Paper Menu >>
Journal Menu >>
 International Journal of Geosciences, 2010, 1, 130-138 doi:10.4236/ijg.2010.13017 Published Online November 2010 (http://www.SciRP.org/journal/ijg) Copyright © 2010 SciRes. IJG Geochemistry of Termite Hills as a Tool for Geochemical Exploration of Glass Sand in the Iraqi Western Desert Salih Muhammad Awadh Earth Science Department, College of Science, University of Baghdad, Baghdad, Iraq E-mail: Salihauad2000@yahoo.com Received September 21, 2010; revised September 30, 2010; accepted October 30, 2010 Abstract Sand glass deposits was located in the mid of the Western Desert of Iraq. It is situated within Rutba Forma- tion (Ceno-manian). Ancient traditional mining method is still used in exploitation the unconsolidated white glass sand from glass sand quarry. The overburden thickness ranges from 2 to 4 m in average. Termite hills were observed around the glass sand quarry extending far from the quarry area. Termites could burrows down and penetrate the sand glass bringing it up to the surface. The depth of penetration reaches more than 35 m. The field observation of the white color of termite hills which are built up by sand glass gave a good indicator for the hidden subsurface deposit and it appears to be a surface signature for finding glass sand di- rectly under the termite hills. The scattered white hills of glass sand on the surface with high content of SiO2, concordant Zr/Hf and Th/U ratios and heavy mineral distribution in both of quarry and termite hills provide a strong evidence of that those termite hills could be an effective tool for exploring subsurface hidden glass sand up to 35 m depth. Keywords: Geochemistry, Glass Sand, Geochemical Exploration, Termite Hill, Iraqi Western Desert 1. Introduction Glass sand and unconsolidated sand stone scatter in the Iraqi Western Desert within many geological formations like Rutba, Msaad, Nahr Umar and Najma Formations. A reconnaissance study h ad done by [1] who was expressed a pioneer in investigation of sand glass in Iraq; he had found a white glass sand in Wadi Amij in the mid of the Iraqi Western Desert and he mentioned to its unsuitabil- ity for glass industry. Erindus which is a Belgian Com- pany had achieved detailed classic study in 1959 for glass sand investigating and determined pure white glass sand near Al-Rutba city. These actions were followed by the Soviiet geologists who found that the glass sand which is located near Al-Rutba city (Rutba Formation) is suitable for glass manufacturing; and they also concluded the delta was a depositional environment [2] The deposi- tional environment also described as continental and flu- viatile in most [3-5]. The state of geological survey and mineral investiga- tion in 1985 started seeking and investigating within Najma formation (Upper Jurassic) for new locations of glass sand around Al-Rutba city. The quarry of glass sand which is a part of the study area located south west of Al-Rutba city in the mid of the Iraqi Western desert (Figure 1) which was discovered by Erindus in 1959 is industrially evaluated by Soviiet geologists in 1961. Glass sand is still traditionally mined and transported to the factory of glass production in Al-Ramadi city which situated of about 320 km eastward the quarry (Figure 1). Termite able to penetrate down the water table at the depth between of 5 to 25 m bringing excavated uranifer- ous material up to the surface [6]. Termite which can penetrate down tens of meters form- ing enormous excavation capable to transport up 1 kg/m2 per day of material from their burrows [7,8]. Many geo- chemical studies have been carried out on termite and its role in geochemical exploration; some of these studies are that of [9,10] which are done with respect to both gold and zinc, but they are inconclusive. Mineral anoma- lies were found in termite hills over known gold ore bodies in Zimbabwe [11]. This study based on direct field observations and ca- pability of termite in penetrating down bringing glass sand to the surface then it is going to discuss the use of termite hills as a geochemical tool for exploring hidden glass sand. 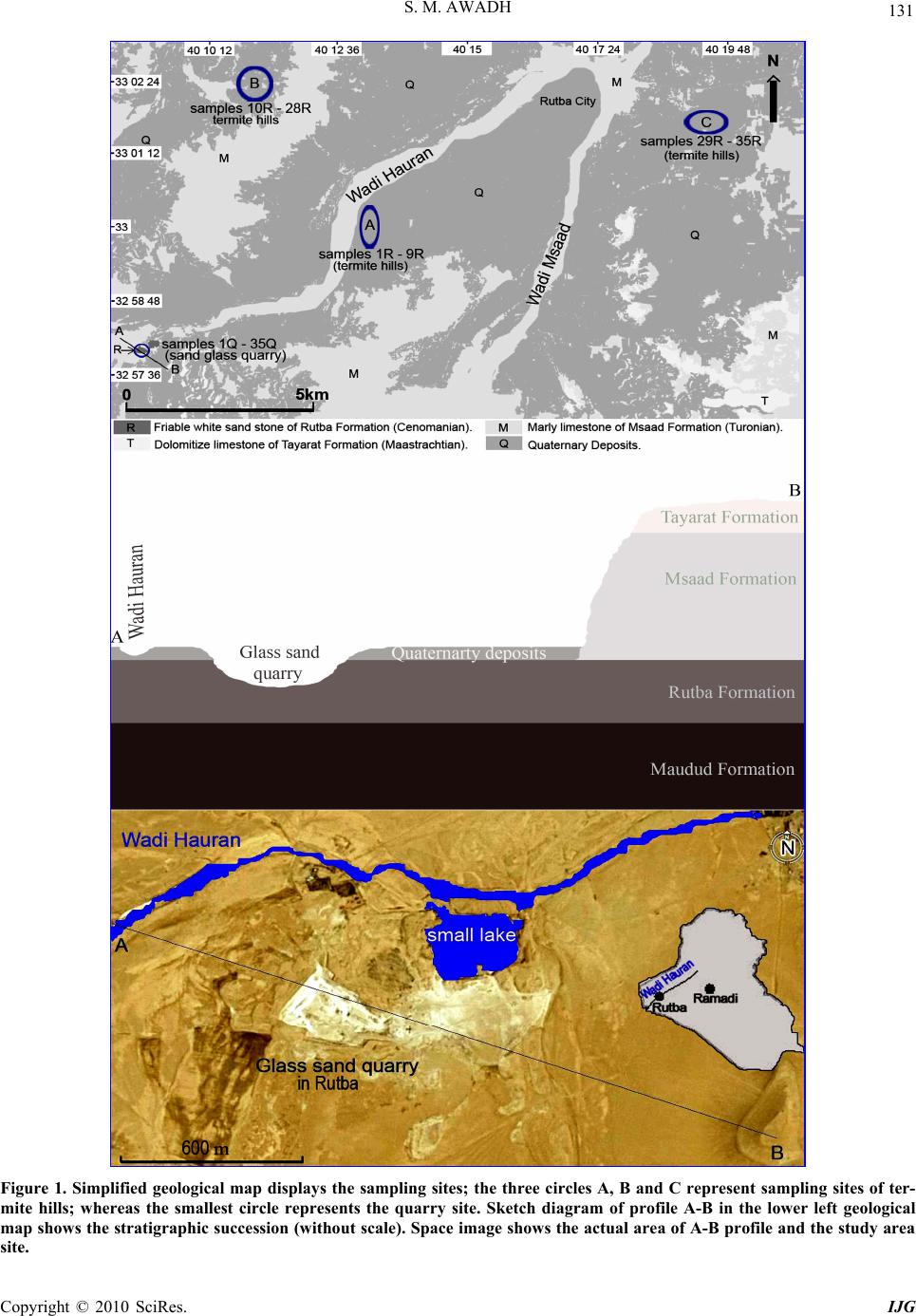 S. M. AWADH Copyright © 2010 SciRes. IJG 131 Figure 1. Simplified geological map displays the sampling sites; the three circles A, B and C represent sampling sites of ter- mite hills; whereas the smallest circle represents the quarry site. Sketch diagram of profile A-B in the lower left geological map shows the stratigraphic succession (witho ut scale). Space image shows the actual area of A-B profile and the study area site.  S. M. AWADH Copyright © 2010 SciRes. IJG 132 2. Location and Nature of the Study Area This work is done on Rutba Formation in the mid of Western Desert of Iraq (Figure 1) which is one of geo- logical formations exposed in several sites in the West- ern Desert. Glass sand quarry in Rutba Formation situ- ated of 16 km due west of Rutba city which is an open quarry covered by 3 m (average) overburden of Quater- nary deposits. Sampling included both Rutba Formation represented by glass sand quarry and termite hills scattered around quarry and extended farther of 15 km due north and east. Longitude of E 40º 09 20 and latitude of N 32º 57 40 with elevation of 640 m exactly determine the lo cation of glass sand quarry. Termite trenches exist as network of tubes extended from surface perpendicularly downward penetrating Quaternary deposits (overburden) as well as the hidden ore (glass sand) (Figure 2). Termite trenches tend to have brown color due to the biological activities and weathering processes. The ancient colony trenches ap- pear to be filled mostly with clay minerals and calcite descended from sediments of adjacent surface (Figure 3). Sometime walls of termite trenches tend to be harder than adjacent glass sand and form small cone like-shapes on the ground of quarry (Figure 4). Figure 2. Network of termite trenches penetrating the overburden (brown color) and glass sand (white color). Figure 3. Termite hill trenches filled with the surface sedi- ments. Figure 4. Remnant trace of termite trenches on the ground of the glass sand quarry. 3. Geology of Study Area Iraqi Western Desert is an extensive semi-flat area which gradually rises westward to maximum elevation of 987 m above the sea level [12]. Tectonically, it lies within the stable shelf according to the tectonic division of Iraq [13]. Net of seasonal valleys running generally north-east ward intervention the Western Desert; on e of these called Hauran valley (Wadi Hauran) which passing closely ad- jacent the quarry of glass sand then connects with valley of Wadi Msaad in the north east Rutba city (Figure 1), which eventually drain into Euphrates River. Small scat- tered hills not exceeding more than 60 m are common morphological features. Physical weathering is active by wind action and high variation in temperature between the night and the day, while the chemical weathering is becoming weak due to the scarcity of seasonal rainfall. Small sand dunes scatter in the Western Desert on the slope of hills within the shadow zone of the w ind’s face.  S. M. AWADH 133 The exposed sedimentary sequence in the Western Desert ranges from Permocarboniferous to Pleistocene with several regional unconformities punctuating the co- lumn [14]. The Cretaceous age in the stratigraphic suc- cession is represented by eight formations deposited during six sedimentary cycles [15]. Rutba Formation (Cenomanian) appears to be the third formation in the second sedimentary cycle in the stratigraphic succession of the Cretaceous p eriod [15]; it overlies un conformabily Permocarboniferous, Triassic, Jurassic formations; also it unconformabily emplaces on Maudud Formation (Figure 1) which forms mainly from gray limestone of Albian – Cenomanian age [16]; the contact is covered by thick Quaternary sediments [17]. Msaad Formation (Cenoma- nian – Turonian) represents the fourth formation in the second sedimentary cycle of the Cretaceous period; gen- erally, it consists of shallow marine limestone, marl and sand stone [5] and it overlies gradationally and con- formably Rutba Formation (Figure 1) [16-18]. Msaad Formation is exposed in Hauran Valley (Wadi Hauran), Msaad Valley (Wadi Msaad) and around Rutba city, it extends along H auran Valley n orthward for ab out 18 km, then extend eastwards parallel the older Cretaceous for- mations for about 130 km [15]. Sand glass is a deposit of white coarse sand and sand- stone locally cemented to quartzite locatin g within Rutba Formation (Figure 1); it is exposed on surface after re- moval the quaternary overburden which ranges from 2 to 4 m thickness by mining work. 4. Material and Methods A detail field work was done seeking of termite hill after the determination of three sites of termite hills. Thirty five samples were collected randomly from termite hills which scattered in three sites (A, B and C) on the surface of the Quaternary deposits; nine samples of them (1 R to 9 R), nineteen samples (10 R to 28 R) and seven samples (29 R to 35 R) were collected from sites A, B and C re- spectively (Figure 1). Another 35 samples of glass sand were collected from the quarry itself. All of these sam- ples were analyzed for major oxides (SiO2, Al2O3, Fe2O3, CaO and K2O) by using Atomic Absorption Spectropho- tometer (AAS) as well as trace elements (Zr, Hf, U and Th) by using Neutron Activation Analyses (NAA). Re- sults of major oxides and trace elements for termite hills and quarry are listed in Tables 1-2 respectively. In both sample types (termite hills and quarry) sand with a grain size from 0.063 to 0.250 mm was sieved from the bulk samples. The sand grains were immersed in a bromoform liquid to separate the heavy minerals (which sink in the liquid) from the light minerals (which float in the liquid). The heavy sand grains were mounted on glass slides, and were identified. Quantitative and qualitative mineral analyses were done and illustrated as a flow chart (Figure 5) by using Flow 4 program. 5. Mineral Indicators Most of grain tend to be quartz formed about 97% and 93% of the mineral constitutes in quarry and termite hills respectively (Figure 5) and tends to be sub-rounded to rounded in most. Calcite presences as a friable replace- ment cement. Feldspar is very rare and seems altered. It is possible that originally these sediments were rich in feldspar and due to the reworking in many sedimentary cycles within fluviatile and shallow marine env ironments, climatic effects, and diagenesis. These sediments were changed to orthoquartzite. Heavy minerals contributed of 0.05% and 0.03% from bulk samples of quarry and termite hills respectively (Figure 5); this may give precise information about the types of rock from which the sediment was eroded [19,20]. Quantity of heav y minerals in tegrates the qu ality of light minerals (quartz, calcite, clay minerals and or- thoclase) which formed 99.5% and 97.5% respectively (Figure 5). The opaque heavy minerals (mostly hematite) formed about 40%, whereas the translucent heavy min- erals (tourmaline, zircon, rutile, hornblende, staurolite, biotite, epidote, pyroxene and monazite) formed about 60% approximately from the total heav y minerals in both termite hills and quarry samples. The same heavy mineral suite existed in both quarry and termite hill samples; their content within termite hill appears to be less than of their content within quarry samples (Figure 5) due to the additive minerals (calcite and clay minerals) from overburden to the sand glass during penetrating processes by termite for building up their mounds. The ultrastable heavy minerals (zircon, tourmaline and rutile) and stable heavy minerals monazite exist mainly in subrounded to rounded shapes referring to reworked and long transportation from source of acidic igneous rocks. The moderate stable heavy minerals (epidote and staurolite, hornblende and biotite) originally come from source of metamorphic rocks. The scarce presence of pyroxene doesn’t indicate basic igneous rocks were ma- jor source for Rutba Formation, but they have a simple contribution. Biotite has the greatest tendency to include accessories, thereby ind irectly controllin g the geochemistry of Th and U [21], but the little occurrence of biotite in comparison with zircon and tourmaline made it less important. 6. Geochemistry: Results and Discussion Major oxides composition of the different sites of termite Copyright © 2010 SciRes. IJG  S. M. AWADH Copyright © 2010 SciRes. IJG 134 hills and glass sand quarry is listed in Tables 1-2. Alu- mina, iron and potassium present very low contents throughout the quarry. Concentration of alumina, iron, calcium and potassium in termite hills which were built on overburden appear to be higher than of their contents in glass sand. This attributed to the relative abundance of clay minerals and ease of sand to wash and leach. Calcium mainly originated from calcite appears to oc- cupy the second order after silica. In respect to the silica, basically a common oxide SiO2 which formed of about Table 1. Results of chemical composition of termite hill samples. SiO2 Al2O3 Fe2O3 CaO K2O Zr Hf Th U Sample no. % ppm Zr/Hf Th/U 1R 91.2 0.9 0.23 6.2 0.21 20 0.5 1.2 0.41 40 3.0 2R 92.1 1.5 0.41 5.5 0.22 13 0.4 1.2 0.30 33 4.0 3R 94.7 1.2 0.90 2.2 0.31 80 2.1 0.9 0.36 38 2.5 4R 93.9 0.7 0.21 4.1 0.20 100 2.5 1.5 0.41 40 3.6 5R 90.4 1.6 0.25 7.3 0.18 15 0.4 7.2 2.40 36 3.0 6R 93.8 1.6 0.26 3.4 0.12 200 6.2 3.3 0.97 32 3.4 7R 96.4 0.2 0.11 2.7 0.02 40 1.1 1.4 0.43 36 3.2 8R 95.5 0.1 0.04 4.1 0.01 419 11.7 5.4 3.30 36 1.6 9R 95.7 0.2 0.03 3.3 0.04 95 2.2 9.9 4.00 43 2.7 10R 96.2 0.5 0.22 2.6 0.21 175 4.3 3.1 0.73 41 4.2 11R 93.9 1.1 0.33 3.9 0.31 175 3.9 2.8 0.90 45 3.1 12R 94.1 1.5 0.34 3.5 0.32 35 1.1 4.3 1.50 32 2.9 13R 94.2 2.2 0.32 3.0 0.17 425 11 5.8 1.60 39 3.6 14R 94.3 0.5 0.45 4.1 0.20 560 14 6.0 2.60 40 2.3 15R 94.9 1.1 0.11 3.7 0.10 415 12 5.5 1.44 35 3.9 16R 95.4 0.7 0.21 2.9 0.16 165 4.3 1.9 0.48 38 3.9 17R 93.5 0.9 0.53 4.5 0.11 10 0.2 3.2 2.00 50 1.6 18R 93.8 1.2 0.21 4.6 0.10 66 1.8 3.6 1.00 37 3.6 19R 94.0 0.7 0.12 4.2 0.13 205 6.7 3.4 1.70 31 2.1 20R 93.9 0.3 0.18 4.7 0.09 80 2.3 10.1 1.50 35 6.6 21R 93.4 1.2 0.22 4.3 0.11 440 9.4 6.3 3.20 46 2.0 22R 90.1 1.4 0.24 7.7 0.21 311 7.9 4.4 1.40 39 3.1 23R 93.3 0.2 0.44 5.5 0.10 185 4.7 2.5 0.77 39 3.2 24R 90.1 1.0 0.25 7.3 0.94 88 2.1 2.8 0.75 42 3.7 25R 89.3 2.2 0.66 6.8 0.88 320 7.3 3.7 0.92 44 4.0 26R 94.3 2.1 0.33 2.6 0.23 140 3.8 1.6 0.57 37 2.8 27R 90.9 1.9 0.33 7.6 0.65 225 4.7 2.2 1.62 48 1.4 28R 94.3 0.3 0.22 4.3 0.34 30 0.9 3.3 1.50 33 2.2 29R 93.0 1.9 0.11 4.1 0.07 10 0.3 4.2 2.20 33 1.9 30R 93.8 0.4 0.05 4.8 0.03 301 7.7 4.3 1.26 39 3.4 31R 95.2 0.09 0.03 3.4 0.01 43 1.2 2.6 0.86 36 3.0 32R 94.5 1.0 0.3 3.8 0.15 490 12.1 6.6 1.90 40 3.5 33R 96.4 0.02 0.9 2.2 0.09 610 18.4 9.4 3.85 33 2.4 34R 93.7 2.1 1.1 3.0 0.23 144 3.8 1.8 0.63 38 2.8 35R 92.6 2.6 2.1 3.7 0.11 87 2.4 3.2 0.85 36 3.7 Range 10-6100.4-18.4 0.9-9.9 0.3-4 31-50 1.4-6.6 Average 93.0 1.1 0.4 4.3 0.2 192 5.0 4.0 1.44 38.3 3.1 S D 1.82 0.71 0.39 1.54 0.21 171.6 4.58 2.44 0.99 4.60 0.96  S. M. AWADH 135 Table 2. Results of chemical composition of quarry samples. SiO2 Al2O3 Fe2O3 CaO K2O Zr Hf Th U Sample no. % ppm Zr/Hf Th/U 1Q 97.2 0.3 0.03 1.1 0.00122 0.7 0.4 0.13 31 3.1 2Q 98.1 0.5 0.0.1 0.5 0.00517 0.4 1.2 0.33 42 3.6 3Q 96.7 0.2 0.05 2.2 0.00481 2.1 0.9 0.36 39 2.5 4Q 96.9 0.1 0.02 0.2 0.00980 2.0 0.6 0.18 40 3.3 5Q 98.1 0.1 0.02 0.3 0.002360 10 7.2 2.32 36 3.1 6Q 95.8 0.1 0.06 2.2 0.002600 17 9.5 2.87 35 3.3 7Q 97.4 0.3 0.02 1.7 0.00666 1.6 0.4 0.15 40 2.6 8Q 97.5 0.5 0.06 1.1 0.001532 14 7.8 4.30 38 1.8 9Q 96.7 0.3 0.11 2.0 0.00747 1.2 0.5 0.16 39 3.1 10Q 98.2 0.1 0.0 0.2 0.005220 5.5 5.3 1.26 40 4.2 11Q 96.9 0.8 0.04 1.6 0.003111 3.4 3.1 1.00 33 3.1 12Q 97.1 0.4 0.09 1.0 0.002444 11 7.4 2.55 40 2.9 13Q 98.2 0.1 0.05 0.7 0.002405 9.9 6.9 1.91 41 3.6 14Q 98.3 0.1 0.02 0.4 0.001109 2.9 1.1 0.39 37 2.8 15Q 97.9 0.1 0.01 1.4 0.005640 20 10.3 2.57 32 4.0 16Q 98.4 0.1 0.01 0.9 0.003169 4.2 1.9 0.54 40 3.5 17Q 96.5 1.0 0.11 2.5 0.00577 2.2 0.8 0.50 35 1.6 18Q 97.8 0.4 0.07 1.6 0.00666 2.6 0.6 0.20 35 3.0 19Q 98.0 0.1 0.03 0.8 0.002355 9.6 7.1 2.95 37 2.4 20Q 97.9 0.6 0.02 0.8 0.00126 0.7 0.9 0.17 37 5.1 21Q 98.4 0.3 0.01 0.7 0.004523 12 6.3 2.33 43 2.7 22Q 98.1 0.2 0.01 0.5 0.003221 5.6 4.2 1.27 40 3.3 23Q 96.3 0.1 0.12 2.5 0.005203 4.8 3.5 1.10 42 3.2 24Q 98.1 0.2 0.03 1.0 0.00878 2.1 1.2 0.34 37 3.5 25Q 97.3 0.4 0.05 1.8 0.009215 6.3 5.5 1.34 34 4.1 26Q 97.3 0.1 0.01 1.7 0.004211 5.3 4.8 1.92 40 2.5 27Q 96.9 0.2 0.04 1.8 0.003569 16 8.5 3.10 36 2.7 28Q 97.3 0.1 0.02 0.9 0.00699 3.3 1.1 0.46 43 2.4 29Q 99.0 0.05 0.004 0.3 0.00168 2.0 0.8 0.42 34 1.9 30Q 98.8 0.3 0.003 0.5 0.004295 7.6 4.3 1.45 39 3.1 31Q 98.2 0.1 0.02 0.1 0.004725 18 11.1 3.46 40 3.2 32Q 96.5 0.6 0.09 1.6 0.007615 14 9.8 2.80 43 3.5 33Q 96.4 0.7 0.06 1.7 0.010500 14 9.4 2.41 36 3.9 34Q 97.7 0.1 0.08 1.8 0.006333 8.3 6.8 1.88 40 3.6 35Q 97.6 0.4 0.04 1.5 0.00275 2.0 0.7 0.20 35 3.5 Range 95.8-99 17-7250.4-18 0.4-11.1 0.3-4 31-43 1.6-5.1 Average 97 0.28 0.04 1.19 0.004261.66.92 4.34 1.41 38 3.1 S.D 0.77 0.23 0.03 0.7 0.002214.35.68 3.52 1.17 3.2 0.7 94% and 97% (Tables 1-2; and Figure 5) of the total composition of sand glass in termite hill and quarry re- spectively, is represented by quartz which is considered as a major constituent. Beside quartz there are little amount of clay minerals and heavy minerals which inte- grate the chemical co mposition. Clay minerals con tribute a little additional amount of SiO2 and Al2O3 as well as other oxides such as Fe2O3, CaO and K2O. The content of clay minerals in termite hill samples is greater than those in quarry samples because of the sediments adding from the surface, which especially increases during it is windy and rainy, The added sediments appear to be mainly formed from calcite, this has supported by the correlation coefficient (–0.89) between CaO and SiO2 Copyright © 2010 SciRes. IJG  136 S. M. AWADH (Table 3). Zircon appears to be an essential mineral containing Hf, U, and Th which show the greatest affinity to Zr. In termite mounds, the relationships of Zr with Hf (0.99); and Th with U (0.81) are positively strong (Table 3). The concordant of the regression curve between Zr - Hf (Figure 6) and Th - U (Figure 7) for both termite hill and quarry samples indicates same origin. Zr/Hf in both of quarry and termite hill samples seems to be similar with slightly increasing within termite hill samples due Quarry samples Sieve analyses SiltClay Sand Separatio n Heavy miner al 0.5% 0.3% Opaque minerals 0.2% 0.1% Translucent minerals 0.3% 0.2% Quartz 97% 93.% Clay minerals from clay fraction 1.9% 2.9% Calcite 0.8% 4.0% Orthoclase 0.3% 0.1% Tourmal i ne 0.09% 0.08% Zircon 0.06% 0.04% Rutile 0.04% 0.02% Hornbl ende 0.03% 0.008% Staurolite 0.03% 0.025% Biotite 0.03% 0.015% Light minerals 99.5% 99.7% Epidote 0.008% 0.001% Pyroxexe 0.004% 0.003% Monazite 0.002% nil Figure 5. Flow chart displays quantitative and qualitative mineral analyses in both termite hill and quarry samples (values in the upper side represent values in quarry samples, whereas values in the lower represent the values in termite hill samples). Table 3. Correlation coefficient between elements in sand glass of termite hill samples. SiO2 Al2O3 Fe2O3 CaOK2O Zr Hf ThU SiO2 1.00 Al2O3 –0.54 1.00 Fe2O3 –0.13 0.48 1.00 CaO –0.89 0.19 –0.15 1.00 K2O –0.64 0.36 0.12 0.531.00 Zr –0.18 –0.10 0.02 –0.12–0.05 1.00 Hf 0.24 –0.14 0.03 –0.17–0.11 0.99 1.00 Th 0.17 –0.25 –0.13 –0.04–0.26 0.46 0.47 1.00 U 0.21 –0.27 –0.14 –0.05–0.26 0.49 0.50 0.811.00 Figure 6. Relationship between Zr and Hf in termite hills and quarry samples. Figure 7. Relationship between Th and U in termite hills and quarry samples. Copyright © 2010 SciRes. IJG  S. M. AWADH 137 to surficial processes. Consequently Th/U in both types of samples (quarry and termite hills) is the same (Figure 8). This supports a presence of a hidden ore of glass sand deposits directly beneath the termite hills. In quarry samples, the correlation coefficient revealed a strong positive relationship between Zr and each of Hf (0.99), Th (0.96) and U (0.93) (Table 4); whereas in termite hill samples, Zr just linearly correlated with Hf (0.99) but has a weak correlation with each of Th (0.46) and U (0.49) (Table 3). In termite hills and quarry Zr/Hf is 38.3 and 38 and Th/U is 3.1 and 3.1 respectively (Ta- bles 1-2); as independent percentage ratio of Zr/Hf (92%) and Th/U (8%) in quarry samp les but th ese ratios slig htly differed to become Zr/Hf (93%) and Th/U (7%) in ter- mite hill samples (Figure 8); this may be attributed to the uranium dissolution by surficial processes. Zircon, rutile and monazite are resistant carrier minerals for U, Th and Hf. Uranium may loses from hosted minerals such as UO2 due to the high mobility of uran yl ion UO2+2 but thorium has low mobility mainly d ue to the high sta- bility of insoluble oxide ThO2. Figure 8. Zr/Hf and Th/U percentage comparison between quarry samples and termite hill samples. Table 4. Correlation coefficient between elements in quarry sand glass samples. SiO2 Al2O3 Fe2O3 CaOK2O Zr Hf ThU SiO2 1.00 Al2O3 –0.36 1.00 Fe2O3 0.13 0.16 1.00 CaO –0.77 0.35 –0.17 1.00 K2O –0.32 0.16 0.05 0.271.00 Zr –0.10 –0.12 –0.20 –0.02–0.05 1.00 Hf –0.11 –0.13 –0.20 0.010.04 0.99 1.00 Th –0.11 –0.10 –0.16 –0.01–0.01 0.96 0.96 1.00 U –0.09 –0.07 –0.16 –0.02–0.16 0.93 0.92 0.941.00 The tropical to subtropical climate and low relief na- ture of the area produced chemical weathering, high enough to reach the alteration of feldspar which appeared in rare quantities. The presence of altered feldspar beside fresh grains indicates more than one source. The mineral and chemical composition of sediments from the Rutba Formation in the study area define three provenance; these are acidic igneous rocks, metamorphic rocks and basic igneous rocks; the first is represented by granitic rocks of the Arabian-African shield which sup- plied the ultrastable heavy minerals (zircon, tourmaline, rutile and monazite) as well as quartz and orthoclase; and it was considered the main source of glass sand of Rutba formation. Zr/Hf in granitic rocks is 39 but in basic and intermediate igneous rocks is 45 and in pegmatite is 25 in the sedimentary rocks is 35 [22]. The Th/U ratio in most upper crustal rocks is typically between 3.5 and 4.0 [23]. In sedimentary rocks, Th/U values are higher than 4.0 and may indicate intense weathering in source areas or sediment recycling. High Th/U ratios favor the old upper continental crust provenance [24]. In the study area Th/U is 3.1 in both of termite hill and quarry sam- ples; Zr/Hf is 38.3 and 38.0 in termite hill and quarry samples respectively indicating granitic igneous rocks origin. Reworked mature quartz with little feldspar pre- fers granitic rocks as source [25]. The second source is metamorphic rocks in the Arabian shield which prov ided the depositional basin with stable and moderate stable heavy minerals such as hornblende and biotite and the third one is basic igneous rocks which provided the de- positional basin with pyroxene. Second and third sources (metamorphic rocks and basic igneous rocks respectively) are quite nearest the main source (granitic rocks). Reference [26] suggested that only cratonic and pas- sive continental margins have sedimentation rates low enough to allow sufficient reworking to produce first cycle quartzarenite. This can be applied to the Rutba sandstones, which appear to be a craton interior prove- nance. The same heavy and light minerals in the termite hills were found also in quarry indicating that they have the same source and origin. Crystalline parent rocks of the Arabian Shield to the west of Saudi Arabia consist of granites and low and high grade metamorphic rocks [27] support this suggestion. 7. Conclusions Mineralogy and geochemistry of both termite hill and quarry samples appear to be concordant. In general, heavy minerals confirm that the granitic rocks of the Arabian-African Shield were a main source for Rutba Formation. The Zr/Hf supports the concept of granitic rocks origin. Beside this origin, metamorphic and basic igneous rocks located nearest granite rocks contributed in processes of supplying the stable craton with eroded clastics which ultimately formed the Rutba Formation, but they were considered as a secondary origin. The chemical resemblance of major and trace elements, mineralogical constituents and the similarity of source Copyright © 2010 SciRes. IJG  S. M. AWADH Copyright © 2010 SciRes. IJG 138 and origin of sand glass for both of termite hill and quarry provided us with convincing evidence for accept- ing the termite sampling and take it in consideration for exploring the subsurface hidden ore especially glass sand under 36 m depth approximately. 8. References [1] M. J. McCarthy, “Report on an Investigation of Sand- stones and Quartzite in Wadi Amij Area,” Unpublished Internal Report, S.O.M., Baghdad, No. 263, 1954. [2] Technoexport, “Report on Geological Prospecting and Investigation Carried out Rutba Glass Sand Deposits and Estimation of Reserves,” Unpublished Internal Report, S.O.M., Baghdad, No. 303, 1961. [3] Z. Kukal and A. Saadalah, “Paleo-Current in Mesopot- Mian Geocyncline, Soder Druck Ausder Geolgischen,” Rundschan, Band 59, 1970, pp. 666-686. [4] A. Saadalah and J. S. Al-Badri, “Sedimentological Study of Rutba Formation for Glass Industry,” Iraq Journal of Science, Vol. 20, No. 3, 1979, pp. 448-478. [5] T. Buday and J. Hak, “Report on Geological Survey of the Western Part of the Western Desert,” In: V. K. Sis- sakian and B. S. Mohammed, “Stratigraphy,” Iraqi Bulle- tin of Geology and Mining, Special issue of Geology of Iraqi Western Desert, 2007, pp. 51-124. [6] J. P. Le Roux and B. B. Hambleton-Jones, “The Analysis of Termite Hills to Locate Uranium Mineralization in the Karoo Basin of South Africa,” Journal of geochemical exploration, Vol. 41, 1991, pp. 341-347. [7] M. D. Picker, “Burrow and Nest Characteristics of the Harvester Termite (Hodo-Termes Mossambicus) and the Seed Storing Activities of the Common Harvester ant (Messor Barbatus),” Report PER, Atomic Energy Corpo- ration of SA Ltd., No. 145, 1987, pp. 1-28. [8] M. D. Picker, “Soil Movement and Trophic Importance of the Harvester Termite Hodo-Termes Mossambicus, with Recommendations for the Monitoring of Biotic Soil Transport,” Report AEC- 89/03, Atomic Energy Corpora- tion of SA Ltd., 1989, pp. 1-20. [9] J. P. Waston, “Contribution of Termite to Development of Zinc Anomaly in Kalahari Sand,” Institute of Mining and Metallurgical, Vol. 79, 1970, pp. 53-59. [10] J. P. Waston, “The Distribution of Gold in Termite Mounds and Soil at a Gold Anomaly in Kalahari Sand,” Soil Science, Vol. 113, No. 5, 1972, pp. 315-321. [11] W. F. West, “Termite Prospecting: The Bulawayo Symp,” Papers. Chamber of Mines Journal, 1970, pp. 35-322. [12] N. M. Hamza, “Geomorphology,” Iraqi Bulletin of Ge- ology and Mining, Special Issue: Geology of Iraqi West- ern Desert, 2007, pp. 9-27. [13] S. Z. Jassim and G. C. Goff, “Geology of Iraq,” Pub- lished by Dloin, Prague and Moravian Museum, Brno, Printed in the Czech Republic, 2006. [ 14] S. F. A. Fouad, “Tectonic and Structural Evolution,” Iraqi Bulletin of Geology and Mining, Special Issue: Geology of Iraqi Western Desert, 2007, pp. 29-50. [15] V. K. Sissakian and B. S. Mohammed, “Stratigraphy,” Iraqi Bulletin of Geology and Mining, Special Issue: Ge- ology of Iraqi Western Desert, 2007, pp. 51-124. [16] A. M. N. Al-Azawi and R. M. Dawood, “Report on De- tailed Geological Survey in Northwest of kilo 160 – Rutba Area,” In: V. K. Sissakian and B. S. Mohammed, “Stratigraphy,” Iraqi Bulletin of Geology and Mining, Special issue of Geology of Iraqi Western Desert, 2007, pp. 51-124. [17] M. AL-Mubarak and R. M. Amin, “Report on the Geo- logical Mapping of the Eastern Part of the Western De- sert-Western Part of the Southern Desert,” In: V. K. Sis- sakian and B. S. Mohammed, “Stratigraphy,” Iraqi Bulle- tin of Geology and Mining, Special issue of Geology of Iraqi Western Desert, 2007, pp. 51-124. [18] R. C. Van Bellen, H. V. Dunnigton, R. Witzel and D. Morton, “Lexique Stratigraphy International,” Asie, Fasc. 10a, Iraq. Paris, 1959. [19] ] M. J. Hibbard, “Mineralogy,” A View Point of Geology, McGraw-Hill, 2002, [20] E. F. Lener, “Mineral Chemistry of Heavy Minerals in the Old Hickory Deposits,” Sussex and Dinwiddie Coun- ties, Virgenia. M.Sc Thesis, Faculty of the Virginia, Polytechnic Institute, State University, 1997. [21] F. Bea, “Residence of REE, Y, Th and U in Granites and Crustal Protoliths; Implications for the Chemistry of Crustal Melts,” Journal of Petrology, Vol. 37, No. 3, 1996, pp. 521-552. [22] A. J. Erlank, “Zirconium, 40-K, Abundance in Common Sediments and Sedimentary Rocks,” In: K. H. Wedepole Ed., “Handbook of geochemistry,” Springer Verlag, Ber- lin, Vol. 2, 1979. pp. 1-8. [23] S. M. McLennan, S. Hemming, D. K. McDaniel and G. N. Hanson, “Geochemical Approaches to Sedimentation, Provenance and Tectonics. Processes Controlling the Composition of Clastic Sediments,” In: M. J. Johnsson, and A. Basu, Eds., Geological Society of America, Spe- cial Paper 284, 1993, pp. 21-40. [24] K. Daniel, S. S. Asiedu, N. Kenji and S. Tsugio, “Geo- chemistry of Lower Cretaceous Sediments, Inner Zone of Southwest Japan: Constraints on Provenance and Tec- tonic Environment,” Geochemical Journal, Vol. 34, 2000, pp. 155-173. [25] S. Koksal, M. Cemal Goncuoglu, F. Toksoy-Koksal and A. H. Moller, “Zircon Typologies and Internal Structures as Petrogenetic Indicators in Contrasting Granitoid Types From Central Anatolia Turkey,” Mineral Petrol, Vol. 93, 2008, 185-211. [26] F. L. Schwab, “Modern and Ancient Sedimentary Utah,” Sedimentology, Vol. 25, 1978, pp. 97-109. [27] O. A. Al-Harbi and M. M. Khan, “Mineralogy and Geo- chemistry of Unayzah Formation, Central SaudiArabia: Implications for Provenance Interpretation,” Journal of King Saud University, Vol. 18, No. 1, 2005, pp. 35-49. |

