Paper Menu >>
Journal Menu >>
 Psychology 2010. Vol.1, No.5, 367-369 Copyright © 2010 SciRes. DOI:10.4236/psych.2010.15045 Acquisition of Avoidance Responding in the Fmr1 Knockout Mouse Maria G. Valdovinos1, Kelly Ippolito1, Lauren Nawrocki2, Greg Woods2, Craige C. Wrenn3 1Department of Psychology, Drake University, Des Moines, USA; 2Neuroscience Program, Drake University, Des Moines, USA; 3Department of Pharmaceutical, Biomedical, and Administrative Sciences, Drake University, Des Moines, USA. Email: maria.valdovinos@drake.edu Received August 26th, 2010; revised November 1st, 2010; accepted November 19th, 2010. Fragile X Syndrome (FXS) is the most common inherited cause of mental retardation. Much work has been done characterizing the behavioral phenotype of the animal model of FXS, the Fmr1 knockout mouse. However, very little literature exists on knockout performance in the active avoidance task. This study evaluated if Fmr1 knockouts differed from wild type littermates in avoidance acquisition. Data revealed no difference in acquisi- tion between knockouts and wild types. Keywords: Fragile X Syndrome, Fmr1 Knockout, Active Avoidance In fragile X syndrome (FXS), the most common inherited cause of intellectual disability, a repeat of the trinucleotide sequence CGG (> 200) in the promoter region of the FMR1 gene (located on the X chromosome) leads to a silencing of the gene. FMR1 silencing results in a lack of fragile X mental re- tardation protein (FMRP) production (Brown, 2002) and, in the absence of FMRP, abnormal dendritic development occurs involving an overgrowth of immature spines (Beckel-Mitchener & Greenough, 2004; Irwin, Galvez, & Greenough, 2000). The mechanism by which this occurs is unknown, but is hypothe- sized to be attributed to enhanced mGluR activity (Bear, Huber, & Warren, 2004). Since FXS is an X-linked disorder, it is more common in males than females (Sherman, 2002). Males with FXS present with specific physical and behavioral phenotypes. Physically, males with FXS have long narrow faces, prominent ears, macroorchidism, ophthalmologic problems, and unusual growth patterns (initial rapid growth followed by a decline in adolescence) (Hagerman, 2002). Behaviorally, males with FXS present with hyperarousal and hyperactivity which is some- times manifested in tantrums (Hagerman, 2002). Additionally, individuals with FXS are also commonly diagnosed with aut- ism (Dölen & Bear, 2009). Research has also demonstrated that individuals with FXS engage in a high degree of avoidance behavior. One proposed explanation of this behavior has been the high degree of social anxiety experienced by those with FXS which appears to mani- fests itself in the form of social avoidance (e.g., withdrawal, eye-gaze avoidance, self-injurious behavior (SIB), and aggres- sion) (Kau, Reider, Payne, Meyer, & Freund, 2000). Indeed, in a survey of psychotropic medication use among those diag- nosed with FXS, Valdovinos and colleagues (2009) found that approximately 36% of their sample were reported to engage in aggressive behavior, 42% in refusals or opposition, 43% in SIB, and 25% in withdrawal. These findings are significant as re- search has demonstrated that in a majority of cases problem behaviors such as aggression and SIB are maintained by nega- tive reinforcement or escape from some noxious stimulus (e.g., demands, social interactions) (Iwata et al., 1994). Case in point, in a survey of families with boys diagnosed with FXS, Symons and colleagues (2003) found that a majority of their sample was reported to engage or have engaged in self-injurious behavior and that SIB was more likely to have occurred after the presen- tation of a difficult demand suggesting an escape function. Another possible explanation for avoidance behavior in those diagnosed with FXS is that perhaps those with FXS experience hyperarousal of the sympathetic nervous system in response to stimuli (auditory, visual, and tactile) (Hagerman, 2002) as evi- dence by increased cortisol reactivity (Hessl, Glaser, Dyer- Friedman, & Reiss, 2006) and increased magnitude of electro- dermal activity (Miller et al., 1999). The Fmr1 knockout mouse has been demonstrated to be an appropriate model for the human condition as similarities have been observed in both physical and behavioral characteristics (The Dutch Belgium Consortium, 1994). In assessments of avoidance behavior in the knockout, data published have been on the knockout’s performance on the passive avoidance test, an assessment of learning and memory. Results of have not revealed any differences between wild type and knockout per- formance (The Dutch Belgium Consortium, 1994; Qin, Kang, & Smith, 2005). However, limited data on the performance of the Fmr1 knockout on the active avoidance test exist. The dif- ference between these two tasks is that in one test, the ability to associate one side of a cage (i .e., the dark side as opposed to bright side) with shock is measured whereas with the other test, the ability to associate a cue with the shock and subsequent avoidance of the shock is measured. Thus, with the human condition in mind, we assessed nega- tively reinforced behavior in Fmr1 knockout (KO) and wild type (WT) littermates using an active avoidance test. Given the research on avoidance behavior in individuals with FXS, we 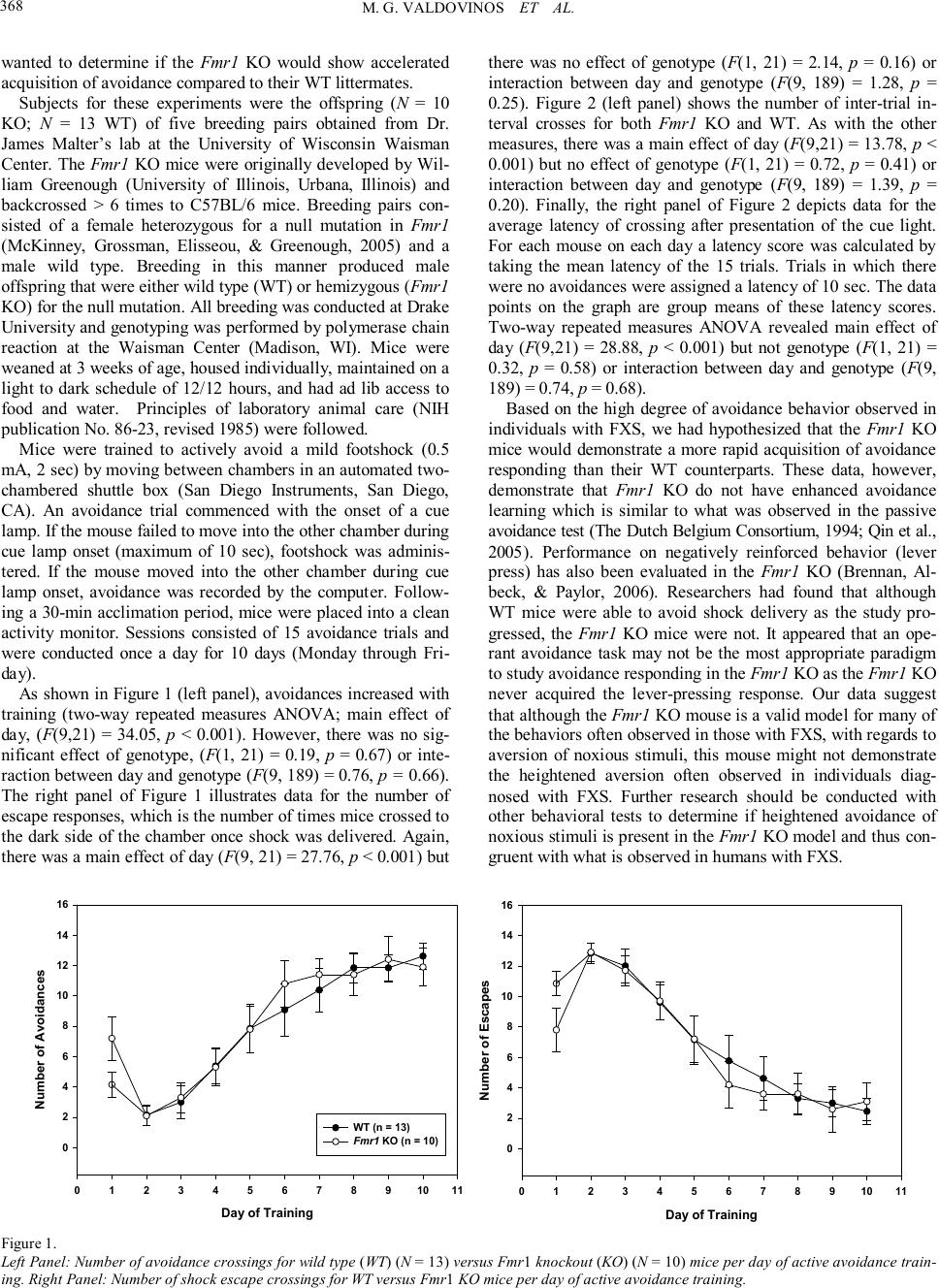 M. G. VALDOVINOS ET AL. 368 wanted to determine if the Fmr1 KO would show accelerated acquisition of avoidance compared to their WT littermates. Subjects for these experiments were the offspring (N = 10 KO; N = 13 WT) of five breeding pairs obtained from Dr. James Malter’s lab at the University of Wisconsin Waisman Center. The Fmr1 KO mice were originally developed by Wil- liam Greenough (University of Illinois, Urbana, Illinois) and backcrossed > 6 times to C57BL/6 mice. Breeding pairs con- sisted of a female heterozygous for a null mutation in Fmr1 (McKinney, Grossman, Elisseou, & Greenough, 2005) and a male wild type. Breeding in this manner produced male offspring that were either wild type (WT) or hemizygous (Fmr1 KO) for the null mutation. All breeding was conducted at Drake University and genotyping was performed by polymerase chain reaction at the Waisman Center (Madison, WI). Mice were weaned at 3 weeks of age, housed individually, maintained on a light to dark schedule of 12/12 hours, and had ad lib access to food and water. Principles of laboratory animal care (NIH publication No. 86-23, revised 1985) were followed. Mice were trained to actively avoid a mild footshock (0.5 mA, 2 sec) by moving between chambers in an automated two- chambered shuttle box (San Diego Instruments, San Diego, CA). An avoidance trial commenced with the onset of a cue lamp. If the mouse failed to move into the other chamber during cue lamp onset (maximum of 10 sec), footshock was adminis- tered. If the mouse moved into the other chamber during cue lamp onset, avoidance was recorded by the computer. Follow- ing a 30-min acclimation period, mice were placed into a clean activity monitor. Sessions consisted of 15 avoidance trials and were conducted once a day for 10 days (Monday through Fri- day). As shown in Figure 1 (l eft panel), avoidances increased with training (two-way repeated measures ANOVA; main effect of day, (F(9,21) = 34.05, p < 0.001). However, there was no sig- nificant effect of genotype, (F(1, 21) = 0.19, p = 0.67) or inte- raction between day and genotype (F(9, 189) = 0.76, p = 0.66). The right panel of Figure 1 illustrates data for the number of escape responses, which is the number of times mice crossed to the dark side of the chamber once shock was delivered. Again, there was a main effect of day (F(9, 21) = 27.76, p < 0.001) but there was no effect of genotype (F(1, 21) = 2.14, p = 0.16) or interaction between day and genotype (F(9, 189) = 1.28, p = 0.25). Figure 2 (left panel) shows the number of inter-trial in- terval crosses for both Fmr1 KO and WT. As with the other measures, there was a main effect of day (F(9,21) = 13.78, p < 0.001) but no effect of genotype (F(1, 21) = 0.72, p = 0.41) or interaction between day and genotype (F(9, 189) = 1.39, p = 0.20). Finally, the right panel of Figure 2 depicts data for the average latency of crossing after presentation of the cue light. For each mouse on each day a latency score was calculated by taking the mean latency of the 15 trials. Trials in which there were no avoidances were assigned a latency of 10 sec. The data points on the graph are group means of these latency scores. Two-way repeated measures ANOVA revealed main effect of day (F(9,21) = 28.88, p < 0.001) but not genotype (F(1, 21) = 0.32, p = 0.58) or interaction between day and genotype (F(9, 189) = 0.74, p = 0.68). Based on the high degree of avoidance behavior observed in individuals with FXS, we had hypothesized that the Fmr1 KO mice would demonstrate a more rapid acquisition of avoidance responding than their WT counterparts. These data, however, demonstrate that Fmr1 KO do not have enhanced avoidance learning which is similar to what was observed in the passive avoidance test (The Dutch Belgium Consortium, 1994; Qin et al., 2005). Performance on negatively reinforced behavior (lever press) has also been evaluated in the Fmr1 KO (Brennan, Al- beck, & Paylor, 2006). Researchers had found that although WT mice were able to avoid shock delivery as the study pro- gressed, the Fmr1 KO mice were not. It appeared that an ope- rant avoidance task may not be the most appropriate paradigm to study avoidance responding in the Fmr1 KO as the Fmr1 KO never acquired the lever-pressing response. Our data suggest that although the Fmr1 KO mouse is a valid model for many of the behaviors often observed in those with FXS, with regards to aversion of noxious stimuli, this mouse might not demonstrate the heightened aversion often observed in individuals diag- nosed with FXS. Further research should be conducted with other behavioral tests to determine if heightened avoidance of noxious stimuli is present in the Fmr1 KO model and thus con- gruent with what is observed in humans with FXS. Day of Training 0 1 2 3 4 5 6 7 8 910 11 Number of Avoidances 0 2 4 6 8 10 12 14 16 WT (n = 13) Fmr1 KO (n = 10) Day of Training 0 1 2 3 4 5 6 7 8 910 11 Number of Escapes 0 2 4 6 8 10 12 14 16 Figure 1. Left Panel: Number of avoidance crossings for wild type (WT) (N = 13) versus Fmr1 knockout (KO) (N = 10) mice per day of active avoidance train- ing. Right Panel: Number of shock escape crossings for WT versus Fmr1 KO mice per day of active avoidance training. 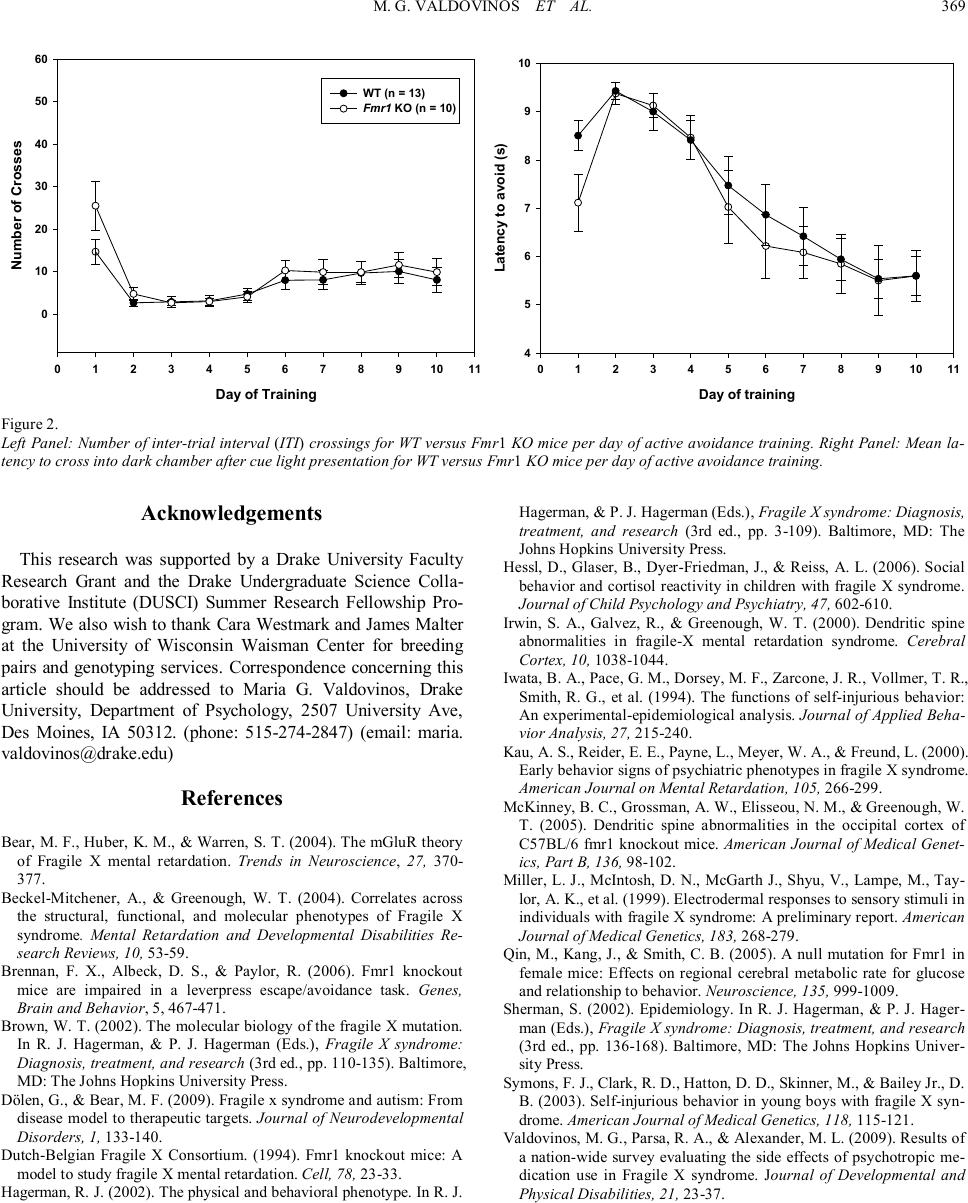 M. G. VALDOVINOS ET AL. 369 Day of Training 0 1 2 3 4 5 6 7 8 910 11 Number of Crosses 0 10 20 30 40 50 60 WT (n = 13) Fmr1 KO (n = 10) Day of training 0 12 34 56 78 910 11 Latency to avoid (s) 4 5 6 7 8 9 10 Figure 2. Left Panel: Number of inter-trial interval (ITI) crossings for WT versus Fmr1 KO mice per day of active avoidance training. Righ t Panel: Mean la- tency to cross into dark chamber after cue light presentation for WT versus Fmr1 KO mice per day of active avoidance training. Ac knowledg ements This research was supported by a Drake University Faculty Research Grant and the Drake Undergraduate Science Colla- borative Institute (DUSCI) Summer Research Fellowship Pro- gram. We also wish to thank Cara Westmark and James Malter at the University of Wisconsin Waisman Center for breeding pairs and genotyping services. Correspondence concerning this article should be addressed to Maria G. Valdovinos, Drake University, Department of Psychology, 2507 University Ave, Des Moines, IA 50312. (phone: 515-274-2847) (email: maria. va ldo vinos@dra ke.edu) Re fere nces Bear, M. F., Huber, K. M., & Warren, S. T. (2004). The mGluR theory of Fragile X mental retardation. Trends in Neuroscience, 27, 370- 377. Beckel-Mitchener, A., & Greenough, W. T. (2004). Correlates across the structural, functional, and molecular phenotypes of Fragile X syndrome. Mental Retardation and Developmental Disabilities Re- search Reviews, 10, 53-59. Brennan, F. X., Albeck, D. S., & Paylor, R. (2006). Fmr1 knockout mice are impaired in a leverpress escape/avoidance task. Genes, Brain and Behavior, 5, 467-471. Brown, W. T. (2002). The molecular biology of the fragile X mutation. In R. J. Hagerman, & P. J. Hagerman (Eds.), Fragile X syndrome: Diagnosis, treatment, and research (3rd ed., pp. 110-135). Baltimore, MD: The Johns Hopkins University Press. Dölen, G., & Bear, M. F. (2009). Fragile x syndrome and autism: From disease model to therapeutic targets. Journal of Neurodevelopmental Disorders, 1, 133-140. Dutch-Belgian Fragile X Consortium. (1994). Fmr1 knockout mice: A model to study fragile X mental retardation. Cell, 78, 23-33. Hagerman, R. J. (2002). The physical and behavioral phenotype. In R. J. Hagerman, & P. J. Hagerman (Eds.), Fragile X syndrome: Diagnosis, treatment, and research (3rd ed., pp. 3-109). Baltimore, MD: The Johns Hopkins University Press. Hessl, D., Glaser, B., Dyer-Friedman, J., & Reiss, A. L. (2006). Social behavior and cortisol reactivity in children with fragile X syndrome. Journal of Child Psychology and Psychiatry, 47, 602-610. Irwin, S. A., Galvez, R., & Greenough, W. T. (2000). Dendritic spine abnormalities in fragile-X mental retardation syndrome. Cerebral Cor te x, 10, 1038-1044. Iwata, B. A., Pace, G. M., Dorsey, M. F., Zarcone, J. R., Vollmer, T. R., Smith, R. G., et al. (1994). The functions of self-injurious behavior: An experimental-epidemiological analysis. Journal of Applied Beha- vior Analysis, 27, 215-240. Kau, A. S., Reider, E. E., Payne, L., Meyer, W. A., & Freund, L. (2000). Early behavior signs of psychiatric phenotypes in fragile X syndrome. American Journal on Mental Retardation, 105, 266-299. McKinney, B. C., Grossman, A. W., Elisseou, N. M., & Greenough, W. T. (2005). Dendritic spine abnormalities in the occipital cortex of C57BL/6 fmr1 knockout mice. American Journal of Medical Genet- ics, Part B, 136, 98-102. Miller, L. J., McIntosh, D. N., McGarth J., Shyu, V., Lampe, M., Tay- lor, A. K., et al. (1999). Electrodermal responses to sensory stimuli in individuals with fragile X syndrome: A preliminary report. American Journal of Medical Genetics, 183, 268-279. Qin, M., Kang, J., & Smith, C. B. (2005). A null mutation for Fmr1 in female mice: Effects on regional cerebral metabolic rate for glucose and relationship to behavior. Neuroscience, 135, 999-1009. Sherman, S. (2002). Epidemiology. In R. J. Hagerman, & P. J. Hager- man (Eds.), Fragile X syndrome: Diagnosis, treatment, and research (3rd ed., pp. 136-168). Baltimore, MD: The Johns Hopkins Univer- sity Press. Symons, F. J., Clark, R. D., Hatton, D. D., Skinner, M., & Bailey Jr., D. B. (2003). Self-injurious behavior in young boys with fragile X syn- drome. American Journal of Medical Genetics, 118, 115-121. Valdovinos, M. G., Parsa, R. A., & Alexander, M. L. (2009). Results of a nation-wide survey evaluating the side effects of psychotropic me- dication use in Fragile X syndrome. Journal of Developmental and Physical Disabilities, 21, 23-37. |

