 Advances in Microbiology, 2013, 3, 333-342 http://dx.doi.org/10.4236/aim.2013.34047 Published Online August 2013 (http://www.scirp.org/journal/aim) Occurrence of Aspergillus Species and Aflatoxin Contamination in Raw and Roasted Peanuts from Formal and Informal Markets in Eldoret and Kericho Towns, Kenya Helene Nyirahakizimana1, Lizzy Mwamburi1, Johnstone Wakhisi2, Charity Kawira Mutegi3, Maria Elisa Christie4, John Maina Wagacha5,6* 1Department of Biological Sciences, University of Eldoret, Eldoret, Kenya 2School of Medicine, Moi University, Eldoret, Kenya 3International Institute of Tropical Agriculture (IITA), Nairobi, Kenya 4Office of International Research, Education and Development, Virginia Technology, Blacksburg, USA 5International Crops Research Institute for the Semi Arid Tropics (ICRISAT), Nairobi, Kenya 6School of Biological Sciences, University of Nairobi, Nairobi, Kenya Email: *maina.wagacha@uonbi.ac.ke Received May 11, 2013; revised June 14, 2013; accepted July 14 2013 Copyright © 2013 Helene Nyirahakizimana et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT The population and diversity of fungal species and levels of aflatoxin contamination were investigated in 228 marketed peanut samples; 140 from formal and 88 from informal markets, in Kericho and Eldoret towns of Kenya. Ground pea- nut samples were cultured on Modified Dichloran Rose Bengal (MDRB) agar while aflatoxin level was quantified based on indirect competitive ELISA. Correlation between the incidence of major aflatoxin-producing fungal species and aflatoxin levels was also established. Fungal species commonly isolated from the peanut samples included Asper- gillus flavus L strain, A. flavus S strain, A. parasiticus, A. tamarii, A. caelatus, A. alliaceus (all of Aspergillus section Flavi) and A. niger. Fungi isolated in low frequency included Fusarium spp., Penicillium spp., Mucor spp. and Rhi- zopus spp. Aflatoxin levels in peanut products ranged from 0 to 2345 µg/kg in raw peanuts, 0 to 382 µg/kg in roasted coated peanuts, and 0 to 201 µg/kg in roasted de-coated peanuts. Overall, levels of total aflatoxin were higher in sam- ples from informal (mean = 97.1 µg/kg) than formal (mean = 55.5 µg/kg) market outlets. There was a positive and sig- nificant correlation (R2 = 0.63; p ≤ 0.05) between aflatoxin levels and the major aflatoxin producing fungi in raw pea- nuts from formal markets in Eldoret town. Additionally, total aflatoxin in raw peanut samples from informal markets in Kericho was positively and significantly correlated (R2 = 0.81; p ≤ 0.05) to the population of A. flavus (L and S strains). In roasted coated peanuts sampled from formal market outlets in Eldoret, aflatoxin levels correlated positively and sig- nificantly (R2 = 0.37; p ≤ 0.05) with A. flavus S strain. There is need to create awareness among peanut traders and con- sumers on proper handling of peanuts and health risks associated with consumption of unsafe peanut products. Keywords: Aflatoxin; Aspergillus Section Flavi; Peanuts; Peanut Market Outlets 1. Introduction Peanut (Arachis hypogeae L) is one of the main crops grown in Kenya [1], primarily for local consumption but also export mainly through the World Food Programme in Kenya [2]. In 2010, FAO statistics indicated produc- tion of 99,072 metric tons of peanuts with shell in Kenya, harvested from 19,291 hectares [3]. Peanut is rich in pro- tein (26% to 39%), fat (47% to 59%), carbohydrates (11%), Na (42.0 mg/100 g), K (705.11 mg/100 g), Mg (3.98 mg/100 g), Ca (2.28 mg/100 g), Fe (6.97 mg/100 g), Zn (3.2 mg/100 g) and P (10.55 mg/100 g) [4,5], as well as vitamins E [6,7] and B [7]. Due to its high nutritional value, it has several uses such as in therapeutic food [8], confectionery [9], and as an animal feed [5]. A major challenge in peanut production is fungal and aflatoxin contamination. Aflatoxins are a group of my- cotoxins that adversely affect food safety, mainly of grains and peanuts, as well as trade and human and ani- *Corresponding authors. C opyright © 2013 SciRes. AiM  H. NYIRAHAKIZIMANA ET AL. 334 mal health. Aflatoxins are the most toxic and carcino- genic compounds among the known mycotoxins [10] and are mainly produced by Aspergillus flavus and A. para- siticus [11-13]. There are four major aflatoxin types: B1, B2, G1 and G2 so designated based on their blue and yel- low-green fluorescence [14]. Aspergillus flavus produces aflatoxin B1 and B2 while A. parasiticus produces B1, B2, G1 and G2 [10]. Other aflatoxin producing species in- clude A. nom ius which produces B and G aflatoxins [15], A. pseudotamarii, A. bombycis and A. ochraceoroseus [16,17]. Peanuts and maize are the main sources of human ex- posure to aflatoxin due to their high level of consumption e.g. 13.3 million tons of peanuts were consumed in Ken- ya in 2001-2003 with a projected consumption of 16.32 million tons in 2030 [14]. Reference [18] found Eurotium repens, A. parasiticus, and A. flavus to be the most potent aflatoxigenic species with average levels of aflatoxin above 100 µg/kg in peanuts from markets within Nairobi. High prevalence of A. flavus L strain (> 77%) and A. fla- vus S stain (> 65%) has been reported in peanuts from Busia and Homa bay counties in Kenya [19]. Marketing of peanuts in Kenya is generally through informal market outlets [1,20], where peanuts are not properly protected from environmental influence and are not properly pac- kaged; making them susceptible to fungal contamina- tion. According to [20,21], peanuts at market level in Kenya are more contaminated with aflatoxin than those stored by farmers. Recent reports indicate that aflatoxin is common in peanuts and grains in different parts of Kenya [1,2,20], posing a serious health challenge. In order to minimize consequences of aflatoxin on food security, trade, health and meet national and international mycotoxin regulatory standards, there is need to monitor fungal species and mycotoxin contamination periodically. The objective of this study was to investigate the incidence and diversity of aflatoxin producing fungal species and aflatoxin levels in marketed raw and roasted peanuts in Eldoret and Keri- cho towns in Kenya. The correlation between the popula- tion of major aflatoxin producing fungi and total afla- toxin levels in peanuts marketed in different outlets was also established. 2. Materials and Methods 2.1. Study Area The study was conducted in Eldoret and Kericho towns within the Rift Valley region in Kenya. Eldoret town (0˚31′54″N, 35˚15′58″E) is located in Western Kenya at 2100 - 2700 m above sea level, 300 km North West of Nairobi on the trans-African highway and 65 km North of the equator. It has a cold and wet climate with an ave- rage temperature of 27˚C and 1124 mm mean annual rainfall. Kericho town (0˚22′0″S, 35˚16′59″E) lies within the highlands west of the Great Rift Valley in Kenya, adjacent to Kenya’s biggest water catchment area, the Mau forest. It is located in the south west of the country at 2096 m above sea level, 263 km North West of Nairobi. The climate in Kericho is characterized by cool tempera- tures ranging from 16˚C to 20˚C, and high rainfall aver- aging between 1400 mm and 2000 mm per annum. 2.2. Market Survey and Collection of Peanut Samples Market survey was conducted in June 2011 and collec- tion of peanut samples took place from June 2011 to January 2012. Two hundred and twenty eight (228) pea- nut samples of 0.5 kg each were collected from formal and informal markets from Eldoret (118) and Kericho (110) towns. Seventy four peanut samples (raw-15, roasted-36, roasted de-coated-23) and 66 samples (raw- 15, roasted-30, roasted de-coated-21) were collected from formal markets in Eldoret and Kericho towns, re- spectively. Additionally, 44 samples (raw-24; roasted-20), and another 44 (raw-24; roasted-15, roasted de-coated-5) were collected from informal markets in Eldoret and Kericho towns, respectively. Within each town, stratified systematic sampling plan was followed in acquiring samples of peanut products on sale. Formal markets referred to stockists, shops and su- permarkets while informal markets included hawkers and open markets. Half a kilogram sample of raw shelled, roasted and roasted de-coated peanuts was collected from each vendor operating formal and informal market out- lets. The peanut samples collected from informal markets were packaged and sealed in a sterile polythene bag. All samples were then transported to the laboratory where they were stored in a cold room at 8˚C until laboratory analyses. Information on the source and handling of peanuts on sale was gathered through direct observation and inter- view using a semi-structured questionnaire that captured the type of peanut product traded, nature of market outlet, packaging material used, source of the peanut products, mode of transport to the market, storage structures and conditions, whether or not peanuts were sorted before selling, the sorting criteria used, and the time interval between buying and selling (data not shown). 2.3. Sample Preparation Each peanut sample was thoroughly mixed and 250 g drawn and ground to a fine powder using a Black and Decker blender machine (BX525-B5 Type 02, Shanghai, China). Two replicates of 100 g each were weighed where one replicate was used for mycological analysis and the other for aflatoxin analysis. Copyright © 2013 SciRes. AiM  H. NYIRAHAKIZIMANA ET AL. 335 2.4. Microbial Analysis 2.4.1. Preparation of Culture Media and Peanut Samples Peanut samples were cultured on modified dichloran rose bengal (MDRB) agar medium [22]. The medium was prepared by mixing 10 g glucose, 2.5 g peptone, 0.5 g yeast extract, 1 g KH2PO4, 0.5 g MgSO4, 7H2O, 20 g agar, and 25 mg rose bengal in 1 L distilled water. The pH of the medium was adjusted to 5.6 using 0.01 M HCl. The medium was autoclaved for 20 minutes at 121˚C and 15 psi, and cooled in a water bath at 60˚C. To inhibit the growth of bacteria and ensuring that the medium was semi selective for Aspergillus species, 5 ml of 4 mg/l dichloran (in acetone), 40 mg/l streptomycin (in 5 ml distilled water) and 1 mg/l chlorotetracycline (in 10 ml distilled water) were added to the medium through a ster- ile 0.25 µm syringe filter paper after cooling to 50˚C. Ap- proximately 20 ml of the medium was dispensed in dis- posable Petri plates, and allowed to settle for two to three days before use. From the 100 g ground peanut sub-sample, two sub- samples of 2.5 g each were weighed and transferred into falcon tubes into which 10 ml of 2% water agar solution (2 g of agar dissolved in 100 ml sterile distilled water) were added and mixed thoroughly. The first sub-sample was serial diluted to 10−1 and the second to 10−2. A 0.2 ml aliquot of the suspension from each dilution was pipetted and spread onto MDRB plates under aseptic conditions. There were six replicates for each sample (three for 10−1 and three for 10−2). The plates were incubated for seven days at 30˚C after which fungal colonies were counted. The fungal colonies were sub-cultured on clean MDRB agar medium Petri plates for identification. 2.4.2. Identification of Fungal Species and Counting of Colonies Pure colonies on MDRB agar medium were sub-cultured onto the Czapek yeast extract agar (CYA; 1 g K2HPO4, 10 mL Czapek concentrate, 5 g powdered yeast extract, 30 g sucrose, 15 g agar), whose pH was adjusted to 7.2 and the plates incubated at 30˚C for 7 days. Species of Aspergillus section Flavi were identified based on cul- tural and morphological characteristics including colony colour, size of sclerotia, texture and conidial morphology characteristics [23], and by comparison with reference strains obtained from the International Crops Research Institute for the Semi-Arid Tropics (ICRISAT) Plant Pa- thology laboratory. The reference cultures were sub-cul- tured at the same time of plating the peanut samples. Co- lonies of other isolated fungal pathogens were identified to genus level. The colony forming units (CFU) of each fungal species were counted using the Gallenkamp col- ony counter (Gallenkamp manufacturer, Frodsham, Eng- land). Equation (1) was used to determine the population (CFU/g peanuts) of the fungal species. colony counts CFUg peanuts=volume plateddilutionfactor (1) The volume plated was 0.2 ml while the dilution fac- tors were 0.25 for the first dilution (10−1) and 0.025 for the second dilution (10−2). 2.5. Aflatoxin Analysis The level of total aflatoxin in each peanut sample was determined by indirect competitive Enzyme Linked Im- munosorbent Assay (ELISA), a method approved by the Association of Analytical Chemists (AOAC) [24]. A 100 g sub-sample peanut powder was well mixed and 20 g taken, triturated in 70% methanol (70 ml absolute metha- nol in 30 ml distilled water, v/v) containing 0.5% (w/v) potassium chloride in blender for 2 minutes. The extracts were transferred to a conical flask and shaken for 30 minutes at 300 rpm. The extract was filtered through Whatman number 41 filter paper and then transferred to a sterile container, stored in a freezer (−20˚C) until analy- sis for total aflatoxin. The extracts were analyzed for aflatoxin level at the Plant Pathology laboratory in IC- RISAT- India. 2.6. Data Analyses Data on fungal population and aflatoxin levels in peanuts were compared based on the type of market (formal and informal), type of peanut product (raw, roasted coated and roasted de-coated) and towns (Eldoret and Kericho). The diversity of fungal species contaminating peanut products sampled from the two market types and towns was compared based on Simpson diversity index (D) values. Equation (2) was used to compute D values. Low index value indicates that a few species dominated over the others. S2 i i1 1 D (2) Where S = number of species; ; i= 1, 2,10 i CFUg peanuts for species i PTotal CFUs Aflatoxin level was not normally distributed and did not have constant variance implying that the assumptions for parametric t-test did not hold (Shapiro-Wilk test for Normality, p < 0.001 and Bartlett’s test for homogeneity of variances, p < 0.001). Therefore, in comparing any two groups of the variables, the non-parametric Mann- Whitney U (Wilcoxon rank-sum) statistical test was used to analyze the data. In comparing more than two groups, data were analyzed using analysis of variance (ANOVA) Copyright © 2013 SciRes. AiM 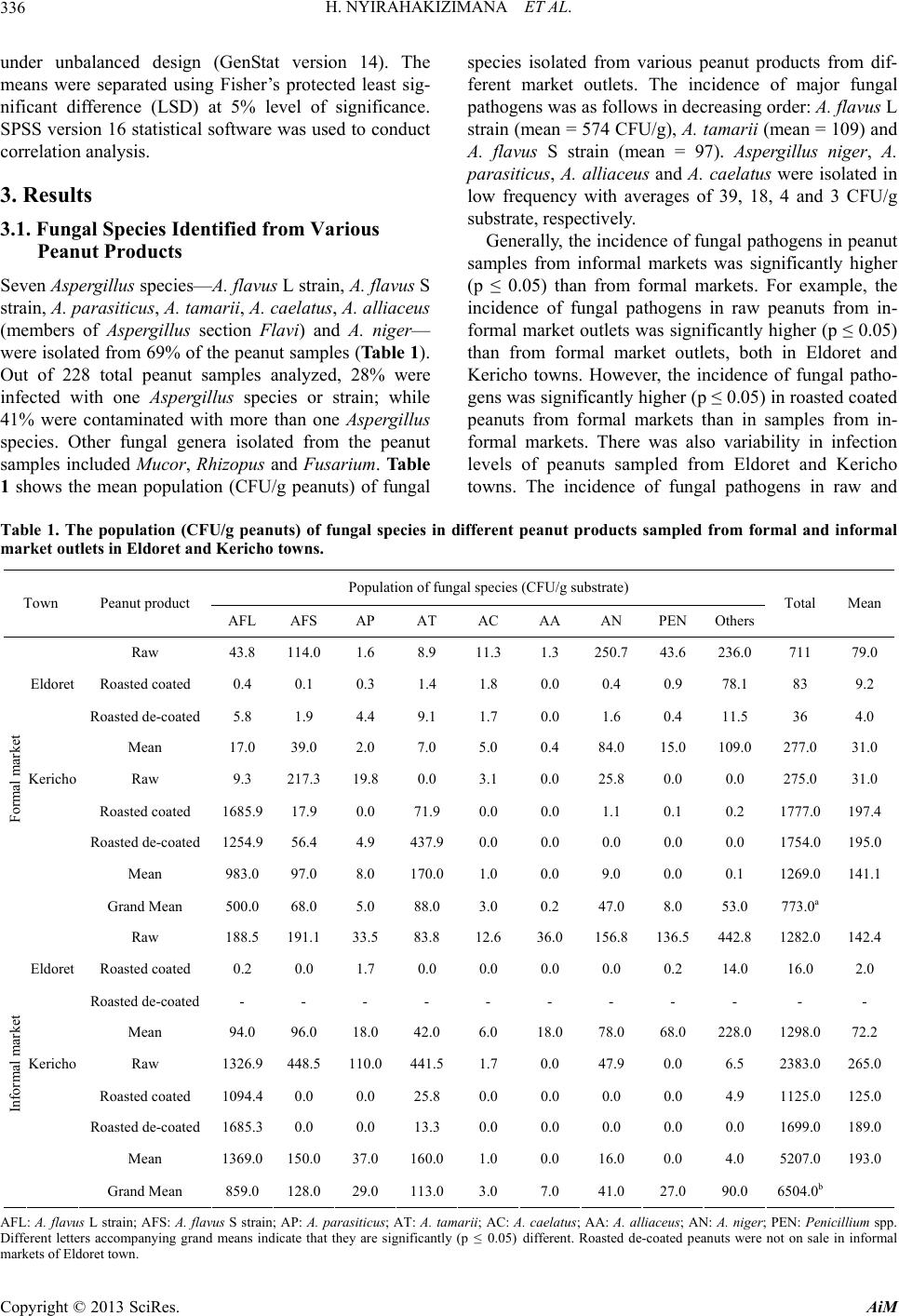 H. NYIRAHAKIZIMANA ET AL. Copyright © 2013 SciRes. AiM 336 under unbalanced design (GenStat version 14). The means were separated using Fisher’s protected least sig- nificant difference (LSD) at 5% level of significance. SPSS version 16 statistical software was used to conduct correlation analysis. 3. Results 3.1. Fungal Species Identified from Various Peanut Products Seven Aspergillus species—A. flavus L strain, A. flavus S strain, A. parasiticus, A. tamarii, A. caelatus, A. alliaceus (members of Aspergillus section Flavi) and A. niger— were isolated from 69% of the peanut samples (Table 1). Out of 228 total peanut samples analyzed, 28% were infected with one Aspergillus species or strain; while 41% were contaminated with more than one Aspergillus species. Other fungal genera isolated from the peanut samples included Mucor, Rhizopus and Fusarium. Table 1 shows the mean population (CFU/g peanuts) of fungal species isolated from various peanut products from dif- ferent market outlets. The incidence of major fungal pathogens was as follows in decreasing order: A. flavus L strain (mean = 574 CFU/g), A. tamarii (mean = 109) and A. flavus S strain (mean = 97). Aspergillus niger, A. parasiticus, A. alliaceus and A. caelatus were isolated in low frequency with averages of 39, 18, 4 and 3 CFU/g substrate, respectively. Generally, the incidence of fungal pathogens in peanut samples from informal markets was significantly higher (p ≤ 0.05) than from formal markets. For example, the incidence of fungal pathogens in raw peanuts from in- formal market outlets was significantly higher (p ≤ 0.05) than from formal market outlets, both in Eldoret and Kericho towns. However, the incidence of fungal patho- gens was significantly higher (p ≤ 0.05) in roasted coated peanuts from formal markets than in samples from in- formal markets. There was also variability in infection levels of peanuts sampled from Eldoret and Kericho towns. The incidence of fungal pathogens in raw and Table 1. The population (CFU/g peanuts) of fungal species in different peanut products sampled from formal and informal market outlets in Eldoret and Kericho towns. Population of fungal species (CFU/g substrate) Town Peanut product AFL AFS AP AT AC AA AN PEN Others Total Mean Raw 43.8 114.0 1.6 8.9 11.3 1.3 250.743.6 236.0 711 79.0 Eldoret Roasted coated 0.4 0.1 0.3 1.4 1.8 0.0 0.4 0.9 78.1 83 9.2 Roasted de-coated 5.8 1.9 4.4 9.1 1.7 0.0 1.6 0.4 11.5 36 4.0 Mean 17.0 39.0 2.0 7.0 5.0 0.4 84.0 15.0 109.0 277.0 31.0 Kericho Raw 9.3 217.3 19.8 0.0 3.1 0.0 25.8 0.0 0.0 275.0 31.0 Roasted coated 1685.9 17.9 0.0 71.9 0.0 0.0 1.1 0.1 0.2 1777.0197.4 Roasted de-coated 1254.9 56.4 4.9 437.90.0 0.0 0.0 0.0 0.0 1754.0195.0 Mean 983.0 97.0 8.0 170.01.0 0.0 9.0 0.0 0.1 1269.0141.1 Formal market Grand Mean 500.0 68.0 5.0 88.0 3.0 0.2 47.0 8.0 53.0 773.0a Raw 188.5 191.1 33.5 83.8 12.6 36.0 156.8136.5 442.8 1282.0142.4 Eldoret Roasted coated 0.2 0.0 1.7 0.0 0.0 0.0 0.0 0.2 14.0 16.0 2.0 Roasted de-coated - - - - - - - - - - - Mean 94.0 96.0 18.0 42.0 6.0 18.0 78.0 68.0 228.0 1298.072.2 Kericho Raw 1326.9 448.5 110.0441.51.7 0.0 47.9 0.0 6.5 2383.0265.0 Roasted coated 1094.4 0.0 0.0 25.8 0.0 0.0 0.0 0.0 4.9 1125.0125.0 Roasted de-coated 1685.3 0.0 0.0 13.3 0.0 0.0 0.0 0.0 0.0 1699.0189.0 Mean 1369.0 150.0 37.0 160.01.0 0.0 16.0 0.0 4.0 5207.0193.0 Informal market Grand Mean 859.0 128.0 29.0 113.03.0 7.0 41.0 27.0 90.0 6504.0b AFL: A. flavus L strain; AFS: A. flavus S strain; AP: A. parasiticus; AT: A. tamarii; AC: A. caelatus ; AA: A. alliaceus; AN: A. niger; PEN: Penicillium spp. Different letters accompanying grand means indicate that they are significantly (p ≤ 0.05) different. Roasted de-coated peanuts were not on sale in informal markets of Eldoret town.  H. NYIRAHAKIZIMANA ET AL. 337 roasted de-coated peanuts sampled from Kericho town was significantly higher (p ≤ 0.05) than in similar sam- ples from Eldoret town. However, there was no signifi- cant (p ≥ 0.05) difference in the incidence of fungal pathogens in roasted de-coated peanuts sampled from formal and informal markets in Kericho town. Similarly, there was no significant (p ≥ 0.05) difference in the inci- dence of fungal pathogens between roasted coated pea- nuts and roasted de-coated peanuts from formal and in- formal markets in Eldoret and Kericho towns. 3.2. Diversity of Fungal Species in Different Peanut Products and Market Types The diversity of fungal species was generally higher in peanut products sampled from Eldoret than Kericho town (Figure 1). However, there was no significant (p ≥ 0.05) difference in the diversity of fungal species among dif- ferent peanut products. 3.3. Population of Major Aflatoxin-Producing Fungi in Peanut Samples The population of major aflatoxin-producing species (A. flavus L strain, A. flavus S strain and A. para siticus) was significantly (p ≤ 0.05) higher in peanuts from Kericho than in Eldoret town. However, the population of these species was not significantly different in roasted coated and roasted de-coated peanuts from both formal and in- formal market outlets (Figure 2). The incidence of A. flavus (L and S strains) and A. parasiticus was significantly lower (p ≤ 0.05) in raw peanuts sampled from formal than informal markets in Eldoret town (Figure 2). Aspergillus flavus L strain was pre-dominant in roasted coated and roasted de-coated peanuts from formal markets with an incidence of 98.9 and 94.9%, respectively (Table 2). The corresponding incidence in raw, roasted coated and roasted de-coated peanuts from informal markets was 68.5, 99.9 and 100%. The incidence of A. flavus S strain was significantly higher (82%) in raw peanuts from formal markets than from informal markets (28%) while Aspergillus para- siticus was isolated in low incidence in same raw peanuts from both formal (5%) and informal (6%) markets. 3.4. Aflatoxin Levels in Different Peanut Products There was variation in total aflatoxin levels between pea- nut samples from formal and informal market outlets, Eldoret and Kericho towns as well as among peanut products (Table 3). Eighty one percent (185 out of 228) of the peanut samples analyzed had detectable levels of total aflatoxin. Aflatoxin levels in peanut products rang- ed from 0 to 2345 µg/kg in raw peanuts, 0 to 382 µg/kg in roasted coated peanuts, and 0 to 201 µg/kg in roasted de-coated peanuts. Generally, raw peanuts were the most Figure 1. Fungal species diversity in peanut products marketed in formal and informal markets in Kericho and Eldoret towns. Bars accompanied by the same letter are not significantly (p ≥ 0.05) different. Copyright © 2013 SciRes. AiM  H. NYIRAHAKIZIMANA ET AL. 338 Figure 2. Colony forming units (CFU/g substrate) of Aspergillus flavus (L and S strains) and A. parasiticus in different peanut products sampled from formal and informal markets in Eldoret and Kericho towns. Bars accompanied by the same letter(s) are not significantly (p ≥ 0.05) different. Table 2. Incidence (%) of major aflatoxigenic species in different peanut products sampled from formal and informal market outlets in Eldoret and Kericho towns. Incidence (%) of aflatoxigenic species Market type Peanut product A. flavus L strain A. flavus S strain A. parasiticus Formal Raw 13.10 81.60 5.30 Roasted coated 98.90 1.10 0.02 Roasted de-coated 94.90 4.40 0.70 Informal Raw 68.50 27.80 6.20 Roasted coated 99.90 0.00 0.10 Roasted de-coated 100.00 0.00 0.00 Table 3. Aflatoxin levels (µg/kg) in different peanut products sampled from formal and informal markets in Eldoret and Kericho towns. Formal market Informal market Grand mean Peanut product Eldoret Kericho Mean Eldoret Kericho Mean Raw 37.8 129.0 83.4 80.1 340.2 210.2 146.8 Roasted coated 93.1 55.5 74.3 48.1 29.4 38.8 56.5 Roasted de-coated 7.9 9.6 8.8 - 42.3 42.3 19.9 Mean 46.3 64.7 55.5 64.1 137.3 97.1 Roasted de-coated peanuts were not on sale in informal markets of Eldoret town. Copyright © 2013 SciRes. AiM  H. NYIRAHAKIZIMANA ET AL. Copyright © 2013 SciRes. AiM 339 contaminated (mean = 146.8 µg/kg), while roasted de- coated peanuts were the least contaminated (mean = 19.9 µg/kg). Similarly, raw peanuts sampled from informal markets had higher levels of aflatoxin (mean = 210.2 µg/kg) than samples from formal market outlets (mean = 83.4 µg/kg). In contrast, roasted coated peanuts from formal markets were more contaminated (mean = 74.3 µg/kg) than samples from informal markets (mean = 38.8 µg/kg). The level of total aflatoxin in roasted coated peanuts was higher in Eldoret than Kericho town. Raw peanuts sampled from informal markets in Kericho town had sig- nificantly higher levels of aflatoxin (mean = 340.2 µg/kg, with 83% contaminated samples), compared to roasted de-coated peanuts from formal markets in Eldoret (mean = 7.9 µg/kg, with 74% contaminated samples). Overall, the levels of total aflatoxin were higher in informal (mean = 97.1 µg/kg) than formal (mean = 55.5 µg/kg) market outlets. 3.5. Correlation between the Population of Major Aflatoxin Producing Species and Aflatoxin Levels The population of A. flavus (S and L strains) and A. pa- rasiticus in raw peanuts had a significant positive corre- lation (R2 = 0.69; p ≤ 0.05) with total aflatoxin level (Figure 3). However, there was no significant (p ≥ 0.05) correlation between the two fungal species and aflatoxin levels in roasted peanuts. The population of A. flavus and A. parasiticus significantly influenced the levels of afla- toxin in peanuts sampled from formal markets in Eldoret town (R2 = 0.63; p ≤ 0.05). On the contrary, aflatoxin levels in roasted coated peanuts from informal markets were not significantly correlated (R2 = 0.09; p ≥ 0.05) to the population of A. flavus and A. parasiticus. In formal markets, the population of A. flavus S strain significantly positively correlated (R2 = 0.37; p ≤ 0.05) with the levels of aflatoxin. However, the level of aflatoxin was not sig- nificantly correlated (R2 = 0.102; p ≥ 0.05) to the popula- tion of A. flavus and A. parasiticus in roasted de-coated peanuts sampled from formal markets. There was a highly significant correlation (R2 = 0.807; p ≤ 0.05) between aflatoxin level and the population of A. flavus (L and S strain) in raw peanuts sampled from in- formal markets in Kericho town. However, aflatoxin level in raw peanuts sampled from formal markets in Kericho was only significantly correlated (R2 = 0.48; p ≤ 0.05) to the population of A. flavus S strain. For roasted coated and de-coated peanuts from both formal and in- formal market outlets, aflatoxin level was not signifi- cantly (p ≥ 0.05) correlated to the population of A. flavus and A. parasiticus. 4. Discussion This study investigated the occurrence of Aspergillus species and aflatoxin contamination in raw and roasted peanuts from formal and informal markets in Eldoret and Kericho towns in Kenya. Six Aspergillus species—A. flavus L and S strains, A. parasiticus, A. tamarii, A. caelatus, A. alliaceus, A. ni- Figure 3. Scatter plot of the population [CFU/g peanuts] of Aspergillus flavus (L and S strains) and A. parasiticus against aflatoxin level in raw peanuts. Aflatoxin level = 31.50 (p ≥ 0.05) + 0.032038 CFU (p ≤ 0.05; R2 = 0.69).  H. NYIRAHAKIZIMANA ET AL. 340 ger—were isolated from marketed raw and roasted pea- nuts in Eldoret and Kericho towns. Sixty-seven percent of the peanut samples analyzed were contaminated with the major aflatoxin producing fungi (A. flavus L strain, A. flavus S strain and A. parasiticus) with A. flavus L strain (> 98%) being the pre-dominant pathogen followed by A. flavus S strain. The occurrence of these fungi especially A. flavus S strain implies a high risk of aflatoxin con- tamination of peanuts marketed in Eldoret and Kericho towns. The incidence of the three fungi in peanuts con- curs with the findings of a recent study in western Kenya [9]. Similar to findings of the current study, two mor- photypes of A. flavus, the S and L strains, have been iso- lated in other studies on peanuts in Kenya [9,19]. The role of A. tamarii, A. alliaceus and A. caelatus as com- mon pathogens of peanuts in Kenya has also been docu- mented [19]. Generally, in both towns, peanuts from in- formal markets had higher fungal species diversity in raw peanuts an observation which concurs with the findings reported in [18]. The type of market outlet influenced the incidence of pathogenic fungi in peanuts. This could be attributed to handling practices including superior packaging, sorting and storage conditions that were characteristic in formal markets. In contrast, raw peanuts sold in informal mar- kets were generally not packaged or sorted and were stored in stalls exposed to weather fluctuations. In addi- tion, some peanuts were sold in open air systems sub- jecting them to weather changes and abrupt rainfall which could promote fungal proliferation. The incidence of aflatoxin producing fungi was sig- nificantly higher in peanuts sampled from markets in Kericho than in Eldoret town. This could be attributed to the fact that raw peanuts sampled from informal markets in Eldoret were sold under covered structures whereas in Kericho, open air markets were more common. Peanut roasting and de-coating reduces fungal population in and/or on kernels [25]. Indeed, during roasting process, peanuts are exposed to dry heat at high temperatures [26] that kill or reduce the population of fungi. However, the incidence of aflatoxin producing fungi was not signify- cantly lower in roasted peanuts sampled from Kericho town. This could be attributed to handling practices which could result in re-contamination of roasted ker- nels. Aspergillus flavus and A. parasiticus are fungal species that have an affinity for nuts and oilseeds, and are the main producers of aflatoxin which are the most toxic and carcinogenic compounds among the known mycotoxins [10]. Aspergillus flavus produces AFB1, AFB2 in addition to cyclopiazonic acid [27] which targets the liver, kidney and gastrointestinal tract in animals, while A. parasiticus produces AFB1, AFB2, AFG1 and AFG2 [10]. Aspergillus tamarii produces AFB1, AFB2 and cyclopiazonic acid [28] while A. alliaceus produces ochratoxin [29]. Ochratoxin is nephrotoxic, hepatotoxic, immunotoxic and possibly neurotoxic [11]. Fusarium spp. produce trichothecenes, fumonisins, and zearalenone among other mycotoxins while Penicillium sp p. produce ochratoxin A and patulin [30]. Although the above toxins were not the subject of investigation in this study, the presence of fungal species known to produce them implies a greater health risk to consumers of peanut products. In addition, this observa- tion reveals the need for management strategies that tar- get the control of both aflatoxin-producing fungi and pathogens that produce other types of mycotoxins. This study also investigated the levels of total afla- toxin in different peanut samples. Aflatoxin levels ranged from 0 to 684.8 μg/kg and 0 to 2344.8 μg/kg in samples from formal and informal markets, respectively. These results are consistent with the findings in [19] where afla- toxin levels ranging from 0 to 2687.6 μg/kg and 0 to 1838.3 μg/kg were reported in peanuts sampled from Busia and Homa bay regions in Western Kenya. High incidence of aflatoxin in raw peanuts (83% of raw peanut samples had levels of aflatoxin averaging 340.2 µg/kg) corroborated findings from Botswana [31], where con- tamination incidence of 78% of raw samples and afla- toxin concentration ranging from 12 to 329 μg/kg were reported. Previous studies [25,32] have shown that pea- nut roasting and de-coating processes reduce the risk of aflatoxin production. This study revealed that there was higher risk of exposure to aflatoxin through raw than roasted peanuts. Roasting kills aflatoxin producing fungi thereby reducing the risk of aflatoxin contamination. Aflatoxin contamination of peanuts should be a public health concern not only in Eldoret and Kericho towns but also in other parts of Kenya as well as other tropical countries. Reference [33] reported a mean content of 40 μg/kg of aflatoxin B1 in over 85% of peanut oil samples from Senegal, while [34] reported high aflatoxin content of 25 to 600 μg/kg in Sudanese peanuts. Different levels of aflatoxin were also reported in peanuts collected from processors, stockers, farmers and traders in Benin [35], while [36] reported total aflatoxin level of 56 μg/kg in unprocessed peanuts in Brazil. Roasted de-coated pea- nuts had high percentage (74%) of contaminated samples whereas the concentration of aflatoxin was relatively low with an average of 7.9 µg/kg. Previous studies have shown that exposure of humans to high levels of afla- toxin leads to acute aflatoxicosis and that long-period of exposure to aflatoxin, even in low concentration, may lead to liver cancer, stunted growth in children and to immune system disorders through chronic aflatoxicosis [14,37]. There was a strong positive correlation between the population of aflatoxin-producing fungi and total afla- toxin levels detected in raw peanuts. However, total afla- Copyright © 2013 SciRes. AiM  H. NYIRAHAKIZIMANA ET AL. 341 toxin levels in roasted peanuts from formal and informal markets were not significantly correlated to the popula- tion of aflatoxigenic fungal species. These findings con- cur with those in [9,25,32] who have reported that roast- ing and de-coating processes reduce fungal population. The population of A. flavus S strain was found to sig- nificantly influence aflatoxin production. This concurs with the findings by [19] who reported that the incidence and population of A. flavus S strain significantly and positively correlated with the levels of total aflatoxin in peanuts. The presence of A. flavus S strain implies a ma- jor health problem to consumers of peanuts because it has been reported to produce greater amount of aflatoxin especially aflatoxin B1 [19] which is also classified as class 1 carcinogen [38]. Aspergillus flavus S strain pro- duces greater quantities of aflatoxin than A. flavus L strains [39]. Reference [40] reported A. flavus S strain to be the primary cause of contamination events in North America and Africa. In conclusion, the incidence of aflatoxin producing fungi in different peanut products analyzed was high (up to 76%), and the levels of aflatoxin differed in peanuts sampled from formal and informal markets. The highest population of aflatoxin-producing fungi was recorded in raw peanuts sampled from informal market outlets. The high incidence of aflatoxin producing fungi in peanuts and peanut products implies poor quality of peanuts marketed in Eldoret and Kericho towns, and cones- quently, a high risk of aflatoxin contamination and health risk to consumers of peanut products. There is therefore need to improve quality standards of peanuts marketed in the two towns. The significantly higher aflatoxin con- tamination of raw peanuts compared to roasted de-coated peanuts implies that processing—combining roasting and de-coating—potentially reduces the incidence of afla- toxin-producing fungi and aflatoxin production in pea- nuts. There is need for raising awareness among peanut traders and consumers on proper handling of peanuts, and the health risks associated with consumption of afla- toxin contaminated products. 5. Acknowledgements This research was funded in part by the Peanut Collabo- rative Research Support Program (Peanut CRSP) funded by USAID under cooperative agreement USAID ECG- A-00-07-00001-00. The authors express their gratitude to all peanut traders who participated in the study. REFERENCES [1] C. K. Mutegi, “The Extent of Aflatoxins and Aspergillus section Flavi, Penicillium Species and Rhizopus species Contamination of Peanuts from Households in Western Kenya and the Causative Factors of Contamination,” PhD Dissertation, University of KwaZulu-Natal, Pietermaritz- burg, 2010. [2] J. Oywa, “Pouring Water on Farmers Rich Grain Har- vest,” Standard Digital News, Thursday, June 3, 2010. http://www.standardmedia.co.ke/?article [3] FAOSTAT, “Production: Crops. Food and Agriculture Organization of the United Nations,” 2012. http://faostat.fao.org/site/567/default.aspx#ancor [4] R. G. Nelson and A. G. Carlos, “Chemical Composition of Aboriginal Peanut (Arachis hypogaea L.) Seed from Peru,” Journal of Agricultural and Food Chemistry, Vol. 43, No. 1, 1995, pp.102-105. doi:10.1021/jf00049a019 [5] V. N. Atasie, T. F. Akinhanmiand and C. C. Ojiodu, “Proximate Analysis and Physiochemical Properties of Groundnut (Arachis hypogaea L.),” Pakistan Journal of Nutrition, Vol. 8, No. 2, 2009, pp. 194-197. doi:10.3923/pjn.2009.194.197 [6] J. Y. Chun, “Vitamin E Content and Stability in Peanuts and Peanut Products during Processing and Storage,” PhD Dissertation, University of Georgia, Athens, 2002. [7] M. Little, “What Vitamins Are in Peanuts?” 2010. http://www.livestrong.com/article/262203-what-vitamins- are-in-peanuts/ [8] D. Nabuuma, D. Nakimbugwe, Y. B. Byaruhanga, F. K. Saalia, R. D. Phillips and J. Chen, “Formulation of a Drinkable Peanut-Based Therapeutic Food for Malnour- ished Children Using Plant Sources,” International Jour- nal of Food Science and Nutrition, Vol. 64, No. 4, 2012, pp. 467-475. doi:10.3109/09637486.2012.746289 [9] B. C. Ajay, M. V. C. Gowda, A. L. Rathnakumar, V. P. Kusuma, R. Abdul Fiyaz, P. Holajjer, K. T. Ramya, G. Govindaraj and H. Prashanth Babu, “Improving Genetic Attributes of Confectionary Traits in Peanut (Arachis hy- pogaea L.) Using Multivariate Analytical Tools,” Journal of Agricultural Science, Vol. 4, No. 3, 2012, pp. 247-258. [10] J. Yu, P. K. Chang, K. C. Ehrlich, J. W. Cary, D. Bhat- nagar, T. E. Cleveland, G. A. Payne, J. E. Linz, C. P. Woloshuk and J. W. Bennett, “Clustered Pathway Genes in Aflatoxin Biosynthesis,” Applied and Environmental Microbiology, Vol. 70, No. 3, 2004, pp. 1253-1262. doi:10.1128/AEM.70.3.1253-1262.2004 [11] J. McLauchlin and C. Little, “Hobbs’ Food Poisoning and Food Hygiene,” Hodder Arnold, Oxford, 2007. [12] J. L. Richard, “Some Major Mycotoxins and Their My- cotoxicoses—An Overview,” International Journal of Food Microbiology, Vol. 119, No. 2, 2007, pp. 3-10. doi:10.1016/j.ijfoodmicro.2007.07.019 [13] R. Russell, M. Paterson and N. Lima, “How Will the Climate Change Affect Mycotoxins in Food?” Food Re- search International, Vol. 43, No. 7, 2010, pp. 1902-1914. doi:10.1016/j.foodres.2009.07.010 [14] F. Wu, C. Narrod, M. Tiongco and Y. Liu, “The Health Economics of Aflatoxins: Global Burden of Disease,” IFPRI Working paper 4, February 2011, pp. 1-20. [15] J. W. Dorner, “Simultaneous Quantitation of Aspergillus flavus/ A. parasiticus and Aflatoxin in Peanuts,” Journal of Association of Official Analytical Chemists Interna- tional, Vol. 85, No. 4, 2002, pp. 911-916. Copyright © 2013 SciRes. AiM  H. NYIRAHAKIZIMANA ET AL. Copyright © 2013 SciRes. AiM 342 [16] J. Varga, K. Rigot, B. Toth, J. Teren and Z. Kozakeiwicz, “Evolutionary Relationship among Aspergillus Species Producing Economically Important Mycotoxins,” Food Technology and Biotechnology, Vol. 41, No. 1, 2003, pp. 29-36. [17] J. W. Cary, M. A. Klich and S. B. Beltz, “Characteriza- tion of Aflatoxins Producing Fungi Outside of Aspergillus section Flavi,” Mycologia, Vol. 97, No. 2, 2005, pp. 425- 432. doi:10.3852/mycologia.97.2.425 [18] E. W. Gachomo, E. W. Mutitu and O. S Kotchoni, “Di- versity of Fungal Species Associated with Peanuts in Storage and the Levels of Aflatoxins in Infected Sam- ples,” International Journal of Agriculture and Biology, Vol. 6, No. 6, 2004, pp. 955-959. [19] C. K. Mutegi, H. K. Ngugi, S. L. Hendiks and R. B. Jones, “Factors Associated with the Incidence of Aspergillus Section Flavi and Aflatoxins Contamination of Peanuts in Busia and Homa bay Districts of Western Kenya,” Plant Pathology, Vol. 61, No. 6, 2012, pp. 1143-1153. doi:10.1111/j.1365-3059.2012.02597.x [20] C. K. Mutegi, J. Kimani, G. Otieno, R. Wanyama, M. E. Christie, K. Mallikarjunan and A. Kaaya, “Market Attrib- utes and their Effect on Levels of Aflatoxin in Peanuts (Arachis hypogaea L.) from Nairobi and Western Ken- ya,” 2010, pp. 237-244. http://www.kari.org/conference/conference12/docs/marke t%20attributes%20and%20their%20effect%20on%20leve ls%20of%20aflatoxin%20in%20peanuts [21] A. Kaaya, “A Review of Past and Present Research on Aflatoxin in Uganda,” African Journal of Food Agricul- ture and Nutritional Development, Vol. 5, No. 1, 2005, pp. 1-18. [22] B. W. Horn, J. W. Dorner, “Soil Populations of Aspergil- lus Species from Section Flavi along a Transect through Peanut-Growing Regions of the United States,” Mycolo- gia, Vol. 90, No. 5, 1998, pp. 767-776. doi:10.2307/3761317 [23] M. A. Klich, “Identification of Common Aspergillus Spe- cies,” Centraalbureau Voor Schimmelculture, Utrecht, 2002. [24] P. Cunniff, “Official Methods of Analysis of AOAC In- ternational,” Association of Official Analytical Chemists, Washington DC, 1995. [25] F. C. Galvez , M. L. Francisco, B. J. Villarino, A. O. Lus- tre and A. V. Resurreccion, “ Manual Sorting to Eliminate Aflatoxin from Peanuts,” Journal of Food Protection, Vol. 66, No. 10, 2003, pp. 1879-1884. [26] D. K. Okello, A. N. Kaaya, J. Bisikwa, M. Were and H. K. Oloka, “Management of Aflatoxins in Groundnuts: A Manual for Farmers, Processors, Traders and Consumers in Uganda,” National Agricultural Research Organization, Entebbe, 2010. [27] H. K. Abbas, M. A. Weaver, B. W. Horn, I. Carbone, J. T. Monacell and W. T. Shier, “Sellection of Aspergillus Iso- lates for Biological Control of Aflatoxins,” Toxin Review, Vol. 30, No. 2-3, 2011, pp. 59-70. doi:10.3109/15569543.2011.591539 [28] T. Goto, D. T. Wcklow and Y. Ito, “Aflatoxin and Cyclo- piazonicacid Production by Sclerotium Producing Asper- gillus tamarii Strain,” Applied and Enironmental Micro- biology, Vol. 62, No. 11, 1996, pp. 4036-4038. [29] P. Bayman, J. L. Baker, M. A. Doster, T. J. Michailides, and N. E. Mahoney, “Ochratoxin Production by Asper- gillus ochraceus and Aspergillus alliaceus,” Applied and Environmental Microbiology, Vol. 68, No. 5, 2002, pp. 2326-2335. doi:10.1128/AEM.68.5.2326-2329.2002 [30] R. A. Samson, E. S. Hoekstra, J. C. Frisvad and O. Fil- tenborg, “Introduction to Food-Borne Fungi,” Pansen and Looyen, Wageningen, 1995. [31] F. Mphande, B. Siame and J. Taylor, “Fungi, Aflatoxins and Cyclopiazonic Acid Associated with Peanut Retailing in Botswana,” Journal of Food Protection, Vol. 67, No. 1, 2004, pp. 96-102. [32] CAC/RCP 55, “Code of Practice for the Prevention and Reduction of Aflatoxin Contamination in Peanuts,” 2004. [33] Y. Diop, B. Ndiaye, A. Diouf, M. Fall, C. Thiaw, A. Thiam, O. Barry, M. Ciss and D. Ba, “Contamination by Aflatoxins of Local Peanut Oils Prepared in Senegal,” Annales Pharmaceutipues Francaises, Vol. 58, No. 6, 2000, pp. 470-474. [34] R. E. Omer, M. I. Bakker, F. Van’t Veer, R. L. Hoogen- boom, T. H. Polman, G. H. Alink, M. O. Idris, A. M. Ka- daru and F. J. Kok, “Aflatoxin and Liver Cancer in Su- dan,” Nutrition and Cancer, Vol. 32, No. 3, 1998, pp. 174-180. doi:10.1080/01635589809514737 [35] C. B. N’Dede, “Economic Risks of Aflatoxin Contamina- tion in the Production and Marketing peanut in Benin,” MSc. Dissertation, Auburn University, Auburn, 2009. [36] C. A. F. Oliveira, N. B. Goncalves, R. E Rosim and A. M. Fernandes, “Determination of Aflatoxins in Peanut Prod- ucts in the Northeast Region of Sao Paulo, Brazil,” In- ternational Journal of Molecular Sciences, Vol. 10, No. 1, 2009, pp. 174-183. [37] C. P. Wild and Y. Y. Gong, “Mycotoxins and Human Di- sease: A Largely Ignored Global Health Issue,” Carcino- genesis, Vol. 31, No. 1, 2010, pp. 71-82. doi;10.1093/carcin/bgp264 [38] IARC, “Overall Evaluation of Carcinogenicity: An Up- dating of IARC Monographs,” Report of an IARC Expert Committee, International Agency for Research on Cancer, Vol. 1-42, Lyon, 1987, pp. 83-87. [39] P. J. Cotty and R. Jaime-Garcia, “Influences of Climate on Aflatoxin Producing Fungi and Aflatoxin Contamina- tion,” International Journal of Food Microbiology, Vol. 119, No. 1-2, 2007, pp. 109-115. doi;10.1016/j.ijfoodmicro.2007.07.060 [40] R. Jaime-Garcia and P. J. Cotty, “Crop Rotation and Soil Temperature Influence the Community Structure of As- pergillus flavus in Soil,” Soil Biology and Biochemistry, Vol. 42, No. 10, 2010, pp. 1842-1847. doi;10.1016/j.soilbio.2010.06.025
|