 Vol.2, No.11, 1264-1273 (2010) Natural Science http://dx.doi.org/10.4236/ns.2010.211153 Copyright © 2010 SciRes. OPEN ACCESS Long-term culture of primary porcine mature hepatocytes in the medium supplemented with ascorbic acid 2-phosphate Yohichi Kumaki1*, Iku Kumaki2, Xiaomei Guo3, Weilin Shang4, Toshie Koyama1, Ai Okamura1, Yoshiaki Shiba1, Toshiyuki Mukaiyama1, Noriko Sasaki1, Makoto Kodama1 1Tissue Engineering Research Center, National Institute of Advanced Industrial Science and Technology, Higashi, Tsukuba, Ibaraki, Japan; *Corresponding author: yohichi.kumaki@usu.edu 2Department of Laboratory Science, Gunma University School of Health Science, Showa-machi, Maebashi, Gunma, Japan 3Laser Vision Center, Suzhou Eye Hospital, 18 Shuyuan Lane, Suzhou City, Jiangsu Province, China 4304th Clinical Department of Chinese PLA General Hospital, Fucheng Road, Haidian District, Beijing, China Received 27 July 2010; revised 28 August 2010; accepted 2 September 2010. ABSTRACT In this study, the effect of ascorbic acid 2-phosphate (Asc2P) was tested on porcine and rat mature hepatocytes in vitro. a). Asc2P in- creased the porcine, but not rat, albumin secre- tion and mRNA expression. The enhancing ef- fect of Asc2P on porcine C/EBP alpha mRNA was observed in porcine mature hepatocytes. These data suggested that Asc2P played an important role in the regulation of porcine al- bumin mRNA level. b). The enhancing effect of Asc2P on ammonium metabolic activity was also observed in porcine, but not rat, mature hepatocytes. The porcine ornithine transcar- bamylase (OTC) and arginase mRNAs were augmented by Asc2P, indicating that Asc2P had a direct effect on the urea cycle. c). The porcine collagen type I and type III mRNA, but not type XII mRNA, were detected as well, sugessting that Asc2P did not have the effect on the non-parenchymal hepatocytes to induce colla- gen type I and III mRNA expression. d). Our RT-PCR analysis demonstrated that the porcine hepatocytes expressed the sodium-ascorbate co-transporters SVCT1 and SVCT2, however, the intensities of porcine sodium-ascorbate co-transporters SVCT1 and SVCT2 bands were not changed markedly. These findings indicated that the Asc2P had no effect on SVCT1 and SVCT2 mRNA expression. e). The enhancing effect of Asc2P on porcine albumin mRNA was inhibited by staurosporine, a portein kinase in- hibitor. We conclude that the enhanced albumin mRNA by Asc2P might be due to activation of tyrosine protein kinase and/or PKC and the Asc2P enhanced porcine albumin mRNA mainly at the transcriptional step. Keywords: Albumin Secretion; Ammonium Metabolic Activity; Ascorbic Acid 2-Phosphate; Porcine Hepatocytes; Reverse Transcriptase-Polymerase Chain Reaction 1. INTRODUCTION Primary cultures of hepatocytes have been used as a model system to investigate liver function, including drug metabolism, hepatotoxicity, protein biosynthesis and gene expression. Primary porcine hepatocytes are currently used in research and therapeutic applications as the biological component of extracorporeal liver assist devices. Tateno and Yoshizato reported that a long-term cultivation of adult rat small hepatocytes had been de- veloped for more than two months without losing their replicative potential and differentiation capacity [1]. They further indicated that the medium used supported the continuous growth of small hepatocytes for an ex- tended period [2]. The culture medium contained four key substances in addition to fetal bovine serum, that is, epidermal growth factor, nicotinamide, dimethyl sulfox- ide and ascorbic acid 2-phosphate. They also co-cultured human parenchymal hepatocytes with Swiss 3T3 cells in the medium containing human serum, EGF, nicontina- mide, and ascorbic acid 2-phosphate [3]. Katsura et al. reported that primary human hepatocytes were cultured in keratinocyte-stimulating factor medium supplemented with 10% human serum, 10 mM nicotinamide, 10 ng/ml epidermal growth factor, 0.5 microg/ml insulin, 10(-7) M dexamethasone [4]. We previously reported that the  Y. Kumaki et al. / Natural Science 2 (2010) 1264-1273 Copyright © 2010 SciRes. OPEN ACCESS 1265 proliferation of porcine mature hepatocytes was ob- served for an extended period using a medium, which contains Williams’ medium E, fetal bovine serum, epi- dermal growth factor, insulin, dexamethasone and ascor- bic acid 2-phosphate. L-ascorbic acid or vitamin C, a naturally occurring major antioxidant, is one of the specialized products from the secondary pathway of glucose oxidation. Ascorbic acid 2-phosphate (Asc2P) is a stable vitamin C derivative. In this study, we first tested the effect of Asc2P on porcine and rat mature hepatocytes and no- ticed that the albumin secretion released into the culture medium from the porcine hepatocytes was increased by Asc2P. Asc2P also increased the ammonium metabolic activity of porcine hepatocytes. However, Asc2P did not give any effect to rat hepatocytes on albumin secretion and ammonium metabolic activity. Based on these ob- servations, we therefore further investigated the mode of action of Asc2P in vitro. Our data had a direct impact on our understanding of the mechanism for vitamin C on the porcine hepatocytes as well as on the transport of the vitamin C across the intestinal barrier. 2. MATERIALS AND METHODS 2.1. Cells and Chemicals Porcine hepatocytes were isolated by perfusion method using dispase and collagenase. Rat hepatocytes were isolated by the standard two-step collagenase per- fusion method from male Wister rats (150-250 g). Iso- lated hepatocytes were cultured in the Williams’ medium E (Gibco BRL) supplemented with 10% fetal bovine serum (FBS) (Sigma). This medium also contained insu- lin (10-7 M) (Wako), dexamethasone (10-6 M) (Wako), epidermal growth factor (EGF) (25 ng/ml) (Wako), penicillin (100 U/ml) and streptomycin (100 µg/ml) (Sigma). The isolated hepatocytes were finally sus- pended at a concentration of 1.5 x 105 cells/ml. Four milliliters of cell suspension were placed into 60-mm culture Iwaki dishes coated with collagen type I and the cells were incubated at 37°C and 5% CO2. The medium was changed every other day. Hep G2 cells are a human cell line, derived from a patient with hepatocellular car- cinoma [5,6]. This cell line was maintained in Dul- becco’s modified Eagle medium (DMEM) (Gibco BRL) containing 10% FBS. Both sera were heat-inactivated (56°C, 30 min) before use. Asc2P (Wako) was dissolved in PBS(-) and sterilized through a 0.2 µm Millipore filter. The solution was stored at –20°C until use. 2.2. Assay for the Albumin Secretion The hepatocytes were seeded as mentioned above and Asc2P was added to the each well at the concentrations of 1.5 and 0.0 mM. The medium was collected during the incubation and stored at –20°C until use. The secreted porcine or rat albumin was determined by enzyme-linked immunosorbent assay (ELISA). We used the two-anti- body sandwich method. Briefly, 50 ng of goat anti-pig or rabbit anti-rat albumin antibody (Bethyl) was bound to each well of a high binding microtiter plate (Costar). After incubation overnight, samples (1000-times-diluted with culture medium) were applied to the wells. Porcine or rat albumin (Sigma) was used as a standard. Then, 50 ng of goat anti-pig or rabbit anti-rat albumin antibody conjugated to the horseradish peroxidase (Bethyl) was added to each well. O-phenylendiamine (Sigma) solution in 0.01% H2O2 (3 mg/ml) was used as a substrate and the reaction was stopped by adding 50 µl of 1.0 mol/l H2SO4. A microplate reader was used for measurement at 490 nm wavelength. 2.3. Assay for Ammonium Metabolic Activity The hepatocytes were incubated in the presence or absence of Asc2P. 0.4 ml of 100 mmol/l NH4Cl (Wako) was added to the medium and 0.1 ml of medium was collected 0, 3 and 6 hours later. Then, the medium was replaced with fresh medium. The ammonium concentra- tion was measured using a commercial kit (AMICHECK™ meter, Arkray Factory Inc., Japan). The same assay sys- tem was carried out during the incubation. 2.4. RNA Extraction The hepatocytes were seeded and Asc2P was added at the concentrations of 15, 7.5, 1.5 and 0.0 mM. Total RNA was isolated from hepatocytes with ISOGEN re- agent (Nippon Gene), according to the manufacturer’s instructions. The RNA was precipitated with 70% etha- nol, resuspended in 20 µl of sterile distilled water con- taining 0.1% (v/v) diethyl pyrocarbonate (DEPC) and stored at –80°C before use. RNA was quantified spec- trophotometrically and tested for integrity by using de- naturing agarose-gel electrophoresis. 2.5. Analysis of Albumin and C/EBP alpha mRNA Expression by RT-PCR Each specific mRNA for the amplification was ana- lysed by reverse transcriptase-polymerase chain reaction (RT-PCR). Reverse transcriptase assay was carried out using total RNA samples and Ready-To-Go RT-PCR Beads (Amersham). Equal amounts of total cellular RNA (2.0 µg) were used to be reverse-transcribed into cDNA at 42°C for 60 min using pd(N)6 Random hexamer (Amersham). Before PCR amplification, the reverse transcriptase enzyme was inactivated at 95°C for 5 min. PCR was performed in a total volume of 50 µl con- 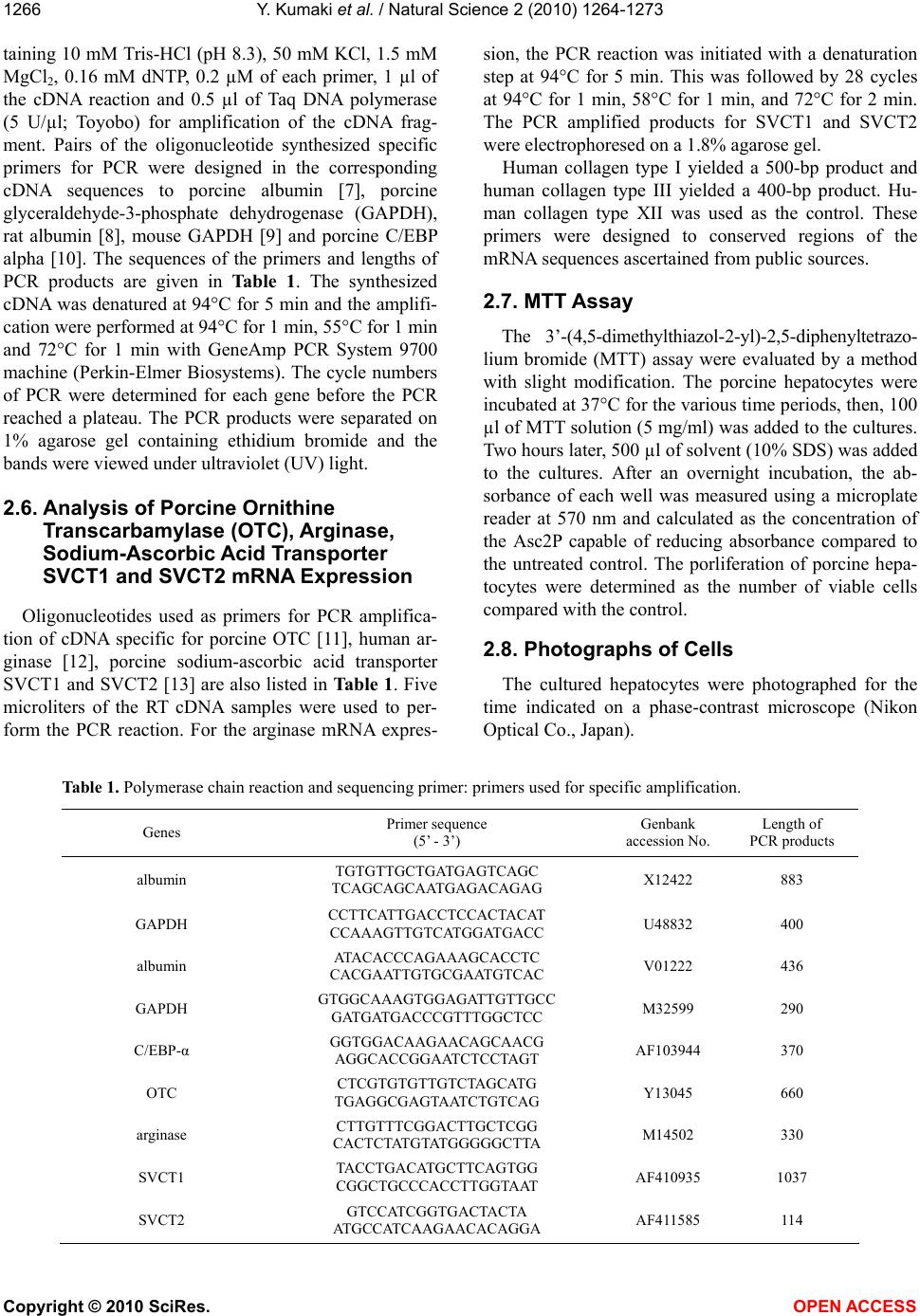 Y. Kumaki et al. / Natural Science 2 (2010) 1264-1273 Copyright © 2010 SciRes. OPEN ACCESS 1266 taining 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.16 mM dNTP, 0.2 µM of each primer, 1 µl of the cDNA reaction and 0.5 µl of Taq DNA polymerase (5 U/µl; Toyobo) for amplification of the cDNA frag- ment. Pairs of the oligonucleotide synthesized specific primers for PCR were designed in the corresponding cDNA sequences to porcine albumin [7], porcine glyceraldehyde-3-phosphate dehydrogenase (GAPDH), rat albumin [8], mouse GAPDH [9] and porcine C/EBP alpha [10]. The sequences of the primers and lengths of PCR products are given in Table 1. The synthesized cDNA was denatured at 94°C for 5 min and the amplifi- cation were performed at 94°C for 1 min, 55°C for 1 min and 72°C for 1 min with GeneAmp PCR System 9700 machine (Perkin-Elmer Biosystems). The cycle numbers of PCR were determined for each gene before the PCR reached a plateau. The PCR products were separated on 1% agarose gel containing ethidium bromide and the bands were viewed under ultraviolet (UV) light. 2.6. Analysis of Porcine Ornithine Transcarbamylase (OTC), Arginase, Sodium-Ascorbic Acid Transporter SVCT1 and SVCT2 mRNA Expression Oligonucleotides used as primers for PCR amplifica- tion of cDNA specific for porcine OTC [11], human ar- ginase [12], porcine sodium-ascorbic acid transporter SVCT1 and SVCT2 [13] are also listed in Table 1. Five microliters of the RT cDNA samples were used to per- form the PCR reaction. For the arginase mRNA expres- sion, the PCR reaction was initiated with a denaturation step at 94°C for 5 min. This was followed by 28 cycles at 94°C for 1 min, 58°C for 1 min, and 72°C for 2 min. The PCR amplified products for SVCT1 and SVCT2 were electrophoresed on a 1.8% agarose gel. Human collagen type I yielded a 500-bp product and human collagen type III yielded a 400-bp product. Hu- man collagen type XII was used as the control. These primers were designed to conserved regions of the mRNA sequences ascertained from public sources. 2.7. MTT Assay The 3’-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazo- lium bromide (MTT) assay were evaluated by a method with slight modification. The porcine hepatocytes were incubated at 37°C for the various time periods, then, 100 µl of MTT solution (5 mg/ml) was added to the cultures. Two hours later, 500 µl of solvent (10% SDS) was added to the cultures. After an overnight incubation, the ab- sorbance of each well was measured using a microplate reader at 570 nm and calculated as the concentration of the Asc2P capable of reducing absorbance compared to the untreated control. The porliferation of porcine hepa- tocytes were determined as the number of viable cells compared with the control. 2.8. Photographs of Cells The cultured hepatocytes were photographed for the time indicated on a phase-contrast microscope (Nikon Optical Co., Japan). Table 1. Polymerase chain reaction and sequencing primer: primers used for specific amplification. Genes Primer sequence (5’ - 3’) Genbank accession No. Length of PCR products albumin TGTGTTGCTGATGAGTCAGC TCAGCAGCAATGAGACAGAG X12422 883 GAPDH CCTTCATTGACCTCCACTACAT CCAAAGTTGTCATGGATGACC U48832 400 albumin ATACACCCAGAAAGCACCTC CACGAATTGTGCGAATGTCAC V01222 436 GAPDH GTGGCAAAGTGGAGATTGTTGCC GATGATGACCCGTTTGGCTCC M32599 290 C/EBP-α GGTGGACAAGAACAGCAACG AGGCACCGGAATCTCCTAGT AF103944 370 OTC CTCGTGTGTTGTCTAGCATG TGAGGCGAGTAATCTGTCAG Y13045 660 arginase CTTGTTTCGGACTTGCTCGG CACTCTATGTATGGGGGCTTA M14502 330 SVCT1 TACCTGACATGCTTCAGTGG CGGCTGCCCACCTTGGTAAT AF410935 1037 SVCT2 GTCCATCGGTGACTACTA ATGCCATCAAGAACACAGGA AF411585 114 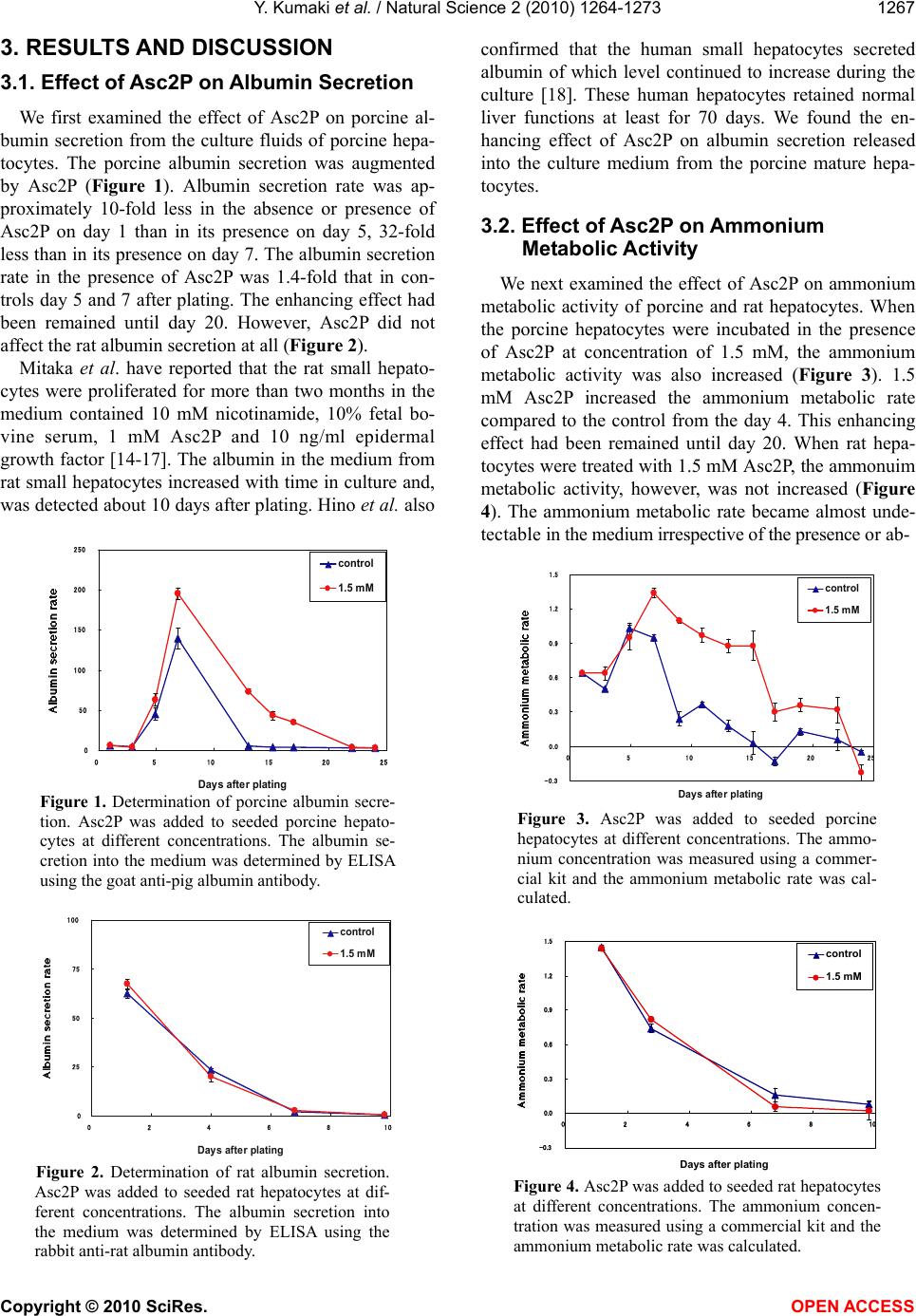 Y. Kumaki et al. / Natural Science 2 (2010) 1264-1273 Copyright © 2010 SciRes. OPEN ACCESS 1267 3. RESULTS AND DISCUSSION 3.1. Effect of Asc2P on Albumin Secretion We first examined the effect of Asc2P on porcine al- bumin secretion from the culture fluids of porcine hepa- tocytes. The porcine albumin secretion was augmented by Asc2P (Figure 1). Albumin secretion rate was ap- proximately 10-fold less in the absence or presence of Asc2P on day 1 than in its presence on day 5, 32-fold less than in its presence on day 7. The albumin secretion rate in the presence of Asc2P was 1.4-fold that in con- trols day 5 and 7 after plating. The enhancing effect had been remained until day 20. However, Asc2P did not affect the rat albumin secretion at all (Figure 2). Mitaka et al. have reported that the rat small hepato- cytes were proliferated for more than two months in the medium contained 10 mM nicotinamide, 10% fetal bo- vine serum, 1 mM Asc2P and 10 ng/ml epidermal growth factor [14-17]. The albumin in the medium from rat small hepatocytes increased with time in culture and, was detected about 10 days after plating. Hino et al. also 0 50 100 150 200 250 0510152025 Days after plating control 1.5 mM Figure 1. Determination of porcine albumin secre- tion. Asc2P was added to seeded porcine hepato- cytes at different concentrations. The albumin se- cretion into the medium was determined by ELISA using the goat anti-pig albumin antibody. 0 25 50 75 100 0246810 Days after plating control 1.5 mM Figure 2. Determination of rat albumin secretion. Asc2P was added to seeded rat hepatocytes at dif- ferent concentrations. The albumin secretion into the medium was determined by ELISA using the rabbit anti-rat albumin antibody. confirmed that the human small hepatocytes secreted albumin of which level continued to increase during the culture [18]. These human hepatocytes retained normal liver functions at least for 70 days. We found the en- hancing effect of Asc2P on albumin secretion released into the culture medium from the porcine mature hepa- tocytes. 3.2. Effect of Asc2P on Ammonium Metabolic Activity We next examined the effect of Asc2P on ammonium metabolic activity of porcine and rat hepatocytes. When the porcine hepatocytes were incubated in the presence of Asc2P at concentration of 1.5 mM, the ammonium metabolic activity was also increased (Figure 3). 1.5 mM Asc2P increased the ammonium metabolic rate compared to the control from the day 4. This enhancing effect had been remained until day 20. When rat hepa- tocytes were treated with 1.5 mM Asc2P, the ammonuim metabolic activity, however, was not increased (Figure 4). The ammonium metabolic rate became almost unde- tectable in the medium irrespective of the presence or ab- -0.3 0.0 0.3 0.6 0.9 1.2 1.5 0510 1520 25 Days after plating control 1.5 mM Figure 3. Asc2P was added to seeded porcine hepatocytes at different concentrations. The ammo- nium concentration was measured using a commer- cial kit and the ammonium metabolic rate was cal- culated. -0.3 0.0 0.3 0.6 0.9 1.2 1.5 0246810 Days after plating control 1.5 mM Figure 4. Asc2P was added to seeded rat hepatocytes at different concentrations. The ammonium concen- tration was measured using a commercial kit and the ammonium metabolic rate was calculated. 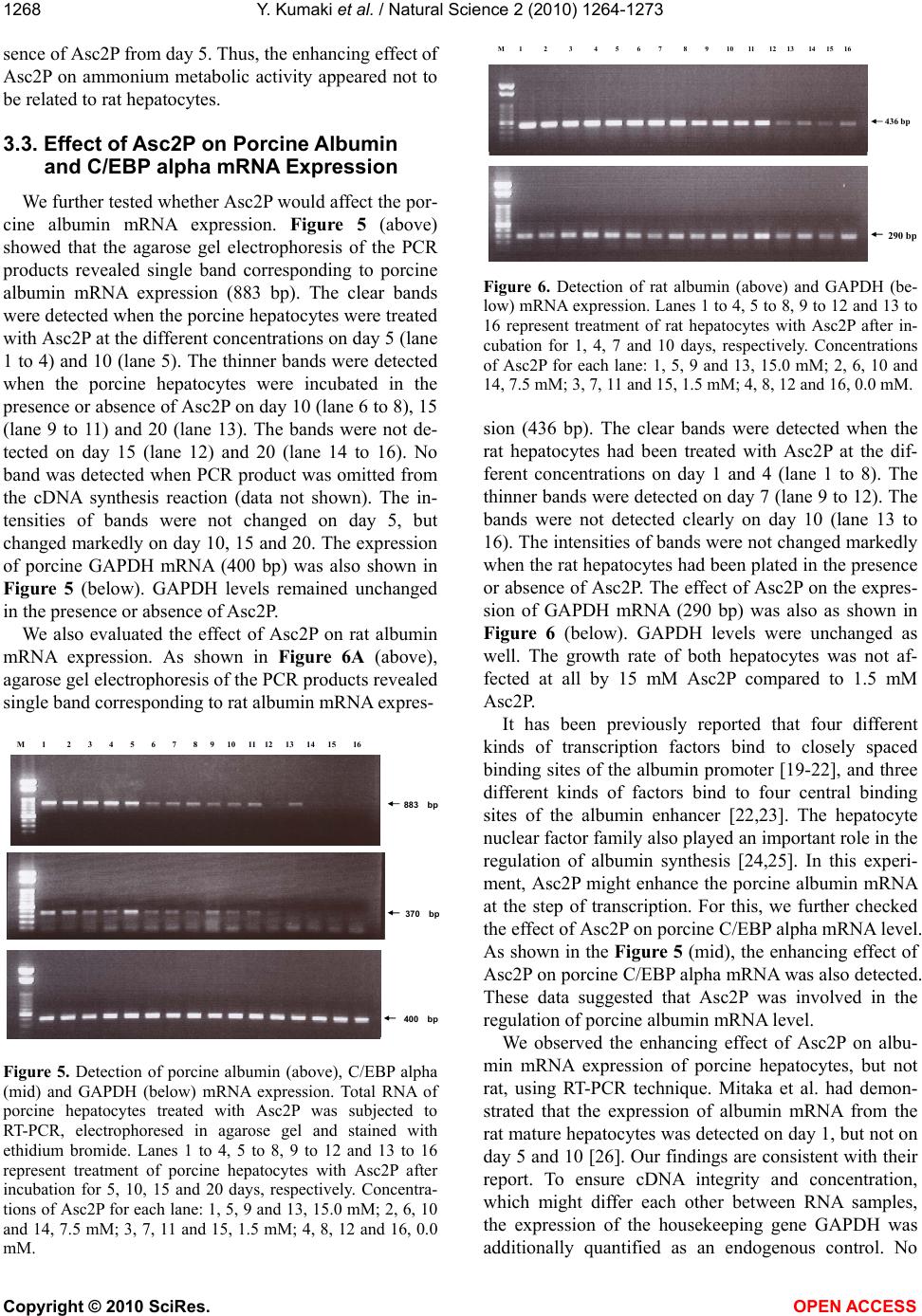 Y. Kumaki et al. / Natural Science 2 (2010) 1264-1273 Copyright © 2010 SciRes. OPEN ACCESS 1268 sence of Asc2P from day 5. Thus, the enhancing effect of Asc2P on ammonium metabolic activity appeared not to be related to rat hepatocytes. 3.3. Effect of Asc2P on Porcine Albumin and C/EBP alpha mRNA Expression We further tested whether Asc2P would affect the por- cine albumin mRNA expression. Figure 5 (above) showed that the agarose gel electrophoresis of the PCR products revealed single band corresponding to porcine albumin mRNA expression (883 bp). The clear bands were detected when the porcine hepatocytes were treated with Asc2P at the different concentrations on day 5 (lane 1 to 4) and 10 (lane 5). The thinner bands were detected when the porcine hepatocytes were incubated in the presence or absence of Asc2P on day 10 (lane 6 to 8), 15 (lane 9 to 11) and 20 (lane 13). The bands were not de- tected on day 15 (lane 12) and 20 (lane 14 to 16). No band was detected when PCR product was omitted from the cDNA synthesis reaction (data not shown). The in- tensities of bands were not changed on day 5, but changed markedly on day 10, 15 and 20. The expression of porcine GAPDH mRNA (400 bp) was also shown in Figure 5 (below). GAPDH levels remained unchanged in the presence or absence of Asc2P. We also evaluated the effect of Asc2P on rat albumin mRNA expression. As shown in Figure 6A (above), agarose gel electrophoresis of the PCR products revealed single band corresponding to rat albumin mRNA expres- 400 bp 883 bp M 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 370 bp Figure 5. Detection of porcine albumin (above), C/EBP alpha (mid) and GAPDH (below) mRNA expression. Total RNA of porcine hepatocytes treated with Asc2P was subjected to RT-PCR, electrophoresed in agarose gel and stained with ethidium bromide. Lanes 1 to 4, 5 to 8, 9 to 12 and 13 to 16 represent treatment of porcine hepatocytes with Asc2P after incubation for 5, 10, 15 and 20 days, respectively. Concentra- tions of Asc2P for each lane: 1, 5, 9 and 13, 15.0 mM; 2, 6, 10 and 14, 7.5 mM; 3, 7, 11 and 15, 1.5 mM; 4, 8, 12 and 16, 0.0 mM. 290 b 436 bp M 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 Figure 6. Detection of rat albumin (above) and GAPDH (be- low) mRNA expression. Lanes 1 to 4, 5 to 8, 9 to 12 and 13 to 16 represent treatment of rat hepatocytes with Asc2P after in- cubation for 1, 4, 7 and 10 days, respectively. Concentrations of Asc2P for each lane: 1, 5, 9 and 13, 15.0 mM; 2, 6, 10 and 14, 7.5 mM; 3, 7, 11 and 15, 1.5 mM; 4, 8, 12 and 16, 0.0 mM. sion (436 bp). The clear bands were detected when the rat hepatocytes had been treated with Asc2P at the dif- ferent concentrations on day 1 and 4 (lane 1 to 8). The thinner bands were detected on day 7 (lane 9 to 12). The bands were not detected clearly on day 10 (lane 13 to 16). The intensities of bands were not changed markedly when the rat hepatocytes had been plated in the presence or absence of Asc2P. The effect of Asc2P on the expres- sion of GAPDH mRNA (290 bp) was also as shown in Figure 6 (below). GAPDH levels were unchanged as well. The growth rate of both hepatocytes was not af- fected at all by 15 mM Asc2P compared to 1.5 mM Asc2P. It has been previously reported that four different kinds of transcription factors bind to closely spaced binding sites of the albumin promoter [19-22], and three different kinds of factors bind to four central binding sites of the albumin enhancer [22,23]. The hepatocyte nuclear factor family also played an important role in the regulation of albumin synthesis [24,25]. In this experi- ment, Asc2P might enhance the porcine albumin mRNA at the step of transcription. For this, we further checked the effect of Asc2P on porcine C/EBP alpha mRNA level. As shown in the Figure 5 (mid), the enhancing effect of Asc2P on porcine C/EBP alpha mRNA was also detected. These data suggested that Asc2P was involved in the regulation of porcine albumin mRNA level. We observed the enhancing effect of Asc2P on albu- min mRNA expression of porcine hepatocytes, but not rat, using RT-PCR technique. Mitaka et al. had demon- strated that the expression of albumin mRNA from the rat mature hepatocytes was detected on day 1, but not on day 5 and 10 [26]. Our findings are consistent with their report. To ensure cDNA integrity and concentration, which might differ each other between RNA samples, the expression of the housekeeping gene GAPDH was additionally quantified as an endogenous control. No 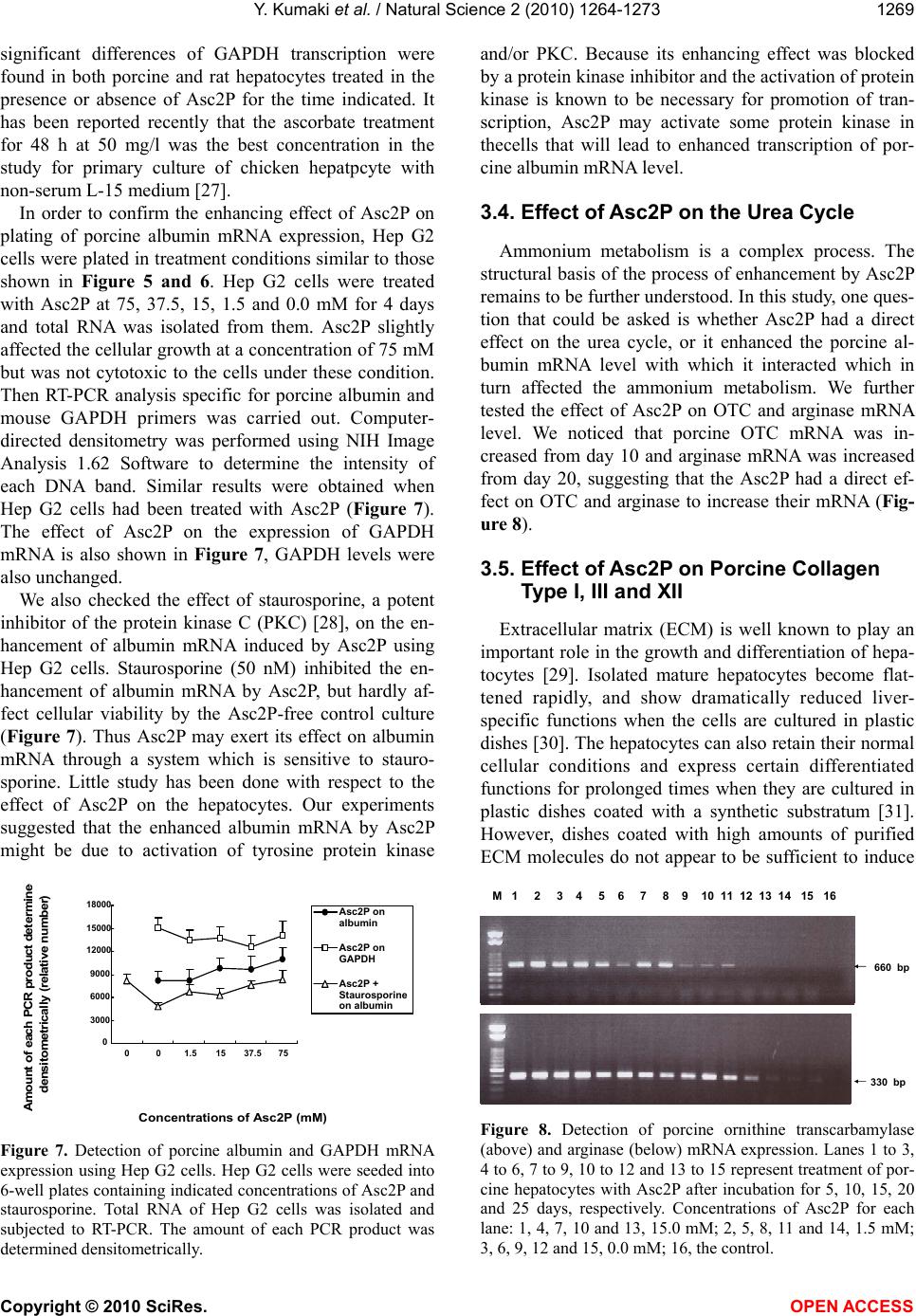 Y. Kumaki et al. / Natural Science 2 (2010) 1264-1273 Copyright © 2010 SciRes. OPEN ACCESS 1269 significant differences of GAPDH transcription were found in both porcine and rat hepatocytes treated in the presence or absence of Asc2P for the time indicated. It has been reported recently that the ascorbate treatment for 48 h at 50 mg/l was the best concentration in the study for primary culture of chicken hepatpcyte with non-serum L-15 medium [27]. In order to confirm the enhancing effect of Asc2P on plating of porcine albumin mRNA expression, Hep G2 cells were plated in treatment conditions similar to those shown in Figure 5 and 6. Hep G2 cells were treated with Asc2P at 75, 37.5, 15, 1.5 and 0.0 mM for 4 days and total RNA was isolated from them. Asc2P slightly affected the cellular growth at a concentration of 75 mM but was not cytotoxic to the cells under these condition. Then RT-PCR analysis specific for porcine albumin and mouse GAPDH primers was carried out. Computer- directed densitometry was performed using NIH Image Analysis 1.62 Software to determine the intensity of each DNA band. Similar results were obtained when Hep G2 cells had been treated with Asc2P (Figure 7). The effect of Asc2P on the expression of GAPDH mRNA is also shown in Figure 7, GAPDH levels were also unchanged. We also checked the effect of staurosporine, a potent inhibitor of the protein kinase C (PKC) [28], on the en- hancement of albumin mRNA induced by Asc2P using Hep G2 cells. Staurosporine (50 nM) inhibited the en- hancement of albumin mRNA by Asc2P, but hardly af- fect cellular viability by the Asc2P-free control culture (Figure 7). Thus Asc2P may exert its effect on albumin mRNA through a system which is sensitive to stauro- sporine. Little study has been done with respect to the effect of Asc2P on the hepatocytes. Our experiments suggested that the enhanced albumin mRNA by Asc2P might be due to activation of tyrosine protein kinase Concentrations of Asc2P (mM) Amount of each PCR product determine densitometrically (relative number) 0 3000 6000 9000 12000 15000 18000 001.51537.575 Asc2P on albumin Asc2P on GAPDH Asc2P + Staurosporine on albumin Figure 7. Detection of porcine albumin and GAPDH mRNA expression using Hep G2 cells. Hep G2 cells were seeded into 6-well plates containing indicated concentrations of Asc2P and staurosporine. Total RNA of Hep G2 cells was isolated and subjected to RT-PCR. The amount of each PCR product was determined densitometrically. and/or PKC. Because its enhancing effect was blocked by a protein kinase inhibitor and the activation of protein kinase is known to be necessary for promotion of tran- scription, Asc2P may activate some protein kinase in thecells that will lead to enhanced transcription of por- cine albumin mRNA level. 3.4. Effect of Asc2P on the Urea Cycle Ammonium metabolism is a complex process. The structural basis of the process of enhancement by Asc2P remains to be further understood. In this study, one ques- tion that could be asked is whether Asc2P had a direct effect on the urea cycle, or it enhanced the porcine al- bumin mRNA level with which it interacted which in turn affected the ammonium metabolism. We further tested the effect of Asc2P on OTC and arginase mRNA level. We noticed that porcine OTC mRNA was in- creased from day 10 and arginase mRNA was increased from day 20, suggesting that the Asc2P had a direct ef- fect on OTC and arginase to increase their mRNA (Fig- ure 8). 3.5. Effect of Asc2P on Porcine Collagen Type I, III and XII Extracellular matrix (ECM) is well known to play an important role in the growth and differentiation of hepa- tocytes [29]. Isolated mature hepatocytes become flat- tened rapidly, and show dramatically reduced liver- specific functions when the cells are cultured in plastic dishes [30]. The hepatocytes can also retain their normal cellular conditions and express certain differentiated functions for prolonged times when they are cultured in plastic dishes coated with a synthetic substratum [31]. However, dishes coated with high amounts of purified ECM molecules do not appear to be sufficient to induce 330 bp M 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 660 bp Figure 8. Detection of porcine ornithine transcarbamylase (above) and arginase (below) mRNA expression. Lanes 1 to 3, 4 to 6, 7 to 9, 10 to 12 and 13 to 15 represent treatment of por- cine hepatocytes with Asc2P after incubation for 5, 10, 15, 20 and 25 days, respectively. Concentrations of Asc2P for each lane: 1, 4, 7, 10 and 13, 15.0 mM; 2, 5, 8, 11 and 14, 1.5 mM; 3, 6, 9, 12 and 15, 0.0 mM; 16, the control.  Y. Kumaki et al. / Natural Science 2 (2010) 1264-1273 Copyright © 2010 SciRes. OPEN ACCESS 1270 hepatocyte differentiation [32]. Mitaka et al reported that the small hepatocytes could differentiate to mature hepatocytes by interacting with ECM [26]. Collagen type I and type III are made by fibroblasts and fat-storing (Ito) cells in normal liver were found to con- tain collagens I and III [33,34]. Collagen Type XII, a fibril-associated collagen, help to organize the fibrils in some other tissues [34]. Since vitamin C is required for the synthesis of collagen, our data showed that the por- cine collagen type I and type III mRNA, but not type XII mRNA, were detected as well (Figure 9), suggesting that Asc2P did not have the effect on the non-parenchy- mal hepatocytes to induce collagen type I and III mRNA expression. 3.6. Effect of Asc2P on the Porcine Sodium-Ascorbate Co-Transporters SVCT1 and SVCT2 Vitamin C exists in two chemically distinct forms in human plasma, the reduced ascorbate ion form (ascorbic acid, AA) and the oxidized non-ionic form (dehy- droascorbic acid, DHA). Human cells acquire both chemical forms of vitamin C by transporting them across cell membrane with the participation of two different transporter systems that show absolute specificity for one or the other vitamin form [35]. These two different transporter systems are first transporter system, the fa- cilitative glucose transporters for dehydroascorbic acid, and second transporter system, the sodium-ascorbic acid co-transporters for ascorbic acid. Second transport sys- tem for vitamin C is a high affinity, low capacity sodium dependent system (SVCTs) which corresponds to a re- 400 bp 500 bp M 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 Figure 9. Detection of porcine collagen type I (above), type III (mid) and type XII (below) mRNA expression. Lanes 1 to 3, 4 to 6, 7 to 9, 10 to 12 and 13 to 15 represent treatment of por- cine hepatocytes with Asc2P after incubation for 5, 10, 15, 20 and 25 days, respectively. Concentrations of Asc2P for each lane: 1, 4, 7, 10 and 13, 15.0 mM; 2, 5, 8, 11 and 14, 1.5 mM; 3, 6, 9, 12 and 15, 0.0 mM; 16, the control. cently described family of mammalian sodium-ascorbic acid co-transporters composed of two members, SVCT1 and SVCT2. These transporters display affinity for re- duced vitamin C [35-39]. There is no information on the mechanism of vitamin C transport across the intestinal barrier. It has been re- cently reported that the colon carcinoma cell line CaCo-2 was used as an in vitro model for vitamin C transport and the RT-PCR analysis indicated that CaCo-2 cells express the sodium-ascorbate co-transporters SVCT1 and SVCT2 [40]. The human vitamin C transporters expressed in COS-1 cells is regulated by protein kinase C [41]. Our RT-PCR analysis showed that the porcine hepatocytes expressed the sodium-ascorbate co-transpor- ters SVCT1 and SVCT2, however, the intensities of por- cine sodium-ascorbate co-transporters SVCT1 and SVCT2 bands were not changed markedly (Figure 10). These findings indicated that the Asc2P had no effect on SVCT1 and SVCT2 mRNA expression. 3.7. Effect of Asc2P on MTT Assay We further examined the effect of Asc2P on the pro- liferative rate by MTT assay at the various concentra- tions. A tetrazolium salt has been used to develop a quantitative colorimetric assay for mammalian cell sur- vival and proliferation. The assay detects living, but not dead cells and the signal generated is dependent on the degree of activation of the cells [42]. In this study, the cellular growth and proleferation were significantly in- creased by Asc2P during the long-term culture (data not shown). 3.8. Phase-Contrast Microscopic Observation Liver cell suspensions were prepared and freshly iso- lated cells were plated on type I collagen-coated 60-mm 1037 bp M 1 2 3 4 5 6 7 8 9 10 11 12 114 bp Figure 10. Detection of porcine sodium-ascorbate co-trans- porters SVCT1 (above) and SVCT2 (below) mRNA expression. Lanes 1 to 3, 4 to 6, 7 to 9 and 10 to 12 represent treatment of porcine hepatocytes with Asc2P after incubation for 5, 10, 15 and 20 days, respectively. Concentrations of Asc2P for each lane: 1, 4, 7 and 10, 15.0 mM; 2, 5, 8 and 11, 1.5 mM; 3, 6, 9 and 12, 0.0 mM.  Y. Kumaki et al. / Natural Science 2 (2010) 1264-1273 Copyright © 2010 SciRes. OPEN ACCESS 1271 Figure 11. Morphological variation of porcine hepatocytes treated with ascorbic acid 2-phosphate. culture dishes. The hepatocytes were checked and pho- tographed on a phase-contrast microscope. The hepato- cytes displayed a normal spreading rate and began to proliferate and scatter on day 3 and 4. The hepatocytes- maintained their normal shapes until day 10. Thereafter, the cells, which were not treated with Asc2P, were mor- phologically changed compared to those treated with Asc2P at 1.5 and 15 mM on day 15. In addition, not only the cells untreated with Asc2P, but also those treated with Asc2P at 1.5 mM were morphologically changed on day 20 and 25. The hepatocytes began to be disappeared gradually and the fibroblasts grew rapidly at this stage. The hepatocytes, which had been treated with Asc2P at 15 mM, remained almost unchanged after further time in this culture system (Figure 11). We developed this culture method by which adult por- cine hepatocytes can be maintained up to at least one month. There was one important improvement in our method. The cells cultured were the porcine mature hepatocytes. Several types of bioartificial livers have been deviced to treat patients with liver failures [43-45]. Demetriou et al. [43,44] and Sussman et al [45]. have developed bioartificial livers that are clinically applica- ble as a bridge to transplants. These investigators have utilized porcine hepatocytes or a transformed human liver cell line (Hep G3), which makes the use of these devices limited to transient treatments. Further im- provements of our method to grow porcine mature hepatocytes should make it possible to obtain prolifera- tive normal hepatocytes more effciently which can be used to reconstruct bioartificial livers. 4. ACKNOWLEDGEMENTS The authors are grateful to Dr. J. Kano (Tsukuba University) for providing porcine C/EBP alpha cDNA and Dr. S. Miyaki (Tissue En- gieering Research Center) for providing human collagen type I, III and XII cDNAs. We also thank Mr. K. Sakurai and Ms. Y. Asanuma for kindly providing technical assistance, and Ms. K. Miyazaki for excel- lent secretarial help. REFERENCES [1] Tateno, C. and Yoshizato, K. (1999) Long-term cultiva- tion of adult rat hepatocytes that undergo multiple cell divisions and express normal parenchymal phenotypes. American Journal of Pathology, 148, 383-392. [2] Tateno, C. and Yoshizato, K. (1999) Growth potential and differentiation capacity of adult rat hepatocytes in vi- tro. Wound Repair Regen, 7, 36-44. [3] Yamasaki, C., Tateno, C., Aratani, A., Ohnishi, C., Kata- yama, S., Kohashi, T., Hino, H., Marusawa, H., Asahara, T. and Yoshizato, K. (2006) Growth and differentiation of colony-forming human hepatocytes in vitro. Journal of Hepatology, 44, 749-757. [4] Katsura, N., Ikai, I., Mitaka, T., Shiotani, T., Yamanoku- chi, S., Sugimoto, S., Kanazawa, A., Terajima, H., Mo- Day 10 Day 15 Day 25 0.0 mM 1.5 mM 15.0 mM Day 20  Y. Kumaki et al. / Natural Science 2 (2010) 1264-1273 Copyright © 2010 SciRes. OPEN ACCESS 1272 chizuki, Y. and Yamaoka, Y. (2002) Long-term culture of primary human hepatocytes with preservation of prolif- erative capacity and differentiated functions. The Journal of surgical research, 106, 115-123. [5] Aden, D.P., Fogel, A., Plotkin, S., Damjanov, I. and Knowles, B.B. (1979) Controlled synthesis of HBsAg in a differentiated human liver carcinoma-derived cell line. Nature, 282, 615-616. [6] Knowles, B.B., Howe, C.C. and Aden, D.P. (1980) Hu- man hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science, 209, 497-499. [7] Weinstock, J. and Baldwin, G.S. (1988) Nucleotide se- quence of porcine liver albumin. Nucleic Acids Research, 16, 9045. [8] Sargent, T.D., Yang, M. and Bonner, J. (1981) Nucleotide sequence of cloned rat serum albumin messenger RNA. Proceedings of National Academy of Science of the United States of America, 78, 243-246. [9] Sabath, D.E., Broome, H.E. and Prystowsky, M.B. (1990) Glyceraldehyde-3-phosphate dehydrogenase mRNA is a major interleukin 2-induced transcript in a cloned T-helper lymphocyte. Gene, 91, 185-191. [10] Ding, S.T., McNeel, R.L. and Mersmann, H.J. (1999) Expression of porcine adipocyte transcripts: tissue dis- tribution and differentiation in vitro and in vivo. Com- parative Biochemistry and Physiology Part B: Biochem- istry and Molecular Biology, 123, 307-318. [11] Koger, J.B., Howell, R.G., Kelly, M. and Jones, E.E. (1994) Purification and properties of porcine liver or- nithine transcarbamylase. Archives of Biochemistry and Biophysics, 309, 293-299. [12] Haraguchi, Y., Takiguchi, M., Amaya, Y., Kawamoto, S., Matsuda, I. and Mori, M. (1987) Molecular cloning and nucleotide sequence of cDNA for human liver arginase. Proceedings of National Academy of Science of the United States of America, 84, 412-415. [13] Clark, A.G., Rohrbaugh, A.L., Otterness, I. and Kraus, V.B. (2002) The effects of ascorbic acid on cartilage me- tabolism in guinea pig articular cartilage explants. Matrix Biology, 21, 175-184. [14] Mitaka, T., Sattler, C.A., Sattler, G.L., Sargent, L.M. and Pitot, H.C. (1991) Multiple cell cycles occur in rat hepa- tocytes cultured in the presence of nicotinamide and epi- dermal growth factor. Hepatology, 13, 21-30. [15] Mitaka, T., Mikami, M., Sattler, G.L., Pitot, H.C. and Mochizuki, Y. (1992) Small cell colonies appear in the primary culture of adult rat hepatocytes in the presence of nicotinamide and epidermal growth factor. Hepatology, 16, 440-447. [16] Mitaka, T., Kojima, T., Mizuguchi, T. and Mochizuki, Y. (1995) Growth and maturation of small hepatocytes iso- lated from adult rat liver. Biochemical and biophysical research communications, 214, 310-317. [17] Mitaka, T., Mizuguchi, T., Sato, F., Mochizuki, C. and Mochizuki, Y. (1998) Growth and maturation of small hepatocytes. Journal of Gastroenterology Hepatology, 13, S70-77. [18] Hino, H., Tateno, C., Sato, H., Yamasaki, C., Katayama, S., Kohashi, T., Aratani, A., Asahara, T., Dohi, K. and Yoshizato, K. (1999) A long-term culture of human hepa- tocytes which show a high growth potential and express their differentiated phenotypes. Biochemical and Bio- physical Research Communications, 256, 184-191. [19] Babiss, L.E., Herbst, R.S., Bennett, A.L. and Darnell, Jr., J.E. (1987) Factors that interact with the rat albumin pro- moter are present both in hepatocytes and other cell types. Genes Development, 1, 256-267. [20] Cereghini, S., Raymondjean, M., Carranca, A.G., Her- bomel, P. and Yaniv, M. (1987) Factors involved in con- trol of tissue-specific expression of albumin gene. Cell, 50, 627-638. [21] Lichtsteiner, S., Wuarin, J. and Schibler, U. (1987) The interplay of DNA-binding proteins on the promoter of the mouse albumin gene. Cell, 51, 963-973. [22] Liu, J.K., DiPersio, C.M. and Zaret, K.S. (1991) Ex- tracellular signals that regulate liver transcription factors during hepatic differentiation in vitro. Molecular Cell Bi- ology, 11, 773-784. [23] Herbst, R.S., Friedman, N., Darnell, J.E. and Babiss, Jr., L.E. (1989) Positive and negative regulatory elements in the mouse albumin enhancer. Proceedings of National Academy of Science of the United States of America, 86, 1553-1557. [24] Pontoglio, M., Barra, J., Hadchouel, M., Doyen, A., Kress, C., Bach, J.P., Babinet, C. and Yaniv, M. (1996) Hepatocyte nuclear factor 1 inactivation results in hepatic dysfunction, phenylketonuria, and renal Fanconi syn- drome. Cell, 84, 575-585. [25] Samadani, U. and Costa, R.H. (1996) The transcriptional activator hepatocyte nuclear factor 6 regulates liver gene expression. Molecular Cell Biology, 16, 6273-6284. [26] Mitaka, T., Sato, F., Mizuguchi, T., Yokono, T. and Mo- chizuki, Y. (1999) Reconstruction of hepatic organoid by rat small hepatocytes and hepatic nonparenchymal cells. Hepatology, 29, 111-125. [27] Sasaki, K., Kitaguchi, Y., Fukuda, T. and Aoyagi, Y. (2000) Ascorbic acid supplementation to primary culture of chicken hepatocytes with non-serum medium. The In- ternational Journal of Biochemistry & Cell Biology, 32, 967-973. [28] Seki, J., Ikeda, R. and Hoshino, H. (1996) Dimethyl sul- foxide and related polar compounds enhance infection of human T cells with HIV-1 in vitro. Biochemical and bio- physical research communications, 227, 724-729. [29] Stamatoglou, S.C. and Hughes, R.C. (1994) Cell adhe- sion molecules in liver function and pattern formation. The FASEB Journal, 8, 420-427. [30] Bissell, D.M., Arenson, D.M., Maher, J.J. and Roll, F.J. (1987) Support of cultured hepatocytes by a laminin-rich gel. Evidence for a functionally significant subendothe- lial matrix in normal rat liver. The Journal of Clinical Investigation, 79, 801-812. [31] Tobe, S., Takei, Y., Kobayashi, K. and Akaike, T. (1992) Receptor-mediated formation of multilayer aggregates of primary cultured adult rat hepatocytes on lac- tose-substituted polystyrene. Biochemical and Biophysi- cal Research Communications, 184, 225-230. [32] Sudhakaran, P.R., Stamatoglou, S.C. and Hughes, R.C. (1986) Modulation of protein synthesis and secretion by substratum in primary cultures of rat hepatocytes. Ex- perimental Cell Research, 167, 505-516. [33] Bentley, S.A. (1982) Collagen synthesis by bone marrow stromal cells: A quantitative study. British Journal of  Y. Kumaki et al. / Natural Science 2 (2010) 1264-1273 Copyright © 2010 SciRes. OPEN ACCESS 1273 Haematology, 50, 491-497. [34] Vuorio, E. and de Crombrugghe, B. (1990) The family of collagen genes. The Annual Review of Biochemistry, 59, 837-872. [35] Daruwala, R., Song, J., Koh, W.S., Rumsey, S.C. and Levine, M. (1999) Cloning and functional characteriza- tion of the human sodium-dependent vitamin C trans- porters hSVCT1 and hSVCT2. FEBS Letters, 460, 480-484. [36] Faaland, C.A., Race, J.E., Ricken, G., Warner, F.J., Wil- liams, W.J. and Holtzman, E.J. (1998) Molecular charac- terization of two novel transporters from human and mouse kidney and from LLC-PK1 cells reveals a novel conserved family that is homologous to bacterial and Aspergillus nucleobase transporters. Biochimica et Bio- physica Acta, 1442, 353-360. [37] Tsukaguchi, H., Tokui, T., Mackenzie, B., Berger, U.V., Chen, X.Z., Wang, Y., Brubaker, R.F. and Hediger, M.A. (1999) A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature, 399, 70-75. [38] Wang, H., Dutta, B., Huang, W., Devoe, L.D., Leibach, F.H., Ganapathy, V. and Prasad, P.D. (1999) Human Na(+)-dependent vitamin C transporter 1 (hSVCT1): primary structure, functional characteristics and evidence for a non-functional splice variant. Biochimica et Bio- physica Acta, 1461, 1-9. [39] Wang, Y., Mackenzie, B., Tsukaguchi, H., Weremowicz, S., Morton, C.C. and Hediger, M.A. (2000) Human vita- min C (L-ascorbic acid) transporter SVCT1. Biochemical and Biophysical Research Communications, 267, 488- 494. [40] Maulen, N.P., Henriquez, E.A., Kempe, S., Carcamo, J.G., Schmid-Kotsas, A., Bachem, M., Grunert, A., Busta- mante, M.E., Nualart, F. and Vera, J.C. (2003) Up-regulation and polarized expression of the so- dium-ascorbic acid transporter SVCT1 in post-confluent differentiated CaCo-2 cells. The Journal of Biological Chemistry, 278, 9035-9041. [41] Liang, W.J., Johnson, D., Ma, L.S., Jarvis, S.M. and Wei-Jun, L. (2002) Regulation of the human vitamin C transporters expressed in COS-1 cells by protein kinase C [corrected]. American Journal of Physiology Cell Physiology, 283, C1696-1704. [42] Mosmann, T. (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cy- totoxicity assays. The Journal of Immunological Methods, 65, 55-63. [43] Watanabe, F.D., Mullon, C.J., Hewitt, W.R., Arkadopou- los, N., Kahaku, E., Eguchi, S., Khalili, T., Arnaout, W., Shackleton, C.R., Rozga, J., Solomon, B. and Demetriou, A.A. (1997) Clinical experience with a bioartificial liver in the treatment of severe liver failure. A phase I clinical trial. Annals of Surgery, 225, 484-491. [44] Detry, O., Arkadopoulos, N., Ting, P., Kahaku, E., Wata- nabe, F.D., Rozga, J. and Demetriou, A.A. (1999) Clini- cal use of a bioartificial liver in the treatment of aceta- minophen-induced fulminant hepatic failure. Annals of Surgery, 65, 934-938. [45] Ellis, A.J., Hughes, R.D., Wendon, J.A., Dunne, J., Lang- ley, P.G., Kelly, J.H., Gislason, G.T., Sussman, N.L. and Williams, R. (1996) Pilot-controlled trial of the extra- corporeal liver assist device in acute liver failure. Hepa- tology, 24, 1446-1451.
|