 Advances in Microbiology, 2013, 3, 317-325 http://dx.doi.org/10.4236/aim.2013.34045 Published Online August 2013 (http://www.scirp.org/journal/aim) Activity of Selected Essential Oils against Candida spp. strains. Evaluation of New Aspects of their Specific Pharmacological Properties, with Special Reference to Lemon Balm Aleksandra Budzyńska1, Beata Sadowska1, Grażyna Lipowczan2, Agnieszka Maciąg3, Danuta Kalemba3, Barbara Różalska1* 1Department of Immunology and Infectious Biology, University of Lodz, Poland 2dr Wł. Biegański Provincial Specialist Hospital in Lodz, Diagnostic Laboratory, Poland 3Institute of General Food Chemistry, Lodz University of Technology, Poland Email: *rozab@biol.uni.lodz.pl Received June 5, 2013; revised July 5, 2013; accepted July 15, 2013 Copyright © 2013 Aleksandra Budzyńska et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT The aim was to investigate the antifungal effectiveness and some of pharmacological properties of essential oils (EOs), which had not yet been thoroughly studied in the planned scope. We first evaluated MIC/MFC of sixteen EOs against C. albicans ATCC 10231. Then, five most active EOs were tested, using 50 clinical Candida spp. strains and additional reference C. albicans ATCC 90028 strain. The time-kill curve, carryover, post-antifungal effects (PAFE), mutant pre- vention concentrations, the susceptibility of reference strains to the cell wall disrupting agents and tolerance to oxidative stress, were evaluated. For these detailed studies, we chose the following four essential oils. Clove oil, Geranium oil, Lemon balm and Citronella oil, with MICs of 0.097% (v/v), resulted concentration- and time-dependent killing and may be therapeutically safe, because they do not generate resistance. The best one was Lemon balm, which caused most ex- tended PAFE, significantly reduced tolerance to oxidative stress and increased susceptibility to Calcofluor White, Congo Red and SDS. Phytochemical analysis of these four EOs has been performed and compared; looking for the rea- son that Lemon balm was the best. Keywords: Candida; Cell Wall Sensitivity; Essential Oils PAFE; Mutant Generation; Oxidative Stress Tolerance; Time-Killing 1. Introduction Numerous members of Can did a spp. are commensal or- ganisms colonizing several ecological niches of healthy individuals. On the other hand, they are also the most frequent pathogens in humans at risk. Depending on the immunological state of the host, candidiasis can develop as mild superficial infection or very dangerous, life threatening invasive one. Epidemiological data also show an increase in local fungal infections of chronic wounds (diabetic foot, burn, bed-sore, cancer ulceration), with the participation of C. albicans biofilm. Such infections, similarly to systemic ones, are very difficult to eradicate since Candida cells living as a biofilm community show extremely high resistance to most of the currently used antifungal drugs [1,2]. Despite the discovery in the last decades of potent antimycotics, most types of systemic and local Cand ida infections still remain a serious medi- cal problem. Thus, it is pivotal to discuss various strate- gies that may become therapeutic tools in future, in which the search for agents with novel mechanisms of action should be considered. The most intensively stud- ied option is using plant products as factors modulating the resistance of microorganisms [3]. Indeed, many com- pounds of plant origin affect some structures and some of the mechanisms responsible for the resistance phenotype in bacteria and fungi. A very interesting trend of world- wide studies is the possible synergism that may occur between, for example, plant-derived agents and antibiot- ics or other chemotherapeutics. It can result in strong growth inhibition even of drug-resistant microbes (bacte- *Corresponding author. C opyright © 2013 SciRes. AiM  A. BUDZYŃSKA ET AL. 318 ria or fungi) and the ability to obtain a stronger post-anti- biotic effect [4-9]. Generally, studies on the possible use of plant-derived compounds for combating human patho- gens are being carried out worldwide and knowledge coming from in vitro studies in this field is already quite extensive. However, the modern approach to observa- tions drawn from folk medicine is to clarify the mecha- nisms of their activity, forming the basis for safe use. The main objective of the present study was to investi- gate the possibility of using essential oils as potential me- dicinal substances active against Candida spp. For this purpose, the essential oils were chosen which had not yet been thoroughly studied in the planned scope. As target microorganisms we used C. albicans reference strains and a group of C. albicans and various non-albicans Candida clinical isolates. More detailed research, concer- ning time-kill curve, carryover and post-antifungal ef- fects as well as the mutant prevention concentration of four selected the most active essential oils, was conduc- ted. Also, the susceptibility of C. albicans to cell wall disrupting agents and yeast cell tolerance to oxidative stress induced by hydrogen peroxide, after treatment with essential oils were estimated. For these preparations the phytochemical analysis has also been performed. 2. Materials and Methods 2.1. Candida Strains and Culture Conditions A total of 52 strains were tested for their susceptibility to essential oils. These comprised two reference C. albicans strains (ATCC 10231, ATCC 90028) and 50 clinical iso- lates: C. albicans (n = 20), C. glabrata (n = 13), C. kru- sei (n = 6), C. parapsilosis (n = 5), C. tropicalis (n = 6). The Cand ida isolates were obtained from cultures of blood, wounds swabs, stool and various mucosal tissue specimens of patients hospitalized at Dr Wł. Biegański Provincial Specialist Hospital in Lodz, Poland. Suspen- sions of the yeasts for each test were prepared from fresh (24 h-old) cultures grown at 35˚C on Sabouraud Dex- trose agar (SDA, Difco Laboratories, USA) or if neces- sary on YPG P-0035 medium (Yeast Extract Peptone Glucose, BTL, Poland). 2.2. Essential oils, MIC and MFC Determination Essential oils (EOs) of plants listed in Table 1. were used, all purchased from Pollena Aroma, Poland. The initial screening of 16 EOs activity was performed by broth microdilution method, according to the guidelines of EUCAST with minor modifications [10,11], using C. al- bicans ATCC 10231 strain as a target organism. Then, the MIC/MFC (minimum inhibitory/fungicidal concen- tration) of 5 most active EOs against 50 clinical Candida spp. and additional reference (C. albicans ATCC 90028) strains was evaluated, using the same experimental pro- Table 1. List of essentials oils tested at different stages of research. Plant species Essential Oil 1 23 Lavandula angustifoliaLavender oil ● ● Melaleuca alternifoliaTea tree oil ● Citrus limon Lemon oil ● Anthemis nobilis Anthemis oil ● Melissa citrata indicaLemon balm ●●● Pinus sylvestris Scott pine ● Ribes nigrum Black currant ● Cymbopogon citratusCitronella oil ●●● Pimpinella anisum Oleum anisi ● Pelargonium graveolens Geranium oil ●●● Eugenia caryophyllataOleum Caryophylli (Clove oil) ●●● Hyssopus officinalisHyssop oil ● Mentha piperita Peppermint oil ● Thymus vulgaris Thyme oil ● Rosmarinus officinalisRosemary oil ● Abies sibirica Abies oil ● 1—MIC/MFC evaluation; C. albicans ATCC 10231, 2—MIC/MFC evalua- tion; 50 clinical Candida spp. strains, C. albicans ATCC 90028, 3—tests evaluating selected pharmacological properties; C. albicans ATCC 10231, C. albicans ATCC 90028. tocol. Briefly, EOs (concentrations at range 6.25% - 0.024%, v/v) were deposited (100 L) in triplicate in the wells of flat-bottom polystyrene 96-well microplates (Nunc, Denmark). Then, 100 L of yeast suspension (105 CFU/mL in RPMI-1640/0.5% Tween 20) was added. The positive control was a suspension of yeasts in the culture medium, and the negative control was the medium. After 48 h incubation at 35˚C, the absorbance at A600 (multi- counter Victor 2, Wallac, Finland) was determined. The endpoint was defined as the lowest concentration of the compound resulting in total inhibition (MIC100) of yeast growth, compared to the growth in the control wells. The lowest concentration of essential oils fungicidal to ≥99.9% of the original inoculum (MFC) was determined paralelly, by subculturing 10 L from the wells with suspected MIC, 2x, 4x MIC, on the SDA without any antimicrobial agents. No visible colony growth after subsequent 24 - 48 h incubation was accepted as MFC. All experiments were conducted in duplicate. 2.3. GC-FID-MS Analysis of Selected EOs Essential oils were analyzed using Trace GC Ultra (The- rmo Electron Corporation) equipment combined with Copyright © 2013 SciRes. AiM  A. BUDZYŃSKA ET AL. 319 DSQ II mass spectrometer and with flame ionization de- tector (FID) throughout MS-FID Splitter. Analysis was provided using nonpolar chromatography column: Rtx-1 ms (Restek) 60 m length, inner diameter 0.25 mm, film thickness 0.25 μm. Temperature programme: 50˚C (3 min), temperature rise 4˚C /min; 310˚C (10 min); injector temperature 280˚C; detector temperature 310˚C. Hellium was used as a carrier gas which was pressurized to 300 kPa, ionization energy 70 eV, ion source temperature 200˚C. Identification of components was based on the comparison of their MS spectra with those in a labora- tory-made MS library, commercial libraries (NIST 98.1 and Mass Finder 4) along with the retention indices as- sociated with a series of alkanes with linear interpolation (C8-C26). A quantitative analysis (expressed as percent- ages of each component) was carried out by peak area normalization measurements without correction factors. 2.4. EOs Killing Kinetics Assay In time-kill curve studies, four most active essential oils were used against C. albicans ATCC 10231 and C. alb- icans ATCC 90028 strains. These are EOs obtained from the following plants: Cymbopogon citratus (Citronella oil), Pelargonium graveolens (Geranium oil), Eugenia caryophyllata (Clove oil), Melissa citrata indica (Lemon balm). The inoculum suspension (5 × 105 CFU/mL) was prepared in 10 mL of RPMI-1640 (Cytogen, Poland) with or without EOs in the concentrations range from 1/2 MIC to 2x MICs. At predetermined time points (0, 0.5, 1, 2, 4, 6, 8, 24 and 48 h) of incubation at 35˚C, a 100 μL samples were serially diluted in sterile water. Then, each 100 μL aliquot was plated onto SDA plates for CFU counting, after 24 - 48 h incubation at 35˚C. The results were reported as the mean percentage of survival ± stan- dard deviation of four replicates, conducted for each compound twice. 2.5. EOs Carryover Effect Fungal suspensions (C. albicans ATCC 10231 and C. albicans ATCC 90028) of approximately 5 × 103 CFU/ mL were prepared in RPMI-1640 medium, and 100 μL of each was added to 900 μL of sterile water without or with EO (at MIC to 4x MIC). Immediately after the addi- tion of the fungal suspension to the agent solution, the test tubes were vortexed and 50 μL aliquots were plated on the SDA. Following 48 h of incubation at 35˚C, the CFU of Candi da was determined. The mean colony count at each multiple of the MIC tested was compared with the data for the control. A significant antifungal carryover was defined as > 25% reduction in CFU in comparison to the control level. The experiments were conducted in duplicate. 2.6. Post-Antifungal Effect (PAFE) of EOs The PAFE of EOs against C. albicans ATCC 10231 and C. albicans ATCC 90028 strains was estimated. The in- oculum suspensions (5 × 105 CFU/mL) were prepared in 10 mL of RPMI-1640 with or without EO (at MIC and 2x MIC). After 1 h exposure to the EOs, samples were diluted 1:100 in pre-warmed medium to effectively re- move the EOs. The diluted cultures were then incubated with agitation (200 rpm) at 35˚C. At the desired time points, 100 μL from each sample was serially diluted 10-fold in sterile water, and 100 μL was plated on SDA. Following incubation at 35˚C for 48 h, the number of CFU was counted. The PAFE was calculated using the formula: PAFE = T − C, where T represents the time re- quired for the CFU to increase 1 log10 CFU/mL in the test culture above the CFU observed immediately after EO removal, and C represents the time required for the count of the untreated control tube to increase by 1 log10 CFU/mL. The experiments were performed in duplicate. 2.7. Selection of Single-Step EOs-Resistant Mutants In order to evaluate the frequency of spontaneous single- step mutations of C. albicans ATCC 10231 and C. albi- cans ATCC 90028 strains, a fungal suspension contain- ing ~1010 CFU was plated on SDA plates (24 cm in di- ameter) containing each EO at concentrations from 1 to 8x MIC. Mutation frequency was expressed as a number of resistant colonies per inoculum. It was calculated by counting the total number of colonies appearing after 7 days of incubation at 35˚C on a plate containing EOs and by dividing this number by the total number of CFU plated. The experiment was repeated twice with three plates for each EO concentration. 2.8. Candida Susceptibility to Cell Wall Disrupting Agents and Oxidative Stress Tolerance under the Influence of Essential Oils Fresh suspensions of C. albicans ATCC 10231 and C. albicans ATCC 90028 in RPMI-1640 medium, prepared from cultures on SDA, were exposed to EOs at MIC for 1 h, 35˚C. Other set of suspensions were prepared after yeasts culture (24 h, 35˚C) in the presence of 1/2 MIC oils (agar dilution). Control suspensions were left with- out EOs influence. Examination of Candida samples exposed to EOs at MIC, was preceded by washing three times in order to avoid carryover effect. The volume of 5 μL of ready-to-use suspensions with densities of 105, 104, 103 cells/mL were spotted on YPG plates containing one of the following cell surface disrupting agents: Cal- cofluor White (5 μg/mL, 10 μg/mL), sodium dodecyl sulfate (SDS) (0.02%, 0.03%, 0.05%) and Congo Red Copyright © 2013 SciRes. AiM  A. BUDZYŃSKA ET AL. Copyright © 2013 SciRes. AiM 320 (20 μg/ mL, 50 μg/mL); all agents were purchased from Sigma, USA; the above concentrations were chosen after preliminary studies. The plates were incubated at 30˚C and monitored for Cand ida growth during 3 days. Each of the experiments was performed in triplicate. To test the oxidative stress tolerance, the remainder of Candida cell suspensions (105), pre-exposed to essential oils or control suspensions, were transferred to Eppendorf tubes in a volume of 1 mL and treated with hydrogen peroxide (12.5, 25 or 50 mM) for 1 h at 35˚C. Then, fungal sus- pensions were diluted (105 to 103 cells/mL) and spotted (5 μL) onto YPG plates. Their growth was monitored for 3 days and compared to the growth of control cultures not treated with EOs and untreated with hydrogen per- oxide. 2.9. Statistical Analysis If necessary, differences in parameters were tested for significance using “U” Mann-Whitney test and computer program Statistica 5.0. 3. Results Screening of sixteen essential oils (EOs), listed in Table 1, against reference C. albicans ATCC 10231 strain has confirmed their known, usually high fungistatic/fungi- cidal activity. Of the preparations used, five EOs which were most active, and not so well-known when it comes to the scope of our research (Lavender oil, Lemon balm, Citronella oil, Geranium oil, Clove oil), were classified to the second step. In the further experiments, the clinical isolates representing C. albicans (n = 20), C. glabrata (n = 13), C. krusei (n = 6), C. parapsilosis (n = 5), C. tro- picalis (n = 6) species and additional reference strain—C. albicans ATCC 90028, have been tested as targets. Si- milar ranges of MIC (0.024% - 0.39%, v/v) for C. al- bicans, and non-albicans clinical strains were noted. However, the fungicidal activity of tested EOs differed, with respect to C. albicans and other species. The mini- mum fungicidal concentrations (MFCs) for C. glabrata, C. krusei, and C. parapsilosis individual strains varied but usually the concentration ranges were higher than that these active for C. albicans (Table 2). Then, the time kill curve, carryover, post-antifungal effect, mutant pre- vention concentrations, the susceptibility of Candi da reference strains to the cell wall disrupting agents and yeast tolerance to oxidative stress, were evaluated. Clove oil, Geranium oil, Lemon balm and Citronella oil were used for these tests. The results of the experiments on killing kinetics of Lemon balm are shown in Figure 1. The fungicidal endpoint (99.9% CFU reduction) for C. albicans ATCC 90028 was achieved after 4 h at MIC and after 1 h at 2x MIC, while for C. albicans ATCC 10231, respectively, after 6 and 2 h. Similar kinetics of killing was showed by Citronella oil. Clove oil and Geranium oil revealed a 99.9% fungi- cidal activity after a longer time (data not shown). Indi- vidual essential oils showed a concentration-dependent PAFE, which means inhibiting the re-multiplication of Candida. A 1-hour exposure to Lemon balm at MIC re- sulted in a long PAFE against both strains tested (3 h). This essential oil, when used at 2x MIC, caused PAFE which lasted 10 h against C. albicans ATCC 10231 and 5 h against C. albicans ATCC 90028. Citronella oil caused similarly strong PAFE, effectively inhibiting regrowth of C. albicans ATCC 10231 after 1 h at MIC and after 4 h at 2x MIC. The PAFE against C. albicans ATCC 90028 lasted even longer—5 h at MIC and 7 h at 2x MIC. The PAFE of Geranium oil and Clove oil, both used at MIC, was weaker (1 h and 3 h, against C. albicans ATCC 10231 and C. albicans ATCC 90028, respectively). The- se two EOs, when used at 2x MIC, caused PAFE which was comparable strong with that of the above described Lemon balm and Citronella oil. Due to the specific physical and chemical properties of essential oils, such as low density and oiliness, investiga- Table 2. Fungicidal activity (minimal fungicidal concentrations, MFCs—% v/v) of selected essentials oils: 1—Lavender oil, 2—Lemon balm, 3—Citronella oil, 4—Geranium oil, oil, 5—Clove oil. MFC range (% v/v) Candida strains 1 2 3 4 5 C. albicans ATCC 10231 0.19 0.097 0.097 0.19 0.19 C. albicans ATCC 90028 0.19 0.097 0.097 0.19 0.19 C. albicans clin., n = 20 0.097 - 0.78 0.048 - 0.19 0.048 - 0.39 0.048 - 0.39 0.048 - 0.78 C. glabrata clin., n = 13 0.048 - 1.56 0.12 - 1.56 0.024 - 1.56 0.024 - 0.78 0.048 - 1.56 C. krusei clin., n = 6 0.39 - 1.56 0.78 - 1.56 0.048 - 1.56 0.019 - 0.78 0.39 - 1.56 C. parapsilosis clin., n = 5 0.048 - 1.56 0.048 - 0.78 0.048 - 1.56 0.097 - 1.56 0.097 - 1.56 C. tropicalis clin., n = 6 0.024 - 0.19 0.048 - 0.097 0.097 - 0.19 0.048 - 0.097 0.097 - 0.39 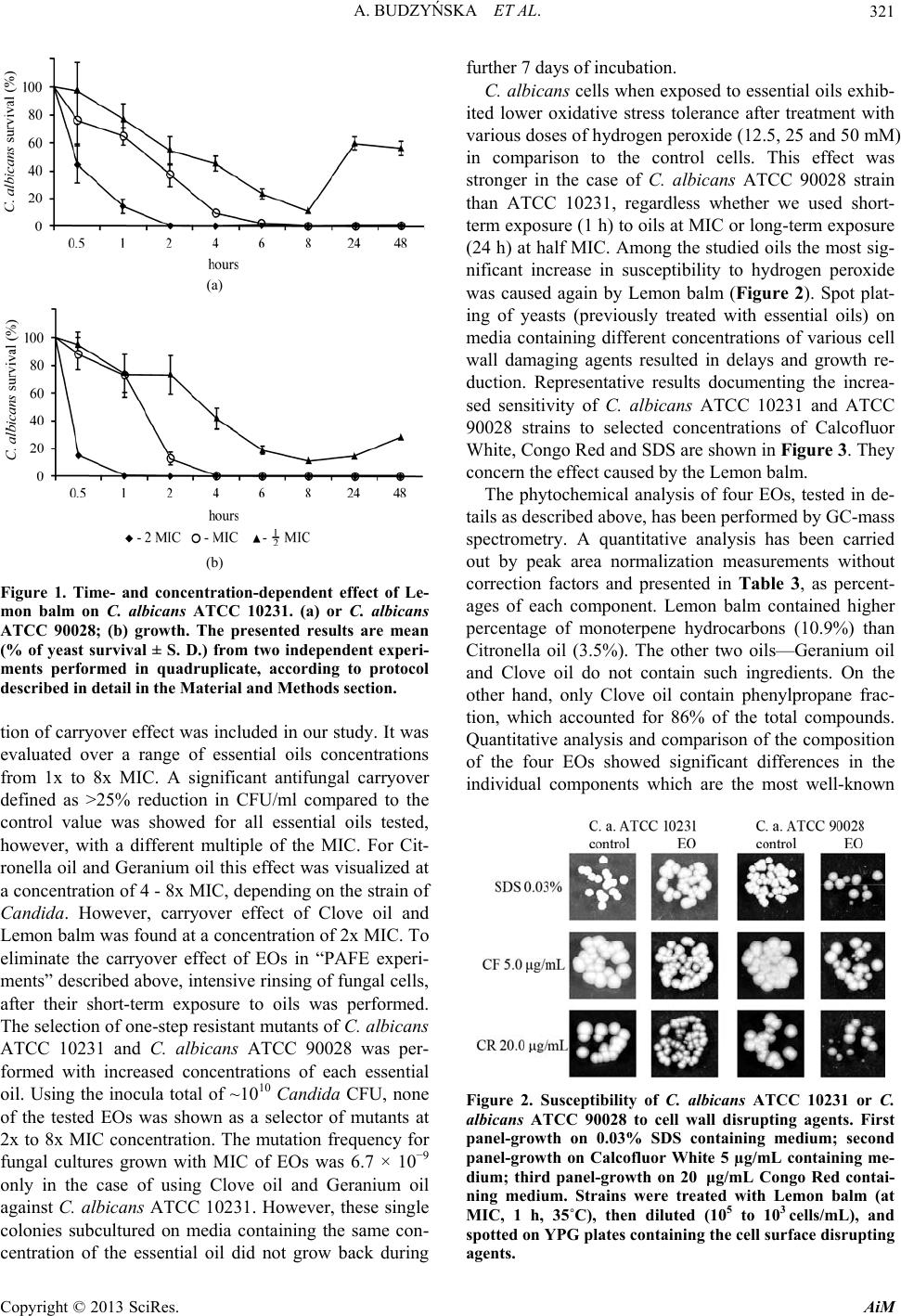 A. BUDZYŃSKA ET AL. 321 (a) (b) Figure 1. Time- and concentration-dependent effect of Le- mon balm on C. albicans ATCC 10231. (a) or C. albicans ATCC 90028; (b) growth. The presented results are mean (% of yeast survival ± S. D.) from two independent experi- ments performed in quadruplicate, according to protocol described in detail in the Material and Methods section. tion of carryover effect was included in our study. It was evaluated over a range of essential oils concentrations from 1x to 8x MIC. A significant antifungal carryover defined as >25% reduction in CFU/ml compared to the control value was showed for all essential oils tested, however, with a different multiple of the MIC. For Cit- ronella oil and Geranium oil this effect was visualized at a concentration of 4 - 8x MIC, depending on the strain of Candida. However, carryover effect of Clove oil and Lemon balm was found at a concentration of 2x MIC. To eliminate the carryover effect of EOs in “PAFE experi- ments” described above, intensive rinsing of fungal cells, after their short-term exposure to oils was performed. The selection of one-step resistant mutants of C. albicans ATCC 10231 and C. albicans ATCC 90028 was per- formed with increased concentrations of each essential oil. Using the inocula total of ~1010 Candida CFU, none of the tested EOs was shown as a selector of mutants at 2x to 8x MIC concentration. The mutation frequency for fungal cultures grown with MIC of EOs was 6.7 × 10−9 only in the case of using Clove oil and Geranium oil against C. albicans ATCC 10231. However, these single colonies subcultured on media containing the same con- centration of the essential oil did not grow back during further 7 days of incubation. C. albicans cells when exposed to essential oils exhib- ited lower oxidative stress tolerance after treatment with various doses of hydrogen peroxide (12.5, 25 and 50 mM) in comparison to the control cells. This effect was stronger in the case of C. albicans ATCC 90028 strain than ATCC 10231, regardless whether we used short- term exposure (1 h) to oils at MIC or long-term exposure (24 h) at half MIC. Among the studied oils the most sig- nificant increase in susceptibility to hydrogen peroxide was caused again by Lemon balm (Figure 2). Spot plat- ing of yeasts (previously treated with essential oils) on media containing different concentrations of various cell wall damaging agents resulted in delays and growth re- duction. Representative results documenting the increa- sed sensitivity of C. albicans ATCC 10231 and ATCC 90028 strains to selected concentrations of Calcofluor White, Congo Red and SDS are shown in Figure 3. They concern the effect caused by the Lemon balm. The phytochemical analysis of four EOs, tested in de- tails as described above, has been performed by GC-mass spectrometry. A quantitative analysis has been carried out by peak area normalization measurements without correction factors and presented in Table 3, as percent- ages of each component. Lemon balm contained higher percentage of monoterpene hydrocarbons (10.9%) than Citronella oil (3.5%). The other two oils—Geranium oil and Clove oil do not contain such ingredients. On the other hand, only Clove oil contain phenylpropane frac- tion, which accounted for 86% of the total compounds. Quantitative analysis and comparison of the composition of the four EOs showed significant differences in the individual components which are the most well-known Figure 2. Susceptibility of C. albicans ATCC 10231 or C. albicans ATCC 90028 to cell wall disrupting agents. First panel-growth on 0.03% SDS containing medium; second panel-growth on Calcofluor White 5 µg/mL containing me- dium; third panel-growth on 20 μg/mL Congo Red contai- ning medium. Strains were treated with Lemon balm (at MIC, 1 h, 35˚C), then diluted (105 to 103 cells/mL), and spotted on YPG plates containing the cell surface disrupting agents. Copyright © 2013 SciRes. AiM 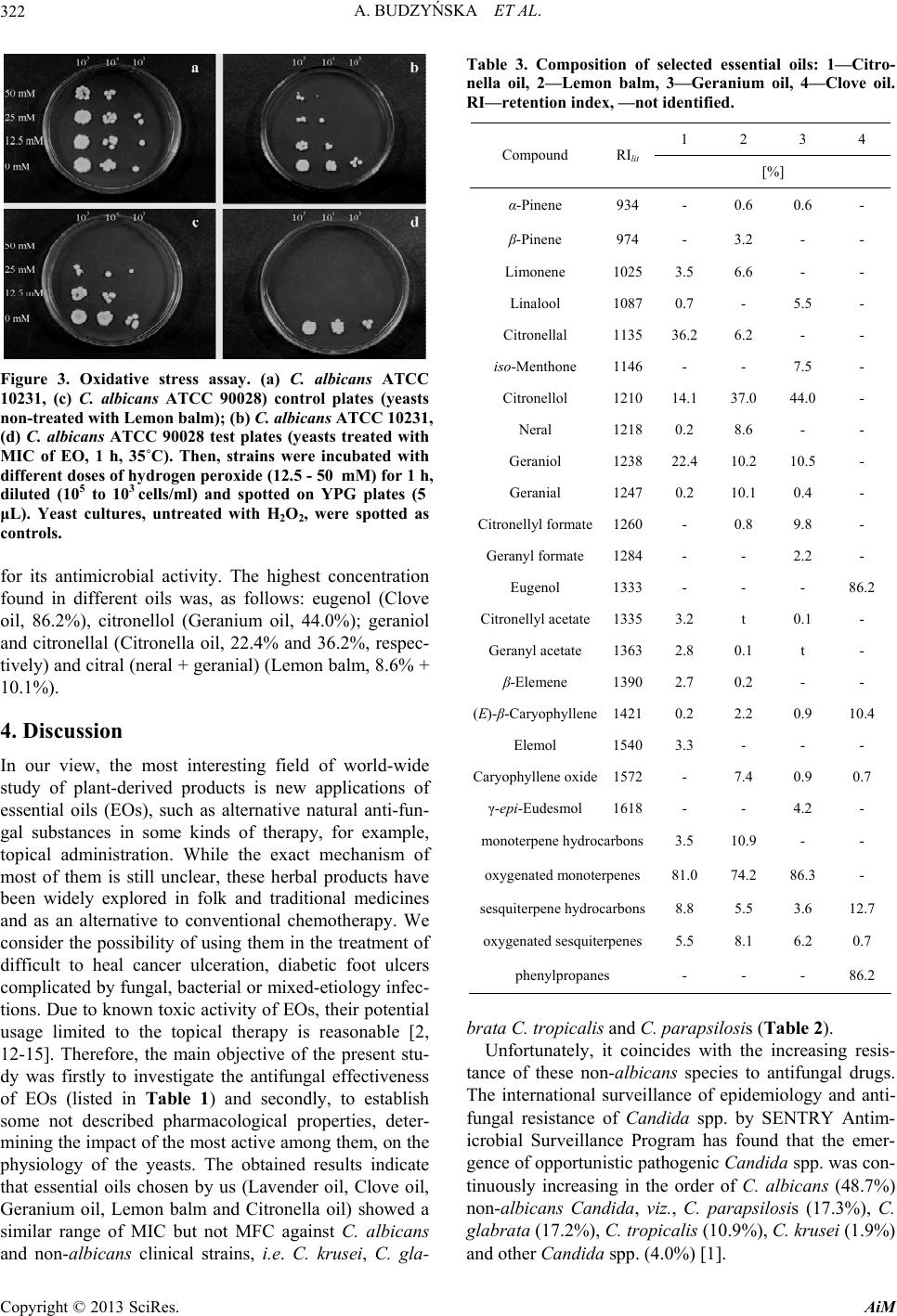 A. BUDZYŃSKA ET AL. 322 Figure 3. Oxidative stress assay. (a) C. albicans ATCC 10231, (c) C. albicans ATCC 90028) control plates (yeasts non-treated with Lemon balm); (b) C. albicans ATCC 10231, (d) C. albicans ATCC 90028 test plates (yeasts treated with MIC of EO, 1 h, 35˚C). Then, strains were incubated with different doses of hydrogen peroxide (12.5 - 50 mM) for 1 h, diluted (105 to 103 cells/ml) and spotted on YPG plates (5 μL). Yeast cultures, untreated with H2O2, were spotted as controls. for its antimicrobial activity. The highest concentration found in different oils was, as follows: eugenol (Clove oil, 86.2%), citronellol (Geranium oil, 44.0%); geraniol and citronellal (Citronella oil, 22.4% and 36.2%, respec- tively) and citral (neral + geranial) (Lemon balm, 8.6% + 10.1%). 4. Discussion In our view, the most interesting field of world-wide study of plant-derived products is new applications of essential oils (EOs), such as alternative natural anti-fun- gal substances in some kinds of therapy, for example, topical administration. While the exact mechanism of most of them is still unclear, these herbal products have been widely explored in folk and traditional medicines and as an alternative to conventional chemotherapy. We consider the possibility of using them in the treatment of difficult to heal cancer ulceration, diabetic foot ulcers complicated by fungal, bacterial or mixed-etiology infec- tions. Due to known toxic activity of EOs, their potential usage limited to the topical therapy is reasonable [2, 12-15]. Therefore, the main objective of the present stu- dy was firstly to investigate the antifungal effectiveness of EOs (listed in Table 1) and secondly, to establish some not described pharmacological properties, deter- mining the impact of the most active among them, on the physiology of the yeasts. The obtained results indicate that essential oils chosen by us (Lavender oil, Clove oil, Geranium oil, Lemon balm and Citronella oil) showed a similar range of MIC but not MFC against C. albicans and non-albicans clinical strains, i.e. C. krusei, C. gla- Table 3. Composition of selected essential oils: 1—Citro- nella oil, 2—Lemon balm, 3—Geranium oil, 4—Clove oil. RI—retention index, —not identified. 1 2 3 4 Compound RIlit [%] α-Pinene 934 - 0.6 0.6 - β-Pinene 974 - 3.2 - - Limonene 10253.5 6.6 - - Linalool 10870.7 - 5.5 - Citronellal 113536.2 6.2 - - iso-Menthone 1146- - 7.5 - Citronellol 121014.1 37.0 44.0 - Neral 12180.2 8.6 - - Geraniol 123822.4 10.2 10.5 - Geranial 12470.2 10.1 0.4 - Citronellyl formate1260- 0.8 9.8 - Geranyl formate 1284- - 2.2 - Eugenol 1333- - - 86.2 Citronellyl acetate13353.2 t 0.1 - Geranyl acetate 13632.8 0.1 t - β-Elemene 13902.7 0.2 - - (E)-β-Caryophyllene14210.2 2.2 0.9 10.4 Elemol 15403.3 - - - Caryophyllene oxide1572- 7.4 0.9 0.7 γ-epi-Eudesmol 1618- - 4.2 - monoterpene hydrocarbons3.5 10.9 - - oxygenated monoterpenes 81.0 74.2 86.3 - sesquiterpene hydrocarbons8.8 5.5 3.6 12.7 oxygenated sesquiterpenes5.5 8.1 6.2 0.7 phenylpropanes - - - 86.2 brata C. tropicalis and C. parapsilosis (Table 2). Unfortunately, it coincides with the increasing resis- tance of these non-albicans species to antifungal drugs. The international surveillance of epidemiology and anti- fungal resistance of Cand ida spp. by SENTRY Antim- icrobial Surveillance Program has found that the emer- gence of opportunistic pathogenic Candida spp. was con- tinuously increasing in the order of C. albicans (48.7%) non-albicans Candida, viz., C. parapsilosis (17.3%), C. glabrata (17.2%), C. tropicalis (10.9%), C. krusei (1.9%) and other Candida spp. (4.0%) [1]. Copyright © 2013 SciRes. AiM  A. BUDZYŃSKA ET AL. 323 Several of the 16 oils tested by us, which were further thoroughly examined (Clove oil, Geranium oil, Lemon balm and Citronella oil), showed strong effect on the cell wall and membrane of C. albicans. Furthermore, they caused long-lasting PAF effect and were shown as safe, because they did not generate resistance in one-step as- say. Time-kill experiments revealed that all these EOs were fungicidal and acted quickly (starting from 0.5 h of co-incubation). The most effective was essential oil of Melissa citrata indica herb (Lemon balm), which caused the quickest decrease in cell density of C. albicans ATCC 90028 and less evident effect towards C. albicans ATCC 10231. What is more interestingly, a 1-hour ex- posure of C. albicans ATCC 90028 to Lemon balm re- sulted in the longest PAFE. To our knowledge, this re- port is the first one describing such effects of essential oils. Furthermore, MPC (mutant prevention concentra- tion) of this oil, tested by single-step methodology was equal to or twice as high as their MICs, thus MSW (mu- tant selection window) was narrow [16-18]. One can ask a question what is so special in the phys- icochemical properties of Melissa essential oil that it exhibits such high activity against Candida. Until re- cently it was known that extracts and essential oils of Melissa sp. are used rather in traditional medicine to treat insomnia, anxiety, gastric conditions, psychiatric condi- tions, migraines, hypertension and bronchial conditions. Their antimicrobial/antiviral activity, although described, is definitely less known and no specific characterization has been provided, especially concerning anti-Candida activity. What was reported earlier the most important identified compounds of Lemon balm showing antimi- crobial effects were geraniol, citral, citronellal, and trans- caryophyllene which are also present in the composition of other essential oils with proven antimicrobial activity [19-21]. Since different chemotypes of the same species may grow in the same place, both the composition and antimicrobial activity may differ, even in the case of commercial essential oils. Therefore, it is important that the composition of the essential oil tested has to be given each time [22]. The main components of Melissa citrata indica essential oils tested in this study were identified by GC-mass spectrometer analyses. The percentage compositions is listed in Table 3. Indeed, citral (neral and geranial) was the main component of Lemon balm and according to many authors these aldehydes show high antimicrobial activity [13,19,22-24]. It is possible, however, that these and other compounds quantity/qua- lity composition is relevant to Lemon balm highest anti-Candidal activity. It is known that the efficacy of the whole herb extract/oil may lie on the low doses of the active constituents present in an herbal product altogether. Quantitative analysis and comparison of the composition of the four EOs tested in the present report showed sig- nificant differences in the individual components which are the most well-known for its antimicrobial activity i.e. eugenol, citronellol, geraniol, citronellal, and citral (neral + geranial). Although oil of Melissa showed the most desirable properties, the other oils are also worthy of consideration as a potentially useful. The results of our study concerning the action of Le- mon balm and three other selected EOs clearly indicate, that the phenotypic features of Cand id a yeasts that de- cide about their success as invasive pathogens, were tar- gets for their activity. The fungal cell wall is composed primarily of polysaccharides such as glucans, mannans, chitin and chitosan. It also contains proteins and lipids, which are often associated with these polysaccharides [25]. The knowledge of the participation of C. albicans products in the pathogenesis of infections has substan- tially expanded, but it is still incomplete in many aspects. Nevertheless, it is known that their multilayer, hard per- meable cell wall is a strong barrier to the operation of antifungal drugs. Thus, it seems advisable to search for products which could weaken their structure. Essential oils, even if used at low concentrations have the desired characteristic, which has been proved in our study. It is generally assumed that the mechanisms by which the constituents of essential oils inhibit the growth of micro- organisms may be partially dependent on their hydro- phobicity. It enables them to embed in the cell wall, damage the lipid layer of the cell membrane and mito- chondria, impair enzyme systems and exhibit side effects on various proteins [4,5,13,14]. Some of them inhibit microbial growth by causing also a global arrest in pro- tein synthesis or inducing cytoplasm coagulation [13]. Our results have shown that essential oils cause changes in the composition of the cell wall of Ca nd ida , since its sensitivity to Calcofluor White, Congo Red, or SDS def- initely have increased. It has been reported that Cal- cofluor binds to β-linked fibrillar polymers, interferes with chitin assembly resulting in growth rate reduction, and alteres incorporation of mannoproteins into cell wall. Growth inhibition by detergent SDS was connected with an increases in wall porosity, solubilization of the plasma membrane, whereas Congo Red altered glucan synthesis and assembly [4,25,26]. However, by weakening the cell wall by essential oil action, effects of the above cell-wall disrupting agents increased, rendering the cell more sus- ceptible to their lower concentrations, which we have shown in our report (Figure 2). Another question which we asked was if an efficient oxidative stress response of Candida will be affected by essential oils action. The response may be of clinical interest, since it is important for C. albicans invasion and colonization of host tissues and survival within the host cells (phagocytes) in the course of an in vivo infection. C. albicans strains show in vitro a great natural resistance to Copyright © 2013 SciRes. AiM  A. BUDZYŃSKA ET AL. 324 H2O2 (10 - 50 mM). It has been reported that various H2O2 treatments have distinct effects on antioxidant en- zymes (catalase, superoxide dismutase, glutathione oxi- dase) [27-29]. Therefore, our observation that preincuba- tion of C. albicans with essential oils (either short with MIC or longer with 1/2 MIC) decreased tolerance to oxi- dative stress induced by all concentrations of H2O2 used (12.5, 25.0, 50.0 mM) suggests, that the activity of vari- ous anti-oxidative enzymes could be decreased. It cannot be ruled out that the architecture of the cell wall pro- teome might be changed by possibly preventing correct positioning and anchoring of cell wall localized super- oxide dismutase or other proteins that are directly or in- directly responsible for countering oxidative stress dam- age [14,20,27-29]. Our results indicate that some essen- tial oils with antifungal activity, such as Lemon balm, can be considered in the future for more clinical evalua- tions and possible applications, other than the one cur- rently used. For example, they could be introduced into modern palliative care in patients with cancer fungating wounds or diabetic foot [22,30-33]. 5. Acknowledgements The work was supported by the National Research Cen- ter, Poland, Grant No. 2011/01/N/NZ6/00317, and by University of Lodz (2012) for A.B. The authors wish to thank M. Więckowska-Szakiel for technical assistance. REFERENCES [1] S. A. Messer, R. N. Jones and T. R. Fritsche, “Interna- tional Surveillance of Candida spp. and Aspergillus spp. Report from the SENTRY Antimicrobial Surveillance Program,” Journal of Clinical Microbiology, Vol. 44, No. 5, 2006, 2003, pp. 1782-1787. doi:10.1128/JCM.44.5.1782-1787.2006 [2] E. Mlinarić-Missoni, S. Kalenić, M. Vukelić, D. De Syo, M. Belicza and V. Vazić-Babić, “Candida Infections of Diabetic Foot Ulcers,” Diabetologia Croatica, Vol. 34, No 1, 2005, pp. 29-35. [3] R. Rajeshkumar and M. Sundararaman, “Emergence of Candida spp. and Exploration of Natural Bioactive Mol- ecules for AntiCandidal Therapy—Status Quo,” Mycoses, Vol. 55, No. 3, 2012, pp. e60-e73. doi:10.1111/j.1439-0507.2011.02156.x [4] F. Silva, S. Ferreira, A. Duarte, D. I. Mendonca and F. C. Domingues, “Antifungal Activity of Coriandrum sativum Essential Oil, Its Mode of Action against Candida Spe- cies and Potential Synergism with Amphotericin B,” Phy- tomedicine, Vol. 19, No. 1, 2011, pp. 42-47. doi:10.1016/j.phymed.2011.06.033 [5] F. Solórzano-Santos and M. G. Miranda-Novales, “Essen- tial Oils from Aromatic Herbs as Antimicrobial Agents,” Current Opinion in Biotechnology, Vol. 23, No. 2, 2012, pp. 136-141. doi:10.1016/j.copbio.2011.08.005 [6] Mikolajewska, S. Schwartz and M. Ruhnke, “Antifungal Treatment Strategies in Patients with Haematological Diseases and Cancer: From Prophylaxis to Empirical, Pre-Emptive and Targeted Therapy,” Mycoses, Vol. 55, No. 1, 2012, pp. 2-16. doi:10.1111/j.1439-0507.2010.01961.x [7] P. Pozzatti, E. S. Loreto, P. G. M. Lopes, M. L. Athayde, J. M. Santurio and S. H. Alves, “Comparison of the Sus- ceptibilities of Clinical Isolates of Candida albicans and Candida dubliniensis to Essential Oils,” Mycoses, Vol. 53, No. 1, 2010, pp. 12-15. doi:10.1111/j.1439-0507.2008.01643.x [8] J. Reichling, P. Schnitzler, U. Suschke and R. Saller, “Essential Oils of Aromatic Plants with Antibacterial, Antifungal, Antiviral, and Cytotoxic Properties—An Over- view,” Forschende Komplementarmedizin, Vol. 16, No. 2, 2009, pp. 79-90. doi:10.1159/000207196 [9] A. Rosato, C. Vitali, D. Gallo, L. Balenzano, R. Malla- maci, “The Inhibition of Candida Species by Selected Essential Oils and Their Synergism with Amphotericin B,” Phytomedicine, Vol. 15, No. 8, 2008, pp. 635-638. doi:10.1016/j.phymed.2008.05.001 [10] A. Budzyńska, M. Więckowska-Szakiel, D. Kalemba, B. Sadowska and B. Różalska, “The Optimization of Meth- ods Utilized for Testing the Antibacterial Activity of Es- sential Oils,” Medycyna Doświadczalna i Mikrobiologia, Vol. 61, No. 3, 2009, pp. 281-287 (in polish). [11] A. Budzyńska, M. Więckowska-Szakiel, B. Sadowska, D. Kalemba and B. Różalska, “Antibiofilm Activity of Se- lected Plant Essential Oils and Their Major Components,” Polish Journal of Microbiology, Vol. 60, No. 1, 2011, pp. 35-41. [12] M. J. Abad, M. Ansuategui and P. Bermejo, “Active An- tifungal Substances from Natural Sources,” Arkivoc: Ar- chive for Organic Chemistry, Vol. vii, No. 7, 2007, pp. 116-145. [13] F. Bakkali, S. Averbeck, D. Averbeck and M. Idaomarb, “Biological Effects of Essential Oils—A Review,” Food and Chemical Toxicology, Vol. 46, No. 2, 2008, pp. 446- 475. doi:10.1016/j.fct.2007.09.106 [14] A. N. Devkatte, G. B. Zore and S. M. Karuppayil, “Po- tential of Plant Oils as Inhibitors of Candida albicans Growth,” FEMS Yeast Research, Vol. 5, No. 9, 2005, pp. 867-873. doi:10.1016/j.femsyr.2005.02.003 [15] R. Khosravi, H. Shokri, S. Kermani, M. Dakhili, M. Ma- dani and S. Parsa, “Antifungal Properties of Artemisia sieberi and Origanum vulgare Essential Oils against Candida glabrata Isolates Obtained from Patients with Vulvovaginal Candidiasis,” Journal of Medical Mycology, Vol. 21, No. 2, 2011, pp. 93-99. doi:10.1016/j.mycmed.2011.01.006 [16] K. Credito, K. Kosowska-Shick and P. C. Appelbaum, “Mutant Prevention Concentration (MPC) of Four Car- bapenems against Gram-Negative Rods,” Antimicrobial Agents and Chemotherapy, Vol. 54, No. 6, 2010, pp. 2692-2695. doi:10.1128/AAC.00033-10 [17] K. Drlica and M. Malik, “Fluoroquinolones: Action and Resistance,” Current Topics in Medicinal Chemistry, Vol. 3, No. 3, 2002, pp. 249-282. Copyright © 2013 SciRes. AiM  A. BUDZYŃSKA ET AL. Copyright © 2013 SciRes. AiM 325 doi:10.2174/1568026033452537 [18] M. E. Klepser, E. J. Ernst, R. E. Lewis, M. E. Ernst and M. A. Pfaller, “Influence of Test Conditions on Antifun- gal Time-Kill Curve Results: Proposal for Standardized Methods,” Antimicrobial Agents and Chemotherapy, Vol. 42, No. 5, 1998, pp. 1207-1212. [19] M. Hăncianu, A. C. Aprotosoaie, E. Gille, A. Poiată, C. Tuchiluş, A. Spac and U. Stănescu, “Chemical Composi- tion and in Vitro Antimicrobial Activity of Essential Oil of Melissa officinalis L. from Romania,” Revista medico- chirurgicala a Societatti de Medici si Naturalisti din Iasi, Vol. 112, No. 3, 2008, pp. 843-847. [20] N. Mimica-Dukic, B. Bozin, M. Sokovic and N. Simin, “Antimicrobial and Antioxidant Activities of Melissa of- ficinalis L. (Lamiaceae) Essential Oil,” Journal of Agri- cultural and Food Chemistry, Vol. 52, No. 9, 2004, pp. 2485-2489. doi:10.1021/jf030698a [21] A. K. Tyagi and A. Malik, “Liquid and Vapour-Phase Antifungal Activities of Selected Essential Oils against Candida albicans: Microscopic Observations and Chemi- cal Characterization of Cymbopogon citratus,” BMC Complementary and Alternative Medicine, Vol. 10, 2010, p. 65. doi:10.1155/2012/692625 [22] A. E. Edris, “Pharmaceutical and Therapeutic Potentials of Essential Oils and their Individual Volatile Constitu- ents: A Review,” Phytotherapy Research, Vol. 21, No. 4, 2007, pp. 308-323. doi:10.1002/ptr.2072 [23] D. Kalemba and A. Kunicka, “ Antibacterial and Anti- fungal Properties of Essential Oils,” Current Medicinal Chemistry, Vol. 10, No. 10, 2003, pp. 813-829. doi:10.2174/0929867033457719 [24] J. Kim, M.R Marschal, C. Wei, “Antibacterial Activity of Some Essential Oil Components against Five Foodborne Pathogens,” Journal of Agricultural Chemistry, Vol. 43, No. 11, 1995, pp. 2839-2845. doi:10.1021/jf00059a013 [25] R. Hashash, S. Younes, W. Bahnan, J. El Koussa, K. Maalouf, H. I. Dimassi and R. A. Khalaf, “Characterisa- tion of Pga1, a Putative Candida albicans Cell Wall Pro- tein Necessary for Proper Adhesion and Biofilm Forma- tion,” Mycoses, Vol. 54, No. 6, 2011, pp. 491-500. doi:10.1111/j.1439-0507.2010.01883.x [26] R. K. Shields, M. H. Nguyen, E. Press and C. J. Clancy, “Five-Minute Exposure to Caspofungin Results in Pro- longed Post-Antifungal Effects and Eliminates the Para- doxical Growth of Candida albicans,” Antimicrobial Agents and Chemotherapy, Vol. 55, No.7, 2011, pp. 3598-3602. doi:10.1128/AAC.00095-11 [27] T. Missall, J. K. Lodge and J. E. McEwen, “Mechanisms of Resistance to Oxidative and Nitrosative Stress. Impli- cations for Fungal Survival in Mammalian Hosts,” Eu- karyotic Cell, Vol. 3, No. 4, 2004, pp. 835-846. doi:10.1128/EC.3.4.835-846.2004 [28] A. Bink, D. Vandenbosch, T. Coenye, H. Nelis, B. P. A. Cammue and K. Thevissen, “Superoxide Dismutases Are Involved in Candida albicans Biofilm Persistence against Miconazole,” Antimicrobial Agents and Chemotherapy, Vol. 55, No. 9, 2011, pp. 4033-4037. doi:10.1128/AAC.00280-11 [29] A. Enjalbert, D. M. MacCallum, F. C. Odds and A. J. P. Brown, “Niche-Specific Activation of the Oxidative Stress Response by the Pathogenic Fungus Candida albi- cans,” Infection and Immunity, Vol. 75, No. 5, 2007, pp. 2143-2151. doi:10.1128/IAI.01680-06 [30] P. H. Warnke, E. Sherry, P. A. J. Russo, Y. Acil, J. Wilt- fang, S. Sivananthan, J. C. Roldàn, S. Schubert, J. P. Bredee and I. N. Springer, “Antibacterial Essential Oils in Malodorous Cancer Patients: Clinical Observations in 30 Patients,” Phytomedicine, Vol. 13, No. 7, 2006, pp. 463- 467. doi:10.1016/j.phymed.2005.09.012 [31] S. Hampton, “Malodorous Fungating Wounds: How Dre- ssings Alleviate Symptoms,” British Journal of Commu- nity Nursing, Vol. 13, No. 6, 2008, pp. S34-S36. [32] D. Mercier and A. Knevitt, “Using Topical Aromatherapy for the Management of Fungating Wounds in a Palliative Care Unit,” Journal of Wound Care, Vol. 14, No. 10, 2005, pp. 497-498. [33] E. Sherry, H. Boeck and P. H. Warnke, “Topical Applica- tion of a New Formulation of Eucalyptus Oil Phytoche- mical Clears Methicillin-Resistant Staphylococcus aureus Infection,” American Journal of Infection Control, Vol. 29, No. 5, 2001, pp. 346. doi:10.1067/mic.2001.117403
|