory mediators molecules promoting cyto-
kines, adhesion molecules, chemokines, inflammatory
factors, oxidative stress-related enzymes released in large
quantities, so as to promote organ inflammation and fi-
brosis[5]. MCP-1 is a chemokine CC subfamily, its main
function is the monocyte-macrophage cell chemotaxis, in
addition, MCP-1 except took one kind of front inflam-
mation cell factor participates in the adjustment white
blood cell outside the chemotaxy, it also has stimulates
the ciliary cell to multiply, the collagen synthesizes as
well as induces certain presses the function which the
fibrosis cell factor or the medium produce[6]. Our studies
found that positive cells of NF-κb and MCP-1 expres-
sions increase in silicotic model group compared with
control group, it is shown that in the stimulation of SiO2
dust, the production and expression of NF-κb and MCP-1
increased in silicosis lungs of rats. More important dis-
coveries found that in silicosis lungs of rats, the positive
cells of NF-κb and MCP-1 were significantly decreased
after given AcSDKP intervention. This indicated that,
AcSDKP can suppress the production and expression of
NF-κb and MCP-1 in the lung with the SiO2 dust stimu-
lation, reduced the inflammatory response in the lungs of
silicotic rats.
5. Conclusions
Our data indicate that AcSDKP possibly through inhibi-
tion of NF-κb and MCP-1 production and expression,
reduce the synthesis and release of pro-fibrotic cytokines,
thereby inhibiting or reducing extracellular matrix syn-
thesis and deposition of the lungs, reducing the extent of
silicosis fibrosis and inhibiting the progression of fibro-
sis.
6. Acknowledgements
This work was funded by National Natural Science
Foundation of China (No. 81072254) and Hebei Province
Natural Science Foundation of China (No. C 20114010
24).
REFERENCES
[1] G. Semenzalo, F. Adami, N. Maschio, et al., “Immune
Copyright © 2013 SciRes. IJCCE