 Vol.3, No.3, 101-110 ( 2013) Journal of Diabetes Mellitus http://dx.doi.org/10.4236/jdm.2013.33015 12/15-Lipoxygenase inhibition counteracts MAPK phosphorylation in mouse and cell culture models of diabetic peripheral neuropathy Roman Stavniichuk1, Alexander A. Obrosov1, Viktor R. Drel1, Jerry L. Nadler2, Irina G. Obrosova1, Mark A. Yorek3* 1Pennington Biomedical Research Center, Louisiana State University System, Baton Rouge, USA 2Department of Internal Medicine, Eastern Virginia Medical School, Norfolk, USA 3Department of Veterans Affairs Iowa City Health Care System and Department of Internal Medicine, University of Iowa, Iowa City, USA; *Corresponding Author: mark-yorek@uiowa.edu Received 14 May 2013; revised 16 June 2013; accepted 23 June 2013 Copyright © 2013 Roman Stavniichuk et al. This is an open access article distributed under the Creative Commons Attribution Li- cense, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Background: Increased mitogen-activated pro- tein kinase (MAPK) phosphorylation has been detected in peripheral nerve of human subjects and animal models with diabetes as well as high-glucose exposed human Schwann cells, and have been implicated in diabetic peripheral neuropathy. In our recent studies, leukocyte- type 12/15-lipoxygenase inhibition or gene defi- ciency alleviated large and small nerve fiber dysfunction, but not intraepidermal nerve fiber loss in streptozotocin-diabetic mice. Methods: To address a mechanism we evaluated the po- tential for pharmacological 12/15-lipoxygenase inhibition to counteract excessive MAPK phos- phorylation in mouse and cell culture models of diabetic neuropathy. C57Bl6/J mice were made diabetic with streptozotocin and maintained with or without the 12 /1 5-lipoxygenase inhibitor c innamyl-3,4-dihydroxy-α-cyanocinnamate (CDC). Human Schwann cells were cultured in 5.5 mM or 30 mM glucose with or without CDC. Results: 12(S) HETE concentrations (ELISA), as well as 12/15-lipoxygenase expression and p38 MAPK, ERK, and SAPK/JNK phosphorylation (all by Western blot analysis) were increased in the peripheral nerve and spinal cord of diabetic mice as well as in high glucose-exposed human Schwann cells. CDC counteracted diabetes-in- duced increase in 12(S)HETE concentrations (a measure of 12/15-lipoxygenase activity), but not 12/15-lipoxygenase overexpression, in sciatic nerve and spinal cord. The inhibitor blunted excessive p38 MAPK and ERK, but not SAPK/ JNK, phosphorylation in sciatic nerve and high glucose exposed human Schwann cells, but did not affect MAPK, ERK, and SAPK/JNK phos- phorylation in spinal cord. Conclusion: 12/15- lipoxygenase inhibition counteracts diabetes related MAPK phosphorylation in mouse and cell culture models of diabetic neuropathy and implies that 12/15-lipoxygenase inhibitors may be an effective treatment for diabetic peripheral neuropathy. Keywords: Diabetes; Lipoxygenase; Neuropathy; Schwann Cells; Mitogen -Activated Protein Kinase 1. INTRODUCTION Diabetic peripheral neuropathy (DPN), affects at least 50% of patients with both Type 1 and Type 2 diabetes, and is a leading cause of foot amputation [1-3]. DPN is manifested by motor (MNCV) and sensory (SNCV) nerve conduction velocity deficits as well as microvas- cular dysfunction and by increased vibration and thermal perception thresholds that progress to sensory loss, oc- curring in conjunction with degeneration of all fiber types in the peripheral nerve [1-4]. A significant propor- tion of patients with DPN also describe abnormal sensa- tions such as paresthesias, allodynia, hyperalgesia, and spontaneous pain [3]. The pathogenesis of DPN has ex- tensively been studied in animal and cell culture models. Multiple biochemical changes have been attributed to the etiology of diabetic neuropathy including, but not limited to, increased activity of the sorbitol pathway [5-7], gen- Copyright © 2013 SciRes. OPEN A CCESS  R. Stavniichuk et al. / Journal of Diabetes Mellitus 3 (2013) 101-110 102 eration of methylglyoxal [8,9] and non-enzymatic glyca- tion/glycoxidation [10,11], oxidative-nitrosative stress [12-16], impaired neurotrophic support [17,18], and ac- tivation of protein kinase C [19,20], poly(ADP-ribose) polymerase [21,22], and of the enzymes of arachidonic acid metabolism, cyclooxygenase-2 [23] and leukocyte- type 12/15-lipoxygenase [24,25]. Increased mitogen- activated protein kinase (MAPK) phosphorylation was detected in peripheral nerve of human subjects with dia- betes [26], several animal models of diabetes [26-31], and high-glucose exposed cultured human Schwann cells [32], and has been implicated in the pathophysiology of diabetic peripheral neuropathy. Multiple specific small molecule MAPK inhibitors are now in clinical trials for chronic diseases including sev- eral types of cancer, inflammatory and autoimmune dis- eases, neuropathic pain following nerve trauma, as well as Parkinson’s and Alzheimer’s diseases [33]. Regardless of these efforts and outcomes, the search for alternative approaches to inhibit excessive MAPK phosphorylation in specific pathological conditions including diabetic neuropathy is highly warranted. In our previous experi- ments [24,25,31], 12/15-lipoxygenase inhibition and gene deficiency improved several diabetic neuropathy associated endpoints in streptozotocin-diabetic mice in- cluding nerve conduction deficits and behavioral changes suggesting that preventing 12/15-lipoxygenase activation may be an effective treatment for diabetic neuropathy. 2. MATERIALS AND METHODS 2.1. Reagents Unless otherwise stated, all chemicals were of re- agent-grade quality, and were purchased from Sigma Chemical Co., St. Louis, MO, USA. Cinnamyl-3,4- dihydroxy-alpha-cyanocinnamate (CDC) was obtained from Enzo Life Sciences International, Plymouth Meet- ing, PA, USA. For Western blot analyses in mouse tis- sues, rabbit polyclonal (clone H-100) anti-12-lipoxy- genase (LO) antibody, rabbit polyclonal (clone H-147) anti-p38 MAPK antibody, mouse monoclonal (clone MK1) anti-ERK antibody, and rabbit polyclonal (clone C17) anti-JNK1 antibody were obtained from Santa Cruz Biotechnology, Santa Cruz, CA, USA. Rabbit polyclonal anti-phospho-p38 MAPK antibody, rabbit monoclonal (clone D13.14.4E) anti-phospho-ERK antibody, and rab- bit polyclonal anti-phospho-SAPK/JNK antibody were purchased from Cell Signaling Technology, Boston, MA, USA. For Western blot analyses in human Schwann cells, rabbit polyclonal (clone C16) antibody against total ERK and mouse monoclonal (clone E4) antibody against phosphorylated ERK were obtained from Santa Cruz Biotechnology, Santa Cruz, CA. For other MAPKs, the antibodies listed above were used. 2.2. Animals The experiments were performed in accordance with The Guide for the Care and Handling of Laboratory Animals (NIH Publication No. 85-23) and Pennington Biomedical Research Center Protocol for Animal Studies. Mature male C57Bl6/J mice were purchased from Jack- son Laboratories. All the mice were fed standard mouse chow (PMI Nutrition International, Brentwood, MO, USA) and had ad libitum access to water. After a 7-day acclimation in a new environment, the mice were ran- domly divided into two groups. In one group, diabetes was induced by streptozotocin (STZ) as we described previously [24,25]. The mice with blood glucose ≥13.8 mM, three days post streptozotocin were considered dia- betic. The control and diabetic mice were kept for 12 weeks without treatment, and then divided into two sub- groups that were maintained with or without treatment with cinnamyl-3,4-dihydroxy-α-cyanocinnamate (CDC), 8 mg kg/d, s.c., for another 4 weeks. CDC, at the afore- mentioned dose, counteracted multiple manifestations of diabetic neuroapthy and oxidative-nitrosative stress in peripheral nerve and spinal cord in our previous study [24]. Non-fasting blood glucose measurements were performed after induction of diabetes and at the end of the study period. 2.3. Anesthesia, Euthanasia and Tissue Sampling The animals were sedated by CO2, and immediately sacrificed by cervical dislocation. Sciatic nerves and spinal cords were rapidly dissected and frozen in liquid nitrogen for subsequent assessment of LO as well as total and phosphorylated p38 MAPK, ERK, and SAPK/JNK levels, and 12(S)-HETE concentrations. 2.4. Human Schwann Cell Culture Schwann cells play a key role in the pathology of various inflammatory, metabolic, and hereditary poly- neuropathies, including diabetic neuropathy [34,35]. Pre- vious studies demonstrated that cultured human Schwann cells (cell line cat. #1700, ScienCell, Carlsbad, CA) manifest increased superoxide production, accumulation of nitrated and poly(ADP-ribosyl)ated proteins and 4- hydroxynonenal adducts, inducible nitric oxide synthase overexpression, 12/15-Lipoxygenase overexpression and activation, increased p38 MAPK phosphorylation, down- regulation of taurine transporter, as well as impaired in- sulin signaling early (1 - 7 d) after exposure to high glu- cose [24,36-38]. They therefore represent a good model for studying interactions among individual pathobio- chemical mechanisms in the peripheral nerve. In the present study, human Schwann cells (passages 7 - 10) were cultured in 6-well plates in media containing 5.5 Copyright © 2013 SciRes. OPEN A CCESS  R. Stavniichuk et al. / Journal of Diabetes Mellitus 3 (2013) 101-110 103 mM D-glucose. At ~70% confluence, the media were replaced with those containing either 5.5 mM D-glucose or 30 mM D-glucose with or without CDC, 10 M (6 - 8 plates per condition). After 24 hr, the cells were used for assessment of total and phosphorylated p38 MAPK, ERK, and SAPK/JNK. 2.5. Specific Methods 2.5.1. Western Bl ot A nalyses of LO and Total and Phosphorylated p38 MAPK, ERK, and SAPK/JNK Sciatic nerve and spinal cord materials (~3 - 10 mg) or scraped human Schwann cells were placed on ice in 100 µL of buffer containing 50 mmol/l Tris-HCl, pH 7.2; 150 mmol/l NaCl; 0.1% sodium dodecyl sulfate; 1% NP-40; 5 mmol/l EDTA; 1 mmol/l EGTA; 1% sodium deoxy- cholate and the protease/ phosphatase inhibitors leu- peptin (10 μg/ml), pepstatin (1 μg/ml), aprotinin (20 μg/ ml), benzamidine (10 mM), phenylmethylsulfonyl fluo- ride (1 mM), sodium orthovanadate (1 mmol/l), and ho- mogenized on ice. The homogenates were sonicated and centrifuged at 14,000 g for 20 min. All the afore-men- tioned steps were performed at 4˚C. The lysates (20 μg protein for sciatic nerve and 40 μg for spinal cord and human Schwann cells) were mixed with equal volumes of 2x sample-loading buffer containing 62.5 mmol/l Tris- HCl, pH 6.8; 2% sodium dodecyl sulfate; 5% -mer- captoethanol; 10% glycerol, and 0.025% bromophenol blue, and fractionated in 10 % (total and phosphorylated MAPKs) or 7.5% (lipoxygenase) SDS-PAGE in an elec- trophoresis cell (Mini-Protean III; Bio-Rad Laboratories, Richmond, CA). Electrophoresis was conducted at 15 mA constant current for stacking, and at 25 mA for pro- tein separation. Gel contents were electrotransferred (80 V, 2 hr) to nitrocellulose membranes using Mini Trans- Blot cell (Bio-Rad Laboratories, Richmond, CA) and Western transfer buffer (10X Tris/Glycine buffer, Bio- Rad Laboratories, Richmond, CA) diluted with 20% (v/v) methanol. Free binding sites were blocked in 5% (w/v) BSA in 20 mmol/l Tris-HCl buffer, pH 7.5, containing 150 mmol/l NaCl and 0.05% Tween 20, for 1 h. Primary antibodies against 12/15-lipoxygenase, or phosphory- lated p38 MAPK, ERK, or SAPK/JNK were applied at 4˚C overnight, after which secondary antibodies were applied at room temperature for 1 h. After extensive washing, protein bands detected by the antibodies were visualized with the Amersham ECLTM Western Blotting Detection Reagent (Little Chalfont, Buckinghamshire, UK). Membranes previously probed for phosphorylated MAPKs were then stripped in the 25 mmol/l glycine-HCl buffer, pH 2.5, containing 2% SDS, and reprobed with antibodies against total p38 MAPK, ERK, and SAPK/ JNK, respectively. Membranes previously probed for 12/ 15-lipoxygenase were stripped again and reprobed with β-actin antibody to confirm equal protein loading. 2.5.2. ELISA 12(S)HETE Measurements For assessment of 12(S)HETE, sciatic nerve and spi- nal cord samples were homogenized on ice in 15 mM Tris-HCI buffer (1:100 w/v) containing 140 mM NaCl, pH 7.6, and centrifuged. 12(S)HETE was measured in supernatants with the 12(S)-hydroxyeicosatetraenoic acid [12(S)HETE] Enzyme Immuno Assay kit (Assay De- signs, Ann Arbor, MI) according to manufacturer’s in- structions. 2.6. Statistical Analysis The results are expressed as Mean ± SEM. Data were subjected to equality of variance F test, and then to log transformation, if necessary, before one-way analysis of variance. Where overall significance (p < 0.05) was at- tained, individual between group comparisons for multi- ple groups were made using the Student-Newman-Keuls multiple range test. When between-group variance dif- ferences could not be normalized by log transformation (datasets for body weights and plasma glucose), the data were analyzed by the nonparametric Kruskal-Wallis one- way analysis of variance, followed by the Bonferroni/ Dunn test for multiple comparisons. Individual pair-wise comparisons in experiments 3 and 4 were made using the unpaired two-tailed Student’s t-test or Mann-Whitney rank sum test where appropriate. Significance was de- fined at p < 0.05. 3. RESULTS 3.1. Animal Experiments 3.1.1. Body Weights and Blood Glucose Concentrations The initial (prior to streptozotocin administration) body weights were similar in all experimental groups (Table 1). Weight gain during the 16-wk study was lower in both untreated and CDC-treated diabetic mice than in the non-diabetic control group. CDC treatment did not affect weight gain in either control or diabetic mice. Ini- tial (after streptozotocin administration) non-fasting blood glucose concentrations were 2.0-fold and 1.9-fold higher in untreated and CDC-treated diabetic mice than in the control group. Hyperglycemia progressed with the prolongation of diabetes, and the differences between final blood glucose concentrations in both diabetic groups and non-diabetic controls exceeded 3-fold. CDC treat- ment did not affect non-fasting blood glucose concentra- tions in either non-diabetic or diabetic mice. 3.1.2. 12/15-Lipoxygenase Expression and 12(S)HETE Concentrations Sciatic nerve (Figures 1(a) and (b)) and spinal cord Copyright © 2013 SciRes. OPEN A CCESS 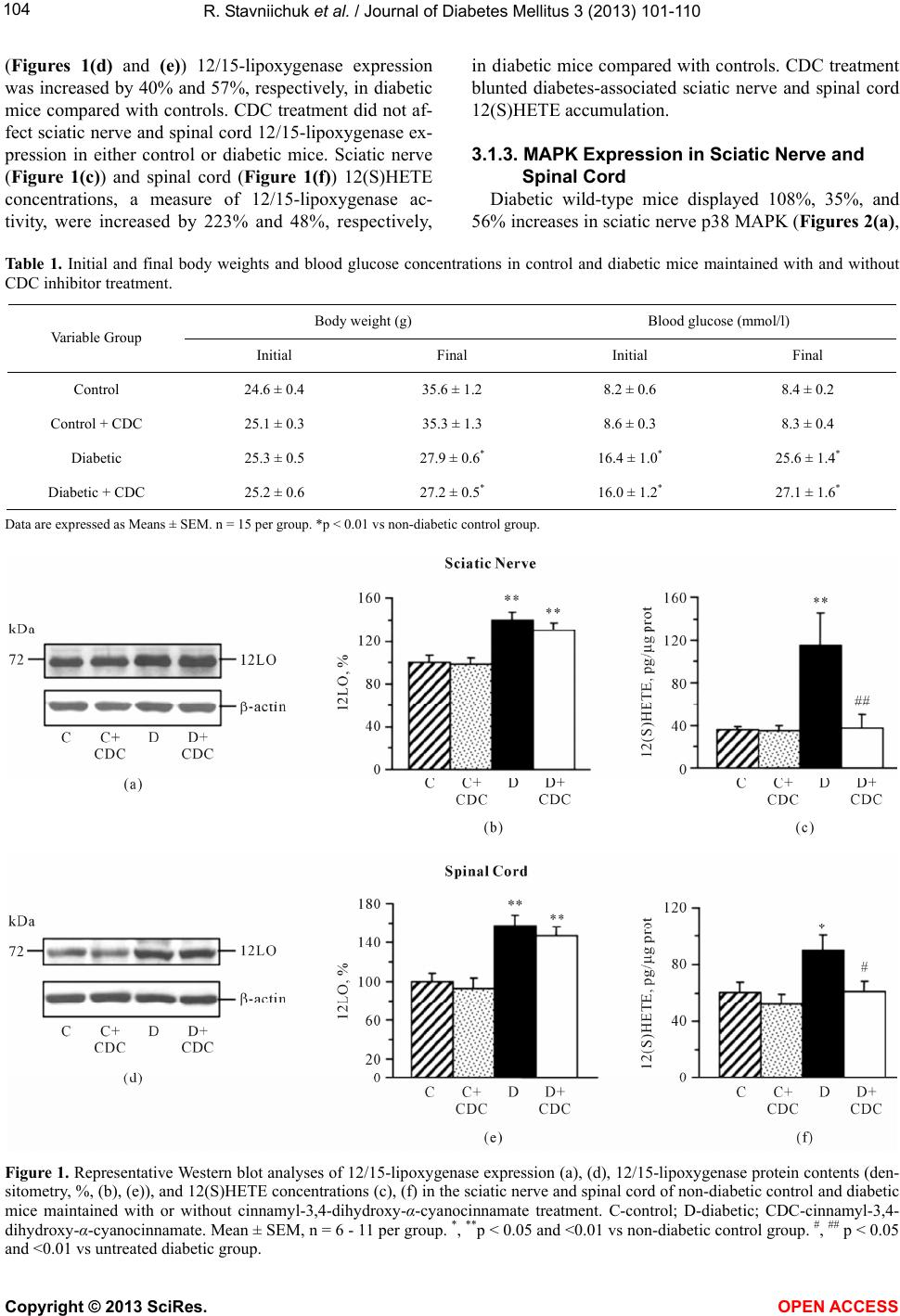 R. Stavniichuk et al. / Journal of Diabetes Mellitus 3 (2013) 101-110 Copyright © 2013 SciRes. 104 (Figures 1(d) and (e)) 12/15-lipoxygenase expression was increased by 40% and 57%, respectively, in diabetic mice compared with controls. CDC treatment did not af- fect sciatic nerve and spinal cord 12/15-lipoxygenase ex- pression in either control or diabetic mice. Sciatic nerve (Figure 1(c)) and spinal cord (Figure 1(f)) 12(S)HETE concentrations, a measure of 12/15-lipoxygenase ac- tivity, were increased by 223% and 48%, respectively, in diabetic mice compared with controls. CDC treatment blunted diabetes-associated sciatic nerve and spinal cord 12(S)HETE accumulation. 3.1.3. MAPK Expression in Sciatic Nerve and Spinal Cord Diabetic wild-type mice displayed 108%, 35%, and 56% increases in sciatic nerve p38 MAPK (Figures 2(a), Ta b le 1 . Initial and final body weights and blood glucose concentrations in control and diabetic mice maintained with and without CDC inhibitor treatment. Body weight (g) Blood glucose (mmol/l) Variable Group Initial Final Initial Final Control 24.6 ± 0.4 35.6 ± 1.2 8.2 ± 0.6 8.4 ± 0.2 Control + CDC 25.1 ± 0.3 35.3 ± 1.3 8.6 ± 0.3 8.3 ± 0.4 Diabetic 25.3 ± 0.5 27.9 ± 0.6* 16.4 ± 1.0* 25.6 ± 1.4* Diabetic + CDC 25.2 ± 0.6 27.2 ± 0.5* 16.0 ± 1.2* 27.1 ± 1.6* Data are expressed as Means ± SEM. n = 15 per group. *p < 0.01 vs non-diabetic control group. Figure 1. Representative Western blot analyses of 12/15-lipoxygenase expression (a), (d), 12/15-lipoxygenase protein contents (den- sitometry, %, (b), (e)), and 12(S)HETE concentrations (c), (f) in the sciatic nerve and spinal cord of non-diabetic control and diabetic mice maintained with or without cinnamyl-3,4-dihydroxy-α-cyanocinnamate treatment. C-control; D-diabetic; CDC-cinnamyl-3,4- dihydroxy-α-cyanocinnamate. Mean ± SEM, n = 6 - 11 per group. *, **p < 0.05 and <0.01 vs non-diabetic control group. #, ## p < 0.05 nd <0.01 vs untreated diabetic group. a OPEN A CCESS  R. Stavniichuk et al. / Journal of Diabetes Mellitus 3 (2013) 101-110 105 (b)), ERK (Figures 2(d), (e)), and SAPK/JNK (Figures 2(g), (h)) phosphorylation, compared with the corre- sponding control group. Total p38 MAPK (Figures 2(a), (c)), ERK (Figures 2(d), (f)), and SAPK/JNK (Figures 2(g), (i)) levels were indistinguishable among the groups. CDC treatment did not affect the phosphorylation state of any of three MAPKs in non-diabetic mice. It reduced p38 and ERK phosphorylation by 58% and 23% (p < 0.05 vs corresponding untreated group for both com- parisons), but did not affect SAPK/JNK phosphorylation, in diabetic mice. Spinal cord p38 MAPK (Figures 3(a), (b)), ERK (Figures 3(d), (e)) and SAPK/JNK (Figures 3(g), (h)) phosphorylation was elevated in both diabetic untreated mice, compared with the non-diabetic controls. Total p38 MAPK (Figures 3(a), (c)), ERK (Figures 3(d), (f)), and SAPK/JNK (Figures 3(g), (i)) levels were simi- lar in control and diabetic mice maintained with or without CDC treatment. The 12/15-lipoxygenase inhibi- tor did not affect the phosphorylation state of any of three MAPKs in either non-diabetic or diabetic mice. 3.2. Human Schwann Cells Experiment MAPK Expression Phosphorylation of p38 MAPK (Figures 4(a), (b)), ERK (Figures 4(d), (e)), and SAPK/JNK (Figures 4(g), (h)) was increased by 47%, 38%, and 95% in human Schwann cells cultured in 30 mM glucose, compared with those cultured in 5.5 mM glucose. Total p38 MAPK (Figure 4(c)), ERK (Figure 4(d)), and SAPK/JNK (Fig- ure 4(i)) levels were indistinguishable among the ex- perimental groups. CDC treatment did not affect levels of any total and phosphorylated MAPKs in human Schwann cells cultured in 5.5 mM glucose, but com- pletely prevented high glucose-induced increase in hu- man Schwann cells p38 MAPK and ERK, but not SAPK/ JNK, phosphorylation. Figure 2. Representative Western blot analyses of phosphorylated and total p38 MAPK, ERK, and SAPK/JNK expression (a), (d), (g), and phosphorylated (b), (e), (h) and total (c), (f), (i) p38 MAPK, ERK, and SAPK/JNK protein contents (densitometry, %) in the sciatic nerve of non-diabetic control and diabetic mice maintained with or without cinnamyl-3,4-dihydroxy-α-cyanocinnamate treat- ment. C-control; D-diabetic; CDC-cinnamyl-3,4-dihydroxy-α-cyanocinnamate. Mean ± SEM, n = 8 - 9 per group. *, **p < 0.05 and <0.01 vs non-diabetic control group. #, ##p < 0.05 and <0.01 vs untreated diabetic group. Copyright © 2013 SciRes. OPEN A CCESS 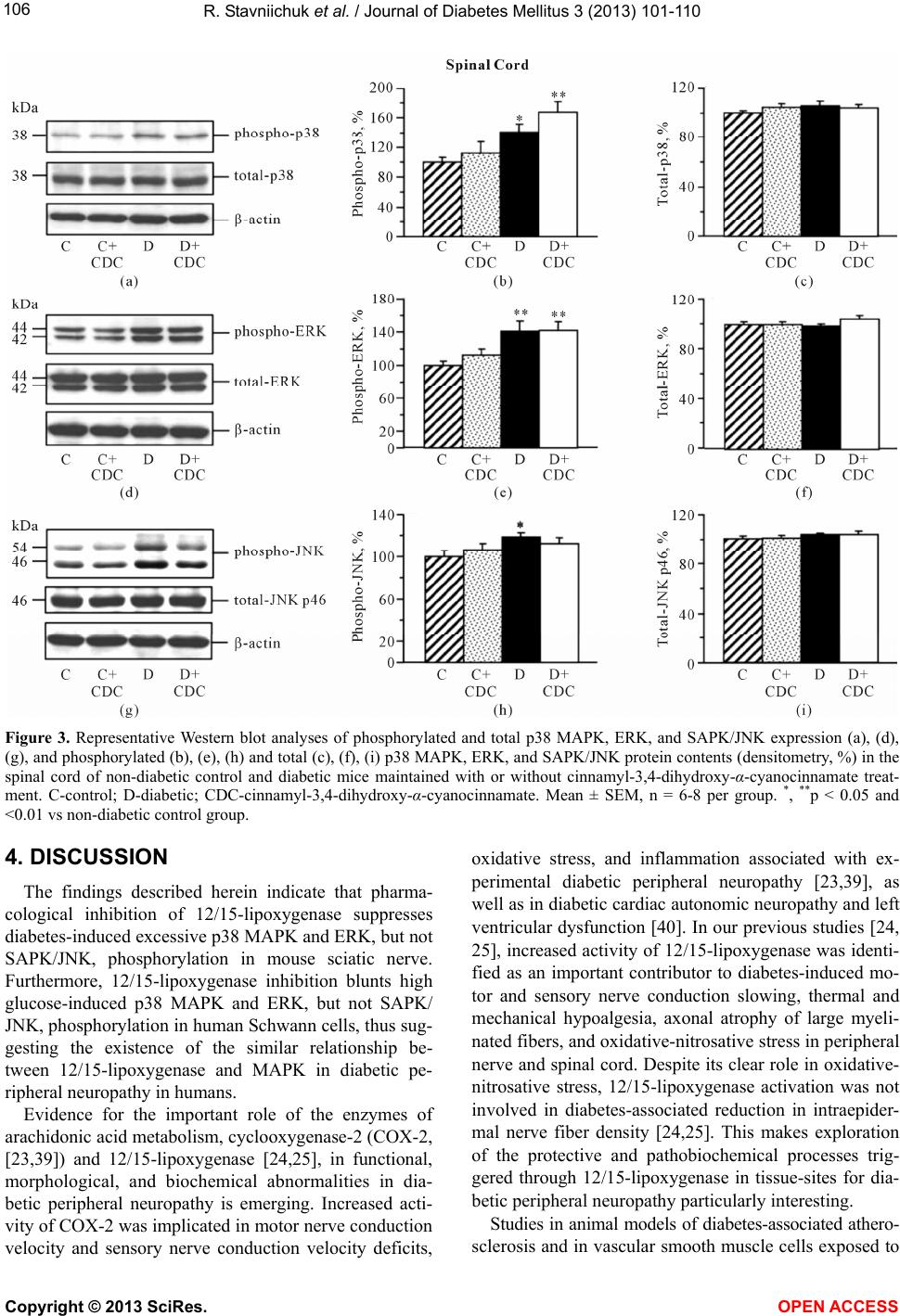 R. Stavniichuk et al. / Journal of Diabetes Mellitus 3 (2013) 101-110 106 Figure 3. Representative Western blot analyses of phosphorylated and total p38 MAPK, ERK, and SAPK/JNK expression (a), (d), (g), and phosphorylated (b), (e), (h) and total (c), (f), (i) p38 MAPK, ERK, and SAPK/JNK protein contents (densitometry, %) in the spinal cord of non-diabetic control and diabetic mice maintained with or without cinnamyl-3,4-dihydroxy-α-cyanocinnamate treat- ment. C-control; D-diabetic; CDC-cinnamyl-3,4-dihydroxy-α-cyanocinnamate. Mean ± SEM, n = 6-8 per group. *, **p < 0.05 and <0.01 vs non-diabetic control group. 4. DISCUSSION The findings described herein indicate that pharma- cological inhibition of 12/15-lipoxygenase suppresses diabetes-induced excessive p38 MAPK and ERK, but not SAPK/JNK, phosphorylation in mouse sciatic nerve. Furthermore, 12/15-lipoxygenase inhibition blunts high glucose-induced p38 MAPK and ERK, but not SAPK/ JNK, phosphorylation in human Schwann cells, thus sug- gesting the existence of the similar relationship be- tween 12/15-lipoxygenase and MAPK in diabetic pe- ripheral neuropathy in humans. Evidence for the important role of the enzymes of arachidonic acid metabolism, cyclooxygenase-2 (COX-2, [23,39]) and 12/15-lipoxygenase [24,25], in functional, morphological, and biochemical abnormalities in dia- betic peripheral neuropathy is emerging. Increased acti- vity of COX-2 was implicated in motor nerve conduction velocity and sensory nerve conduction velocity deficits, oxidative stress, and inflammation associated with ex- perimental diabetic peripheral neuropathy [23,39], as well as in diabetic cardiac autonomic neuropathy and left ventricular dysfunction [40]. In our previous studies [24, 25], increased activity of 12/15-lipoxygenase was identi- fied as an important contributor to diabetes-induced mo- tor and sensory nerve conduction slowing, thermal and mechanical hypoalgesia, axonal atrophy of large myeli- nated fibers, and oxidative-nitrosative stress in peripheral nerve and spinal cord. Despite its clear role in oxidative- nitrosative stress, 12/15-lipoxygenase activation was not involved in diabetes-associated reduction in intraepider- mal nerve fiber density [24,25]. This makes exploration of the protective and pathobiochemical processes trig- gered through 12/15-lipoxygenase in tissue-sites for dia- betic peripheral neuropathy particularly interesting. Studies in animal models of diabetes-associated athero- sclerosis and in vascular smooth muscle cells exposed to Copyright © 2013 SciRes. OPEN A CCESS  R. Stavniichuk et al. / Journal of Diabetes Mellitus 3 (2013) 101-110 107 Figure 4. Representative Western blot analyses of phosphorylated and total p38 MAPK, ERK, and SAPK/JNK expression (a), (d), (g), and phosphorylated (b), (e), (h) and total (c), (f), (i) p38 MAPK, ERK, and SAPK/JNK protein contents (densitometry, %) in human Schwann cells cultured in normal (5.5 mM) or high (30 mM) glucose with or without cinnamyl-3,4-dihydroxy-α-cyanocin- namate. C-control; D-diabetic; CDC-cinnamyl-3,4-dihydroxy-α-cyanocinnamate. Mean ± SEM, n = 5 - 7 per group. *, **p < 0.05 and <0.01 vs cells cultured in normal glucose. ##p < 0.01 vs cells cultured in high glucose without CDC. the diabetic milieu [41-45] revealed that 12/15-lipoxy- genase overexpression and activation is implicated in multiple biochemical changes including increased phos- phorylation of ERK and p38 MAPK, Ras activation, cAMP response element-binding protein (CREB) phos- phorylation, DNA-binding activity, and transactivation, overexpression of intercellular adhesion molecule-1, monocyte chemoattractant protein-1, and interleukin-6, activation of nuclear factor- B, Src tyrosine kinase, fo- cal adhesion kinase, and Akt, and histone H3-Lys-9/14 acetylation. The important role for 12/15-lipoxygenase in increased p38 MAPK, ERK, and CREB phosphorylation, and increased activator protein-1 and CREB DNA bind- ing and transcriptional activities, as well as fibronectin overexpression has been identified in renal mesangial cells isolated from diabetic mice [46]. We have been par- ticularly interested in the relationship between 12/15- lipoxygenase and MAPKs, because both p38 MAPK [26-28,47-49] and, recently, ERK [50,51], have been implicated in neuropathic changes in diabetes. In par- ticular, increased p38 MAPK phosphorylation is in- volved in nerve conduction deficit [27], diabetic erectile autonomic neuropathy and vasculopathy [47], mechani- cal hyperalgesia [28,48], and tactile allodynia [49]. In- creased ERK phosphorylation contributes to tactile allo- dynia [50] and mechanical hyperalgesia [51]. Further- more, inhibition of p38 MAPK with its specific inhibit- tors SB239063 and SB203580 counteracted diabetes- associated GSH depletion in the peripheral nerve and COX-2, inducible nitric oxide synthase, and tumor ne- crosis factor- overexpression in dorsal root ganglion neurons [28,52]. Our recent experiments [31] revealed that 12/15-lipoxygenase gene deficiency prevents dia- betes-associated excessive p38 MAPK, ERK, but not SAPK/JNK phosphorylation, in peripheral nerve, and p38 MAPK, ERK, and SAPK/JNK phosphorylation in Copyright © 2013 SciRes. OPEN A CCESS  R. Stavniichuk et al. / Journal of Diabetes Mellitus 3 (2013) 101-110 108 DRG. Interestingly, spinal cord p38 MAPK, ERK, and SAPK/JNK phosphorylation induced by diabetes, ap- peared independent of the LO mechanism. In the present study, similar findings in peripheral nerve and spinal cord were obtained with the 12/15-lipoxygenase inhibitor CDC. This is consistent with our previous results [24,25] suggesting that CDC, often considered as a relatively weak and non-specific12/15-lipoxygenase inhibitor [53- 55], can be used to dissect the role for leukocyte-type 12/15-lipoxygenase in diabetic peripheral neuropathy, and that the role for 12/15-lipoxygenase in MAPK acti- vation varies in different tissues-12/15-lipoxygenase products have been found to cause SAPK/JNK activation in fibroblasts [56], but, apparently, this mechanism does not mediate high glucose-induced SAPK/JNK activation in human Schwann cells. In conclusion, the present pharmacological study dis- sects the role for12/15-lipoxygenase in diabetes- and high glucose-induced MAPK activation in tissue-sites for diabetic peripheral neuropathy and human Schwann cells. They support interaction between the two mechanisms in the peripheral nerve, but not in spinal cord. The findings are relevant to understanding the mechanisms of diabetic peripheral neuropathy in humans, and suggest, that to- gether with MAPK inhibitors, 12/15-lipoxygenase in- hibitors can be used as pharmacological tool for inhibit- ing excessive MAPK phosphorylation in experimental, and, potentially, future clinical studies. In addition, our findings suggest that the 12/15-lipoxygenase inhibitor CDC can be used for dissection of the pathobiochemical mechanisms triggered by leukocyte-type 12/15-lipoxy- genase, because the biochemical effects of CDC closely mimic those of leukocyte-type 12/15-lipoxygenase gene deficiency. 5. ACKNOWLEDGEMENTS The study was supported in part by the National Institutes of Health Grants DK074517 (to I.G.O.), DK077141 (to I.G.O. and M.A.Y.), DK081147 (to I.G.O. and M.A.Y.), DK073990 (to M.A.Y.) and the American Diabetes Association Research Grant 7-08-RA-102 (to I.G.O.). The authors thank Dr.Rama Natarajan for valuable help with antibodies selections. REFERENCES [1] Boulton, A.J., Vinik, A.I., Arezzo, J.C., Bril, V., Feldman, E.L., Freeman, R., et al. (2005) Diabetic neuropathies: A statement by the American Diabetes Association. Diabe- tes Care, 28, 956-962. doi:10.2337/diacare.28.4.956 [2] Sinnreich, M., Taylor, B.V. and Dyck, P.J. (2005) Diabetic neuropathies. Classification, clinical features, and patho- physiological basis. Neurologist, 11, 63-79. doi:10.1097/01.nrl.0000156314.24508.ed [3] Veves, A., Backonja, M. and Malik, R.A. (2008) Painful diabetic neuropathy: Epidemiology, natural history, early diagnosis, and treatment options. Pain Medicine, 9, 660- 674. doi:10.1111/j.1526-4637.2007.00347.x [4] Tesfaye, S., Boulton, A.J., Dyck, P.J., Freeman, R., Horo- witz, M., Kempler, P., et al. (2010) Diabetic neuropathies: Update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care, 33, 2285-2293. doi:10.2337/dc10-1303 [5] Yagihashi, S., Yamagishi, S.I., Wada, R.R., Baba, M., Hohman, T.C., Yabe-Nishimura, C., et al. (2001) Neu- ropathy in diabetic mice overexpressing human aldose reductase and effects of aldose reductase inhibitor. Brain, 124, 2448-2458. doi:10.1093/brain/124.12.2448 [6] Obrosova, I.G., Van Huysen, C., Fathallah, L., Cao, X.C., Greene, D.A. and Stevens, M.J. (2002) An aldose reduc- tase inhibitor reverses early diabetes-induced changes in peripheral nerve function, metabolism, and antioxidative defense. The FASEB Journal, 16, 123-125. [7] Ho, E.C., Lam, K.S., Chen, Y.S. and Yip, J.C., Arvindak- shan, M., Yamagishi, S., et al. (2006) Aldose reductase- deficient mice are protected from delayed motor nerve conduction velocity, increased c-Jun NH2-terminal kinase activation, depletion of reduced glutathione, increased superoxide accumulation, and DNA damage. Diabetes, 55, 1946-1953. doi:10.2337/db05-1497 [8] Jack, M.M., Ryals, J.M. and Wright, D.E. (2011) Charac- terization of glyoxalase I in a streptozocin-induced mouse model of diabetes with painful and insensate neuropathy. Diabetologia, 54, 2174-2182. doi:10.1007/s00125-011-2196-3 [9] Bierhaus, A., Fleming, T., Stoyanov, S., Leffler, A., Babes, A., Neacsu, C., et al. (2012) Methylglyoxal modification of Na(v)1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nature Medicine, 18, 926-933. doi:10.1038/nm.2750 [10] Bierhaus, A., Haslbeck, K.M., Humpert, P.M., Liliensiek, B., Dehmer, T., Morcos, M., et al. (2004) Loss of pain perception in diabetes is dependent on a receptor of the immunoglobulin superfamily. Journal Clinical Investiga- tion, 114, 1741-1751. [11] Cameron, N.E., Gibson, T.M., Nangle, M.R. and Cotter, M.A. (2005) Inhibitors of advanced glycation end product formation and neurovascular dysfunction in experimental diabetes. Annuals New York Academy Science, 1043, 784- 792. doi:10.1196/annals.1333.091 [12] Nagamatsu, M., Nickander, K.K., Schmelzer, J.D., Raya, A., Wittrock, D.A., Tritschler, H., et al. (1995) Lipoic acid improves nerve blood flow, reduces oxidative stress, and improves distal nerve conduction in experimental diabetic neuropathy. Diabetes Care, 18, 1160-1167. doi:10.2337/diacare.18.8.1160 [13] Cameron, N.E., Tuck, Z., McCabe, L. and Cotter, M.A. (2001) Effect of the hydroxyl radical scavenger, dime- thylthiourea, on peripheral nerve tissue perfusion, con- duction velocity and nociception in experimental diabetes. Diabetologia, 44, 1161-1169. doi:10.1007/s001250100626 [14] Coppey, L.J., Gellett, J.S., Davidson, E.P., Dunlap, J.A., Lund, D.D. and Yorek, M.A. (2001) Effect of antioxidant treatment of streptozotocin-induced diabetic rats on en- doneurial blood flow, motor nerve conduction velocity, Copyright © 2013 SciRes. OPEN A CCESS  R. Stavniichuk et al. / Journal of Diabetes Mellitus 3 (2013) 101-110 109 and vascular reactivity of epineurial arterioles of the sci- atic nerve. Diabetes, 50, 1927-1937. doi:10.2337/diabetes.50.8.1927 [15] Obrosova, I.G., Mabley, J.G., Zsengellér, Z., Charni- auskaya, T., Abatan, O.I., Groves, J.T., et al. (2005) Role for nitrosative stress in diabetic neuropathy: Evidence from studies with a peroxynitrite decomposition catalyst. FASEB Journal, 19, 401-403. [16] Lupachyk, S., Shevalye, H., Maksimchyk, Y., Drel, V.R. and Obrosova, I.G. (2011) PARP inhibition alleviates dia- betes-induced systemic oxidative stress and neural tissue 4-hydroxynonenal adduct accumulation: Correlation with peripheral nerve function. Free Radical Biology Medicine, 50, 1400-1409. doi:10.1016/j.freeradbiomed.2011.01.037 [17] Goss, J.R., Goins, W.F., Lacomis, D., Mata, M., Glorioso, J.C. and Fink, D.J. (2002) Herpes simplex-mediated gene transfer of nerve growth factor protects against peripheral neuropathy in streptozotocin-induced diabetes in the mouse. Diabetes, 51, 2227-2232. doi:10.2337/diabetes.51.7.2227 [18] Bianchi, R., Buyukakilli, B., Brines, M., Savino, C., Cavaletti, G., Oggioni, N., et al. (2004) Erythropoietin both protects from and reverses experimental diabetic neuropathy. Proceedings National Academy Science, 101, 823-828. doi:10.1073/pnas.0307823100 [19] Nakamura, J., Kato, K., Hamada, Y., Nakayama, M., Chaya, S., Nakashima, E., et al. (1999) A protein kinase C-beta- selective inhibitor ameliorates neural dysfunction in strep- tozotocin-induced diabetic rats. Diabetes, 48, 2090-2095. doi:10.2337/diabetes.48.10.2090 [20] Cameron, N.E., Cotter, M.A., Jack, A.M., Basso, M.D. and Hohman, T.C. (1999) Protein kinase C effects on nerve function, perfusion, Na(+), K(+)-ATPase activity and glutathione content in diabetic rats. Diabetologia, 42, 1120-1130. doi:10.1007/s001250051280 [21] Li, F., Drel, V.R., Szabó, C., Stevens, M.J. and Obrosova, I.G. (2005) Low-dose poly(ADP-ribose) polymerase in- hibitor-containing combination therapies reverse early peripheral diabetic neuropathy. Diabetes, 54, 1514-1522. doi:10.2337/diabetes.54.5.1514 [22] Obrosova, I.G., Xu, W., Lyzogubov, V.V., Ilnytska, O., Mashtalir, N., Vareniuk, I., et al. (2008) PARP inhibition or gene deficiency counteracts intraepidermal nerve fiber loss and neuropathic pain in advanced diabetic neuropa- thy. Free Radical Biology Medicine, 44, 972-981. doi:10.1016/j.freeradbiomed.2007.09.013 [23] Kellogg, A.P., Wiggin, T.D., Larkin, D.D., Hayes, J.M., Stevens, M.J. and Pop-Busui, R. (2007) Protective effects of cyclooxygenase-2 gene inactivation against peripheral nerve dysfunction and intraepidermal nerve fiber loss in experimental diabetes. Diabetes, 56, 2997-3005. doi:10.2337/db07-0740 [24] Stavniichuk, R., Drel, V.R., Shevalye, H., Vareniuk, I., Stevens, M.J., Nadler, J.L., et al. (2010) Role of 12/15- lipoxygenase in nitrosative stress and peripheral predia- betic and diabetic neuropathies. Free Radical Biology Medicine, 49, 1036-1045. doi:10.1016/j.freeradbiomed.2010.06.016 [25] Obrosova, I.G., Stavniichuk, R., Drel, V.R., Shevalye, H., Vareniuk, I., Nadler, J.L., et al. (2010) Different roles of 12/15-lipoxygenase in diabetic large and small fiber pe- ripheral and autonomic neuropathies. American Journal Pathology, 177, 1436-1447. doi:10.2353/ajpath.2010.100178 [26] Purves, T., Middlemas, A., Agthong, S., Jude, E.B., Boulton, A.J., Fernyhough, P., et al. (2001) A role for mi- togen-activated protein kinases in the etiology of diabetic neuropathy. FASEB Journal, 15, 2508-2514. doi:10.1096/fj.01-0253hyp [27] Price, S.A., Agthong, S., Middlemas, A.B. and Tomlinson, D.R. (2004) Mitogen-activated protein kinase p38 medi- ates reduced nerve conduction velocity in experimental diabetic neuropathy: Interactions with aldose reductase. Diabetes, 53, 1851-1856. doi:10.2337/diabetes.53.7.1851 [28] Cheng, H.T., Dauch, J.R., Oh, S.S., Hayes, J.M., Hong, Y. and Feldman, E.L. (2010) p38 mediates mechanical allo- dynia in a mouse model of type 2 diabetes. Molecular Pain, 19, 6-28. [29] Stavniichuk, R., Drel, V.R., Shevalye, H., Maksimchyk, Y., Kuchmerovska, T.M., Nadler, J.L., et al. (2011) Bai- calein alleviates diabetic peripheral neuropathy through inhibition of oxidative-nitrosative stress and p38 MAPK activation. Experimental Neurology, 230, 106-113. doi:10.1016/j.expneurol.2011.04.002 [30] Drel, V.R., Pacher, P., Stavniichuk, R., Xu, W., Zhang, J., Kuchmerovska, T.M., et al. (2011) Poly(ADP-ribose)po- lymerase inhibition counteracts renal hypertrophy and multiple manifestations of peripheral neuropathy in dia- betic Akita mice. International Journal Molecular Medi- cine, 28, 629-635. [31] Stavniichuk, R., Shevalye, H., Hirooka, H., Nadler, J.L. and Obrosova, I.G. (2012) Interplay of sorbitol pathway of glucose metabolism, 12/15-lipoxygenase, and mito- gen-activated protein kinases in the pathogenesis of dia- betic peripheral neuropathy. Biochemical Pharmacology, 83, 932-940. doi:10.1016/j.bcp.2012.01.015 [32] Askwith, T., Zeng, W., Eggo, M.C. and Stevens, M.J. (2012) Taurine reduces nitrosative stress and nitric oxide synthase expression in high glucose-exposed human Schwann cells. Experimental Neurology, 233, 154-162. doi:10.1016/j.expneurol.2011.09.010 [33] Roberts, P.J. and Der, C.J. (2007) Targeting the Raf- MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene, 26, 3291-3310. doi:10.1038/sj.onc.1210422 [34] Lehmann, H.C. and Höke, A. (2010) Schwann cells as a therapeutic target for peripheral neuropathies. CNS Neu- rology Disorders Drug Targets, 9, 801-816. doi:10.2174/187152710793237412 [35] Lehmann, H.C., Chen, W., Mi, R., Wang, S., Liu, Y., Rao, M., et al. (2012) Human Schwann cells retain essential phenotype characteristics after immortalization. Stem Cells Development, 21, 423-431. doi:10.1089/scd.2010.0513 [36] Obrosova, I.G., Drel, V.R., Pacher, P., Ilnytska, O., Wang, Z.Q., Stevens, M.J., et al. (2005) Oxidative-nitrosative stress and poly(ADP-ribose) polymerase (PARP) activi- tion in experimental diabetic neuropathy: The relation is revisited. Diabetes, 54, 3435-3441. Copyright © 2013 SciRes. OPEN A CCESS  R. Stavniichuk et al. / Journal of Diabetes Mellitus 3 (2013) 101-110 Copyright © 2013 SciRes. OPEN A CCESS 110 doi:10.2337/diabetes.54.12.3435 [37] Stevens, M.J., Li, F., Drel, V.R., Abatan, O.I., Kim, H., Burnett, D., et al. (2007) Nicotinamide reverses neuro- logical and neurovascular deficits in streptozotocin dia- betic rats. The Journal of Pharmacology and Experimen- tal Therapeutics, 320, 458-464. doi:10.1124/jpet.106.109702 [38] Askwith, T., Zeng, W., Eggo, M.C. and Stevens, M.J. (2009) Oxidative stress and dysregulation of the taurine transporter in high-glucose-exposed human Schwann cells: Implications for pathogenesis of diabetic neuropa- thy. American Journal of Physiology Endocrinology and Metabolism, 297, 620-628. doi:10.1152/ajpendo.00287.2009 [39] Pop-Busui, R., Marinescu, V., Van Huysen, C., Li, F., Sullivan, K., Greene, D.A., et al. (2002) Dissection of metabolic, vascular, and nerve conduction interrelation- ships in experimental diabetic neuropathy by cyclooxy- genase inhibition and acetyl-L-carnitine administration. Diabetes, 51, 2619-2628. doi:10.2337/diabetes.51.8.2619 [40] Kellogg, A.P., Converso, K., Wiggin, T., Stevens, M. and Pop-Busui, R. (2009) Effects of cyclooxygenase-2 gene inactivation on cardiac autonomic and left ventricular function in experimental diabetes. American Journal of Physiology Heart and Circulation Physiology, 296, 453- 461. doi:10.1152/ajpheart.00678.2008 [41] Reddy, M.A., Thimmalapura, P.R., Lanting, L., Nadler, J.L., Fatima, S. and Natarajan, R. (2002) The oxidized lipid and lipoxygenase product 12(S)-hydroxyeicosa-tet- raenoic acid induces hypertrophy and fibronectin tran- scription in vascular smooth muscle cells via p38 MAPK and cAMP response element-binding protein activation. Mediation of angiotensin II effects. Journal of Biological Chemistry, 277, 9920-9928. doi:10.1074/jbc.M111305200 [42] Dwarakanath, R.S., Sahar, S., Reddy, M.A., Castanotto, D., Rossi, J.J. and Natarajan, R. (2004) Regulation of mono- cyte chemoattractant protein-1 by the oxidized lipid, 13-hydroperoxyoctadecadienoic acid, in vascular smooth muscle cells via nuclear factor-kappa B (NF-kappa B). Journal of Molecular and Cell Cardiology, 36, 585-595. doi:10.1016/j.yjmcc.2004.02.007 [43] Reilly, K.B., Srinivasan, S., Hatley, M.E., Patricia, M.K., Lannigan, J., Bolick, D.T., et al. (2004) 12/15-Lipoxy- genase activity mediates inflammatory monocyte/endo- thelial interactions and atherosclerosis in vivo. The Jour- nal of Biological Chemistry, 279, 9440-9450. doi:10.1074/jbc.M303857200 [44] Dwarakanath, R.S., Sahar, S., Lanting, L., Wang, N., Stemerman, M.B., Natarajan, R., et al. (2008) Viral vec- tor-mediated 12/15-lipoxygenase overexpression in vas- cular smooth muscle cells enhances inflammatory gene expression and migration. Journal of Vascular Research, 45, 132-142. doi:10.1159/000109966 [45] Reddy, M.A., Sahar, S., Villeneuve, L.M., Lanting, L. and Natarajan, R. (2009) Role of src tyrosine kinase in the atherogenic effects of the 12/15-lipoxygenase pathway in vascular smooth muscle cells. Arteriosclerosis, Thrombo- sis and Vascular Biology, 29, 387-393. doi:10.1161/ATVBAHA.108.179150 [46] Kim, Y.S., Reddy, M.A., Lanting, L., Adler, S.G. and Natarajan, R. (2003) Differential behavior of mesangial cells derived from 12/15-lipoxygenase knockout mice relative to control mice. Kidney International, 64, 1702- 1714. doi:10.1046/j.1523-1755.2003.00286.x [47] Nangle, M.R., Cotter, M.A. and Cameron, N.E. (2006) Correction of nitrergic neurovascular dysfunction in dia- betic mouse corpus cavernosum by p38 mitogen-activated protein kinase inhibition. International Journal of Impo- tence Research, 18, 258-263. doi:10.1038/sj.ijir.3901414 [48] Daulhac, L., Mallet, C., Courteix, C., Etienne, M., Dur- oux, E., Privat, A.M., et al. (2006) Diabetes-induced me- chanical hyperalgesia involves spinal mitogen-activated protein kinase activation in neurons and microglia via N-methyl-D-aspartate-dependent mechanisms. Molecular Pharmacology, 70, 1246-1254. doi:10.1124/mol.106.025478 [49] Du, Y., Tang, J., Li, G., Berti-Mattera, L., Lee, C.A., Bartkowski, D., et al. (2010) Effects of p38 MAPK inhi- bition on early stages of diabetic retinopathy and sensory nerve function. Investigative Ophthalmology & Visual Science, 51, 2158-2164. doi:10.1167/iovs.09-3674 [50] Daulhac, L., Maffre, V., Mallet, C., Etienne, M., Privat, A.M., Kowalski-Chauvel, A., et al. (2011) Phosphoryla- tion of spinal N-methyl-d-aspartate receptor NR1 sub- units by extracellular signal-regulated kinase in dorsal horn neurons and microglia contributes to diabetes-in- duced painful neuropathy. European Journal of Pain, 15, 169.e1-169.e12. [51] Tsuda, M., Ueno, H., Kataoka, A., Tozaki-Saitoh, H. and Inoue, K. (2008) Activation of dorsal horn microglia con- tributes to diabetes-induced tactile allodynia via extracel- lular signal-regulated protein kinase signaling. Glia, 56, 378-386. doi:10.1002/glia.20623 [52] Price, S.A., Gardiner, N.J., Duran-Jimenez, B., Zeef, L.A., Obrosova, I.G. and Tomlinson, D.R. (2006) Thioredoxin interacting protein is increased in sensory neurons in ex- perimental diabetes. Brain Research, 1116, 206-214. doi:10.1016/j.brainres.2006.07.109 [53] Bürger, F., Krieg, P., Marks, F. and Fürstenberger, G. (2000) Positional- and stereo-selectivity of fatty acid oxy- genation catalysed by mouse (12S)-lipoxygenase iso-en- zymes. Biochemical Journal, 348, 329-335. doi:10.1042/0264-6021:3480329 [54] Gong, Y.Z., Ding, W.G., Wu, J., Tsuji, K., Horie, M. and Matsuura, H. (2008) Cinnamyl-3,4-dihydroxy-alpha cya- nocinnamate and nordihydroguaiaretic acid inhibit human Kv1.5 currents independently of lipoxygenase. European Journal of Pharmacology, 600, 18-25. doi:10.1016/j.ejphar.2008.10.010 [55] Pergola, C., Jazzar, B., Rossi, A., Buehring, U., Luderer, S., Dehm, F., et al. (2011) Cinnamyl-3,4-dihydroxy-α- cyanocinnamate is a potent inhibitor of 5-lipoxygenase. Journal of Pharmacology and Experimental Therapeutics, 338, 205-213. doi:10.1124/jpet.111.180794 [56] Wen, Y., Scott, S., Liu, Y., Gonzales, N. and Nadler, J.L. (1997) Evidence that angiotensin II and lipoxygenase products activate c-Jun NH2-terminal kinase. Circulation Research, 81, 651-655.doi:10.1161/01.RES.81.5.651
|