 Vol.2, No.11, 1189-1194 (2010) Natural Science http://dx.doi.org/10.4236/ns.2010.211147 Copyright © 2010 SciRes. OPEN ACCESS Chiral palladium complexes based on derivatives of benzylamine and 2α-hydroxypinan-3-one Olga A. Zalevskayaa, Yana A. Gur'evab,*, Larisa L. Frolovab, Igor N. Alekseevb, Alexander V. Kutchinb a Syktyvkar State University, Syktyvkar, Petrozavodskaja, Syktyvkar, Russia b Institute of Chemistry of Komi Scientific Centre, Ural Branch of Russian Academy of Sciences, Pervomaiskaja, Syktyvkar, Russia; *Corresponding author: gurjeva-ja@chemi.komisc.ru Received 30 July 2010; revised 2 September 2010; accepted 5 September 2010. ABSTRACT Synthesized and characterized new chiral palla- dium complexes, some of which contain asym- metric donor nitrogen atom. Nitrogen-containing derivatives (+) - and (-)-2α-hydroxypinan-3-one- (1R,2R,5R)-3-(benzylimino)-2,6,6-trimethylbicy- clo[3.1.1]heptane-2-ol (HL1), (1S,2S,3S,5S)-3- (benzylamino)-2,6, 6-trimethylbicyclo[3.1.1]- heptane-2-ol (HL2), (1R,2R,5R)-3-((S)-α-methyl- benzylimino)-2,6,6-trimethylbicyclo[3.1.1]- heptane-2-ol (HL3), (1R,2R,3R,5R)-3-((S)-α-methyl- benzylamino)-2,6,6-trimethylbicyclo[3.1.1]- heptane-2-ol (HL4) -were studied as optically ac- tive ligands. Keywords: Palladium Complexes; Cyclopalladation; Chiral Imine; Amine 1. INTRODUCTION Chiral palladium complexes of various types are widely used in modern asymmetric synthesis, the goal of which is to obtain enantiopure compounds. Cyclopal- ladated complexes (CPCs) form a special group of com- pounds with a σ-connection palladium-carbon [1]. These complexes show reasonably high activity and thermal stability. Chiral CPCs are very successfully used in asymmetric synthesis, both as the original matrix [2-7] and as catalysts [8-12]. They have been used in NMR studies as shifting reagents [13-15] and as effective res- olution agents [16-18]. This is especially significant for obtaining enantiopure phosphines, which are efficient ligands for asymmetric catalysis of transition metal complexes. Currently, the CPCs are received with dif- ferent types of chirality [1]. The compounds of a variety of classes have been tested for their ability to become ligands for CPCs. However, the synthetic accessibility of chiral ligands remains an important problem. 2. EXPERIMENTAL 2.1. General The 1H and 13C NMR spectra were recorded with a Bruker Avance-300 spectrometer operating at the fre- quencies 300 and 75 MHz for 1H and 13C nucleus, re- spectively. The measurements were carried out at ambi- ent temperature in CDCl3. Chloroform signals were used as an internal standard (δН 7.27 ppm, δС 77.00 ppm). The assignment of signals was carried out using 13C NMR spectra recorded in the mode of J-modulation, and ac- cording to two-dimensional correlation spectra of 1H{1H} (COSY) and 1H{ 13C} (HSQC) and NOE experiments. IR spectra were measured in a thin layer or in KBr pel- lets on a device "IR Prestige 21" made by Shimadzu. Optical rotations were measured on a Kruss P3002RS polarimeter (Germany) with a 10 cm cell and were re- ported as follows: tC (concentration in g/10 mL, solvent). Elemental analyses were performed using an automatic analyzer ЕА 1110 CHNS-O. All reactions were monitored on a thin layer chroma- tography (TLC) using Merck silica gel (70-230 mesh) and benzene acetone mixtures as eluents; the TLC spots were visualized with J2 and KMnO4/H2SO 4. Column chromatography was carried out using Merck silica gel (70-230 mesh) and benzene acetone mixtures as eluents. 2.2. Solvents and Starting Reagents Benzene was dried with CaCl2, refluxed with Na, and then distilled from Na. Methanol was distilled from MeONa. Hexane was distilled from Na. Palla- dium chloride was used without additional purifica- tion. (SC)-α-Methylbenzylamine of 99% ee was pur- chased from Merck and used without purification.  O. A. Zalevskaya et al. / Natural Science 2 (2010) 1189-1194 Copyright © 2010 SciRes. OPEN ACCESS 1190 Imines HL1, HL3 and amines HL2, HL4 were pre- pared by a reported method [19]. 2.3. Di-µ-Chlorobis{(1R,2R,5R)-3- (Benzylimino)-2,6,6-Trimethylbicyclo [3.1.1]Heptane-2-ol-C,N} Dipalladium(II), 1 A suspension of palladium chloride (II) (0.09 g, 0.5 mmol) and lithium chloride (0.04 g, 1.0 mmol) in methanol (5 ml) was boiled in a water bath with a reflux condenser for one hour. The resulting solution of lithium tetrachloropalladate (dark-red color) added to a solution of imine HL1 (0.13 g, 0.5 mmol) and so- dium acetate (0.04 g, 0.5 mmol) in methanol (5 ml). Af- ter stirring at room temperature for 1 hour the solvent was removed from the reaction mixture, the complex 1 was extracted with benzene (3×20 mL). The crude product was purified using column chromatography on silica gel with benzene and benzene/acetone 10:1 mix- ture as eluents. After precipitation from benzene by hexane and drying in vacuum, complex 1 was obtained in the yield of 40% (0.080 g, mmol) as a yellow amor- phous powder: mp (dec) 166-167 °C, Rf 0.71 (5:1 ben- zene/acetone), 25 = -415.9 (c 0.06, CHCl3). IR, ν, cm-1: 3441 (OH), 1618 (C = N). 1H NMR (CDCl3, δ / ppm., J / Hz): 0.73 (s, 3H, H9, Me), 0.98 (d, 1H, 7-Hα, J 7α,7β 11.0), 1.10 (s, 3H, H8, Me), 2.12 (ddd, 1H, 7-Нβ, J 2.0, J 5.8, J 7β,7α 11.0), 2.26 (d, 1H, H4α, J 4α,4β 18,3), 2.56 (dd, 1H, H4β, J 4.1, J 4β,4α 18.3), 2.94 (s, 3H, H10, Me), 4.22 (d, 1H, H11α, J 11α,11β 14.5), 4.47 (d, 1H, H11β, J 11β,11α 14.5), 7.15-7.45 m (4H, arom.). 13C NMR (CDCl3, δ, ppm): 23.27 (C9), 26.88 (C8), 28.18 (C7), 32.10 (C10), 32.94 (C 4), 39.17 (C 5), 40.13 (C6), 51.41 (C1), 55.06 (C11), 92.46 (C2), 127.27 (C18), 127.55 (C17), 128.33 (C16), 128.57 (C14), 128.85 (C15), 135.24 (C13), 197.63 (C3). 2.4 Dichloro{(1R,2R,5R)-3- (Benzylimino)-2,6,6-Trimethylbicyclo [3.1.1]Heptane-2-ol- N,N}Palladium(II), 2 A suspension of palladium chloride(II) (0.04 g, 0.2 mmol) and lithium chloride (0.02 g, 0.4 mmol) in methanol (5 ml) was boiled in a water bath with a reflux condenser for one hour. The resulting solution of lithium tetrachloropalladate (dark-red) color was added to a solution of imine HL1 (0.1 g, 0.4 mmol) in methanol (2 ml). After stirring at room temperature for 1 h the solvent was removed from the reaction mixture, the coordinated complex 2 was extracted with benzene (3×20 mL). The crude product was pu- rified using column chromatography on silica gel with benzene and benzene/acetone 10:1 mixture as eluents. After precipitation from benzene by hexane and dry- ing in vacuum, complex 2 was obtained in the yield of 50% (0.070 g, mmol) as a yellow amorphous powder: mp (dec) 153-154 °C, Rf 0.8 (5:1 benzene/acetone), 25 = +113.5 (c 0.07, CHCl3). IR, ν, cm-1: 3410 (OH), 1612 (C = N). 1H NMR (CDCl3, δ / ppm., J / Hz): 0.75 (s, 3H, H9, Me), 1.22 (s, 3H, H8, Me), 1.5 (d, 1H, H7α, J 7α,7β 9.0 ), 1.8 (m, 1H, Н7β), 2.1 (m, 1H, H1), 2.2 (m, 2H, H4), 2.5 (m, 1H, H5), 2.8 (s, 3H, H10, Me), 5.1 (d, 1H, H11α, J 11α,11β 16.0), 5.9 (d, 1H, H11β, J 11β,11α 16.0), 7.4 (m, 5H, arom.). 13C NMR (CDCl3, δ, ppm): 22.97 (C9), 26.71 (C8), 27.61 (C7), 31.06 (C10), 37.97 (C4), 38.13 (C5), 38.54 (C6), 52.87 (C1), 63.00 (C11), 76.61 (C2), 127.21 (C15), 127.55 (C16), 128.83 (C14), 133.81 (C13), 192.35 (C3). 2.5. Dichloro{(1S,2S,3S,5S)-3- (Benzylamino)-2,6,6- Trimethylbicyclo [3.1.1]Heptane-2-ol-N,N} Palladium(II), 3 A suspension of palladium chloride(II) (0.04 g, 0.2 mmol) and lithium chloride (0.02 g, 0.4 mmol) in methanol (5 ml) was boiled in a water bath with a reflux condenser for one hour. The resulting solution of lithium tetrachloropalladate (dark-red color) was added to a solution of imine HL2 (0.12 g, 0.4 mmol) in methanol (2 ml). After stirring at room temperature for 1 h the solvent was removed from the reaction mixture, the coordinated complex 3 was extracted with benzene (3×20 mL).The crude product was puri- fied using column chromatography on silica gel with benzene and benzene/acetone 10:1 mixture as eluents. After precipitation from benzene by hexane and dry- ing in vacuum, complex 3 was obtained in the yield of 60% (0.085 g, mmol) as a yellow amorphous powder: mp (dec) 167-168 °C, Rf 0.85 (5:1 benzene/acetone), 25 = -62.6 (c 0.09, CHCl3). IR, ν, cm-1: 3479 (OH), 3253 (NH). 1H NMR (CDCl3, δ / ppm., J / Hz): 0.75 (s, 3H, H9,Me), 1.24 (s, 3H, H8, Me), 1.43 (s, 3H, H10, Me), 1.46 (d, 1H, H7α, J 7α,7β 11.0), 1.73-1.81 (m, 2H, H1, H5), 1.88 (dd, 1H, H4α, J 4α,3 10.0, J 4α,4β 14.1), 2.10 ddd (1H, H7β, J 5.7, J 6.1, J 7β,7α 11.0), 2.61 (ddd, 1H, H4β, J 4.9, J 4β,3 9.8, J 4β,4α 14.1), 3.08 (dd, 1H, H3, J 3,4β 9.8, J 3,4α 10.0), 4.04 m (2H, H (11)), 7.33 (d, 1H, H16, J 16,15 7.0), 7.40 (dd, 2H, H15, J 15,16 7.0 , J 15,14 7.6), 7.50 (d, 2H, H14, J 14,15 7.6). 13C NMR (CDCl3, δ / ppm.): 23.22 (C9), 23.39 (C10), 24.05 (C7), 27.54 (C8), 32.14 (C4), 39.48 (C6), 40.58 (C5), 57.13 (C1), 57.55 (C11), 65.18 (C3), 76.60 (C2 ), 127.74 (C16), 128.38 (C15), 129.90 (C14), 135.53 (C13).  O. A. Zalevskaya et al. / Natural Science 2 (2010) 1189-1194 Copyright © 2010 SciRes. OPEN ACCESS 1191 2.6. Di-µ-Chlorobis{(1R,2R,5R)-3-((1S)- α-Methylbenzylimino)-2,6,6- Trimethylbicyclo[3.1.1] Heptane-2-ol-C,N}Dipalladium(II), 4 Synthesis is carried out similarly to that described for (1). Dimer 4 as a yellow amorphous powder, yield 50% (0.103 g, mmol), mp (dec) 145-146 °C, Rf 0.4 (5:1 benzene/acetone), 25 = -32.0 (c 0.08, ace- ton). IR, ν, cm-1: 3337 (OH), 1631 (C = N). 1H NMR (CDCl3, δ / ppm., J / Hz): 0.99 (s, 3H, H9, Me), 1.29 (d, 3H, H12, Me, J 12,11 6.8), 1.39 (s, 3H, H8, Me), 1.91 (d, 1H, H7α, J 7α,7β 11.8), 1.95 (s, 3H, H10, Me), 2.12 (m, 1H, H5, J 5,1 5.5 ), 2.27 (m, 1H, H1, J 1,5 5.5, J 5.5), 2.59 (m, 2H, H4α, H7β), J 3.9, J 4β,4α 18.3 ), 2.73 (dd, 1H, H4β, J 3.9, J 4β,4α 18.3 ), 4.05 (sq, 1H, H11, J 11,12 6.8), 6.57 (d, 1H, H18, J 18,17 7.6), 6.84 (dd, 1H, H17, J 17,18 7.6, J 17,16 8.0), 7.04 (dd, 1H, H16, J 16,17 8.0, J 16,15 8.0), 7.44 (d, 1H, H15, J 15,16 8.0). 13C NMR (CDCl3, δ, ppm): 22.43 (C12), 23.18 (C9), 27.23 (C8), 27.90 (C10), 28.87 (C7), 33.90 (C4), 38.41 (C5), 40.52 (C6), 51.78 (C1), 67.00 (C11), 88.64 (C2) ), 122.33 (C18), 124.61 (C16), 124.75 (C17), 135.51 (C15), 138.84 (C13), 156.52 (C14), 185.10 (C3). 2.7. Di-µ-Chlorobis{(1R,2R,3R,5R)-3- ((S)-α-Methylbenzylamino)-2,6,6- Trimethylbicyclo[3.1.1] Heptane-2-ol-C,N}Dipalladium(II), 5 Synthesis is carried out similarly to that described for (1). Dimer 5 as a yellow amorphous powder, yield 55% (0.114 g, mmol), mp (dec) 169-170 °C, Rf 0.3 (5:1 benzene/acetone), 25 = +19.7 (c 0.04, CHCl3). IR, ν, cm-1: 3421 (OH), 3217 (NH). 1H NMR (CDCl3, δ / ppm., J / Hz): 0.96 (s, 3H, H9, Me), 1.29 (s, 3H, H8, Me), 1.56 (s, 3H, H10, Me), 1.66 (dd, 1H, H4α, J 4α,3 11.3, J 4α,4β 13.9), 1.75 (d, 1H, H7α, J 7α,7β 10.9), 1.81 (d, 3H, H12, J 12,11 6.4), 1.95 (m, 1H, H1, J 1,7β 5.6), 2.01 (m, 1H, H5), 2.17 (dd, 1H, H7β, J 7β,1 5.6, J 7β,7α 10.9), 2.46 (s, OH), 2.56 (ddd, 1H, H4β, J 5.6, J 4β,3 9.0, J 4β,4α 13.9), 4.14 (sq, 1H, H11, J 11,12 6.4), 4.18 (dd, 1H, H3, J 3,4β 9.0, J 3,4α 11.3), 4.40 (s, NH)), 6.74 (dd, 1H, H18, J 18,16 1.3, J 18,17 7.2), 6.87 (ddd, 1H, H16, J 16,181.3, J 16,17 7.3, J 16,15 8.5), 6.96 (dd, 1H, H17, J 17,18 7.2, J 17,16 7.3), 7.24 (d, 1H, H15, J 15,16 8.5). 13C NMR (CDCl3, δ, ppm): 22.89 (C9), 24.02 (C7), 24.24 (C10), 25.88 (C12), 28.05 (C8), 28.48 (C4), 40.16 (C6), 40.62 (C5), 55.47 (C1), 64.39 (C11), 67.51 (C3), 77.55 (C2), 119.89 (C18), 124.79 (C17), 125.10 (C16), 134.01 (C15), 141.92 (C13), 156.95 (C14). 3. RESULTS AND DISCUSSION Earlier, we reported on the synthesis of chiral imines and amines on the basis of 2α-hydroxypinan-3-one [19]. These ligands, containing in its composition ben- zylamine fragment, are of interest from the standpoint of the possibility of obtaining ortho-palladated complexes. In the present work we investigated the interaction of the obtained ligands HL1, HL2, HL3, HL4 with lithium tet- rachloropalladate (Li2PdCl4) in the method of Cope [20]. Reaction of cyclopalladation is accompanied by the re- lease of hydrogen chloride. An insertion of an additional base is necessary for the neutralization of the hydrogen chloride. The structure of obtained complex compounds is con- firmed by NMR, IR spectroscopy and elemental analysis data, last are given in Table 1 . On the basis of imine HL1 we managed to get binu- clear palladacycle 1 with the yield of 40% in the pres- ence of sodium acetate as base at a molar ratio of re- agents 1:1. In the absence of the base at a molar ratio of reagents 1:2 mononuclear coordinated complex 2 was obtained in 50% yield (Scheme 1). Complex compounds 1 and 2 were isolated from the reaction mixture by col- umn chromatography and further purified by crystalliza- tion from a mixture of benzene-hexane. The signals of protons of methylene group -CH2N = were observed in the 1H NMR spectrum of compound 1 in the form of two doublets with geminal constant of 14.5 Hz (two-proton singlet was observed in the spec- trum of the initial ligand), which confirms the formation of the cycle. The change of the multiplicity and the inte- grated intensity of signals of protons of benzene ring corresponds to the ortho-disubstituted ring. The preser- vation of the multiplicity and the integral intensity of proton signals of the monosubstituted benzene ring in the 1H NMR spectrum of compound 2 excludes or- tho-palladation. In contrast to the corresponding imine HL1 amine HL2 forms only mononuclear coordinated complex 3 even in the presence of a base (AcONa) (Scheme 2). This result can be explained by the fact that the secon- dary amino group exhibits stronger electron donor prop- erties than the imine, and reduces the electrophilic activ- ity of palladium, preventing the ortho-palladation. The interaction of amine HL2 with Li2PdCl4 may form a mixture of diastereomeric complexes in which the con- figuration of tetragonal nitrogen atom consolidates with the metal and the nitrogen becomes an additional center of chirality. There is only one set of signals in the 1H NMR spectrum of compound 3 that indicates the forma- 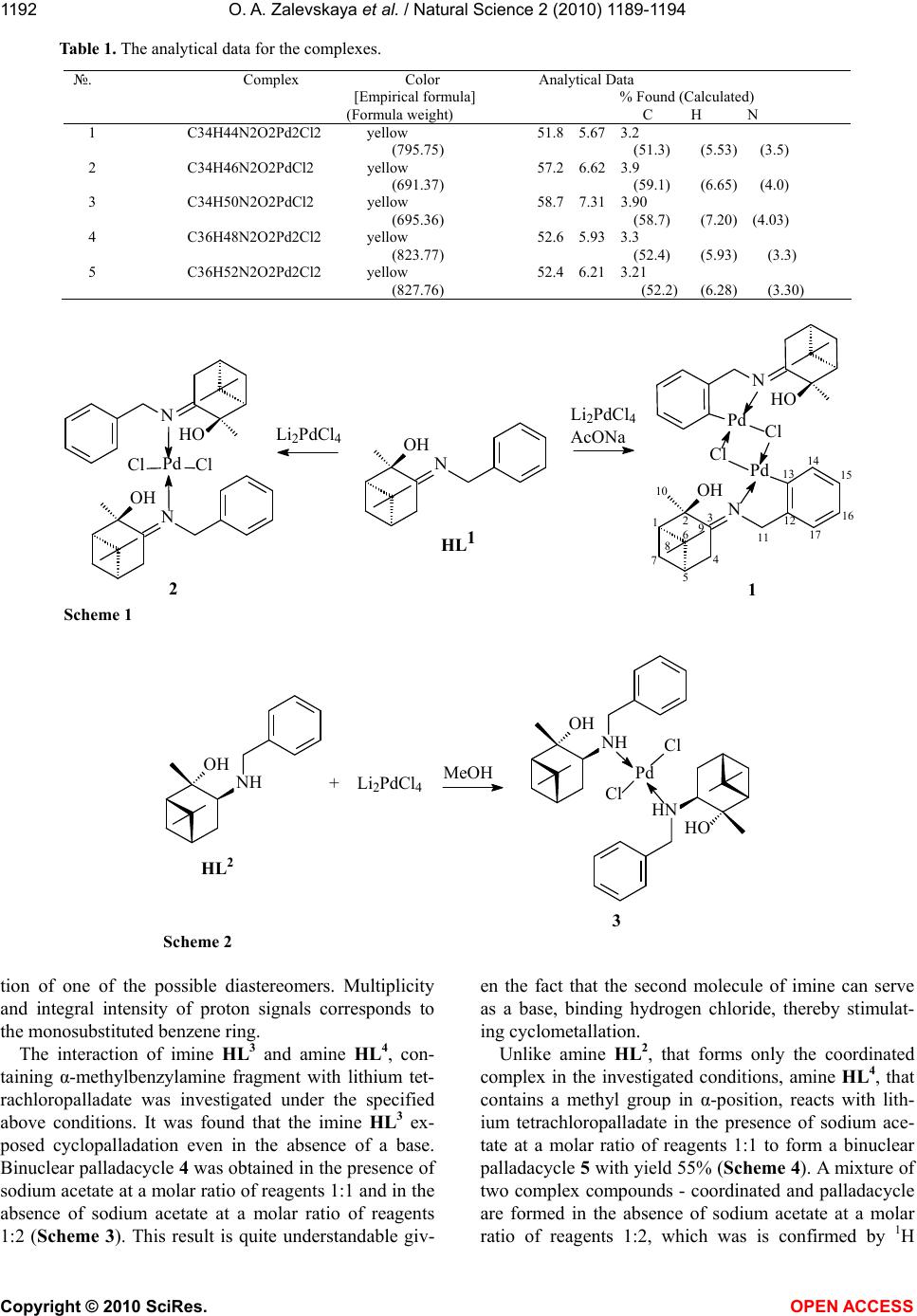 O. A. Zalevskaya et al. / Natural Science 2 (2010) 1189-1194 Copyright © 2010 SciRes. OPEN ACCESS 1192 Table 1. The analytical data for the complexes. №. Complex Color Analytical Data [Empirical formula] % Found (Calculated) (Formula weight) C H N 1 C34H44N2O2Pd2Cl2 yellow 51.8 5.67 3.2 (795.75) (51.3) (5.53) (3.5) 2 C34H46N2O2PdCl2 yellow 57.2 6.62 3.9 (691.37) (59.1) (6.65) (4.0) 3 C34H50N2O2PdCl2 yellow 58.7 7.31 3.90 (695.36) (58.7) (7.20) (4.03) 4 C36H48N2O2Pd2Cl2 yellow 52.6 5.93 3.3 (823.77) (52.4) (5.93) (3.3) 5 C36H52N2O2Pd2Cl2 yellow 52.4 6.21 3.21 (827.76) (52.2) (6.28) (3.30) 11 17 14 16 15 13 2 1 HL1 Li2PdCl4 OH N HO N Pd Cl Cl Li2PdCl4 AcONa HO N Pd Cl OH N Pd Cl OH N 123 4 5 6 7 8 9 10 12 Scheme 1 MeOH + Li2PdCl4 OH NH OH NH Pd HO HN Cl Cl HL2 3 Scheme 2 tion of one of the possible diastereomers. Multiplicity and integral intensity of proton signals corresponds to the monosubstituted benzene ring. The interaction of imine HL3 and amine HL4, con- taining α-methylbenzylamine fragment with lithium tet- rachloropalladate was investigated under the specified above conditions. It was found that the imine HL3 ex- posed cyclopalladation even in the absence of a base. Binuclear palladacycle 4 was obtained in the presence of sodium acetate at a molar ratio of reagents 1:1 and in the absence of sodium acetate at a molar ratio of reagents 1:2 (Scheme 3). This result is quite understandable giv- en the fact that the second molecule of imine can serve as a base, binding hydrogen chloride, thereby stimulat- ing cyclometallation. Unlike amine HL2, that forms only the coordinated complex in the investigated conditions, amine HL4, that contains a methyl group in α-position, reacts with lith- ium tetrachloropalladate in the presence of sodium ace- tate at a molar ratio of reagents 1:1 to form a binuclear palladacycle 5 with yield 55% (Scheme 4). A mixture of two complex compounds - coordinated and palladacycle are formed in the absence of sodium acetate at a molar ratio of reagents 1:2, which was is confirmed by 1H 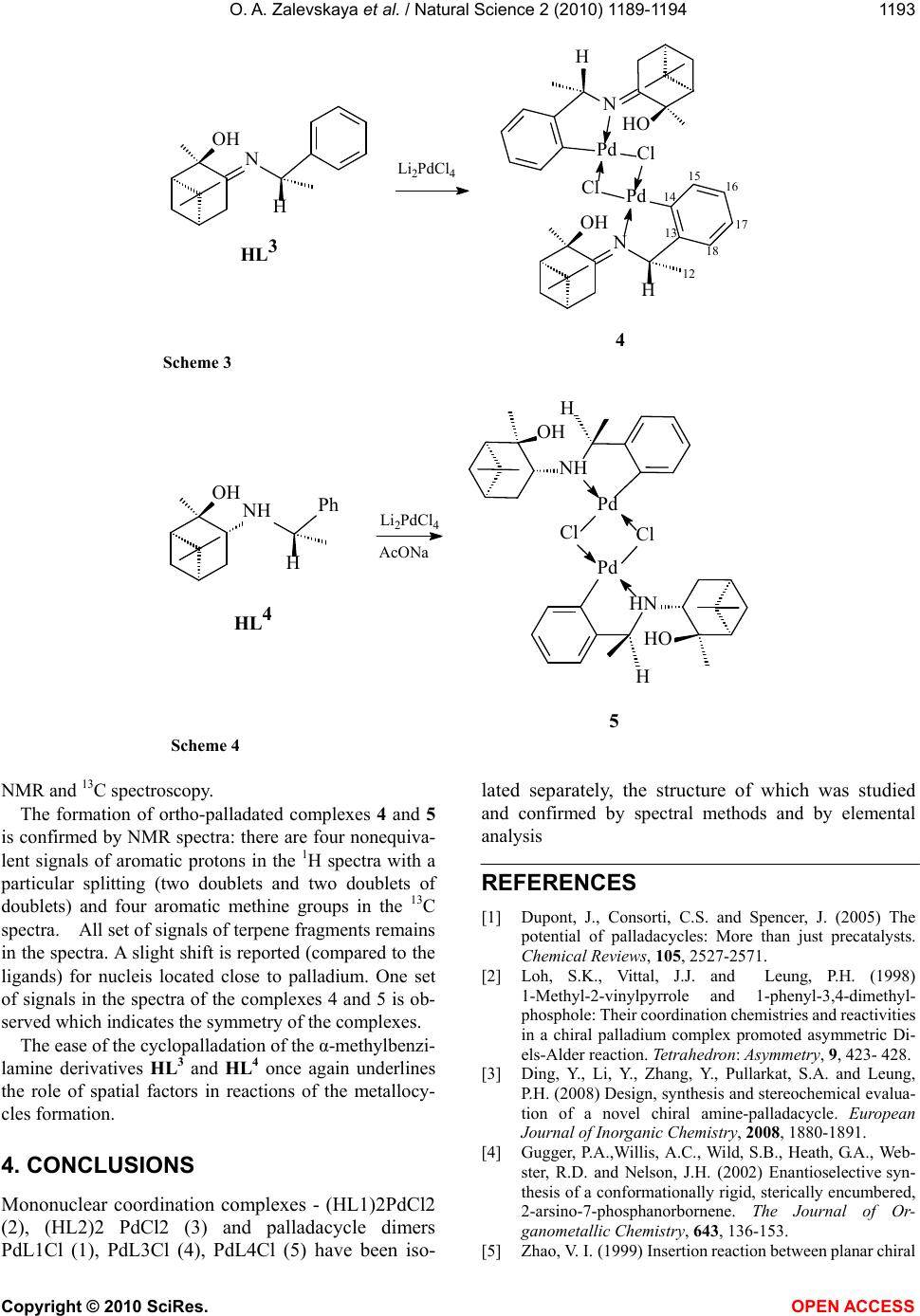 O. A. Zalevskaya et al. / Natural Science 2 (2010) 1189-1194 Copyright © 2010 SciRes. OPEN ACCESS 1193 18 17 14 16 15 13 12 4 OH N H Pd HO N H Pd Cl Cl HL3 OH N H Li2PdCl4 Scheme 3 AcONa Li2PdCl4 HL4 5 OH NH Pd Cl Cl Pd HO HN OH NH Ph H H H Scheme 4 NMR and 13C spectroscopy. The formation of ortho-palladated complexes 4 and 5 is confirmed by NMR spectra: there are four nonequiva- lent signals of aromatic protons in the 1H spectra with a particular splitting (two doublets and two doublets of doublets) and four aromatic methine groups in the 13C spectra. All set of signals of terpene fragments remains in the spectra. A slight shift is reported (compared to the ligands) for nucleis located close to palladium. One set of signals in the spectra of the complexes 4 and 5 is ob- served which indicates the symmetry of the complexes. The ease of the cyclopalladation of the α-methylbenzi- lamine derivatives HL3 and HL4 once again underlines the role of spatial factors in reactions of the metallocy- cles formation. 4. CONCLUSIONS Mononuclear coordination complexes - (HL1)2PdCl2 (2), (HL2)2 PdCl2 (3) and palladacycle dimers PdL1Cl (1), PdL3Cl (4), PdL4Cl (5) have been iso- lated separately, the structure of which was studied and confirmed by spectral methods and by elemental analysis REFERENCES [1] Dupont, J., Consorti, C.S. and Spencer, J. (2005) The potential of palladacycles: More than just precatalysts. Chemical Reviews, 105, 2527-2571. [2] Loh, S.K., Vittal, J.J. and Leung, P.H. (1998) 1-Methyl-2-vinylpyrrole and 1-phenyl-3,4-dimethyl- phosphole: Their coordination chemistries and reactivities in a chiral palladium complex promoted asymmetric Di- els-Alder reaction. Tetrahedron: Asymmetry, 9, 423- 428. [3] Ding, Y., Li, Y., Zhang, Y., Pullarkat, S.A. and Leung, P.H. (2008) Design, synthesis and stereochemical evalua- tion of a novel chiral amine-palladacycle. European Journal of Inorganic Chemistry, 2008, 1880-1891. [4] Gugger, P.A.,Willis, A.C., Wild, S.B., Heath, G.A., Web- ster, R.D. and Nelson, J.H. (2002) Enantioselective syn- thesis of a conformationally rigid, sterically encumbered, 2-arsino-7-phosphanorbornene. The Journal of Or- ganometallic Chemistry, 643, 136-153. [5] Zhao, V. I. (1999) Insertion reaction between planar chiral  O. A. Zalevskaya et al. / Natural Science 2 (2010) 1189-1194 Copyright © 2010 SciRes. OPEN ACCESS 1194 cyclopalladated derivatives of ferrocene and di- phenylacetylene. The Journal of Organometallic Chem- istry, 574, 311-317. [6] Sokolov, V.I. (1995) Optically active organometallic compounds (a personal account from the inside). The Journal of Organometallic Chemistry, 500, 299- 306. [7] Spencer, J. and Pfeffer, M. (1995) The fate of the stereo- genic centre linked to palladium upon reaction with an alkyne. Tetrahedron: Asymmetry, 6, 419-426. [8] Hollis, T.K. and Overman, L.E. (1997) Cyclopalladated ferrocenyl amines as enantioselective catalysts for the rearrangement of allylic imidates to allylic amides. Tet- rahedron Letters, 38, 8837-8840. [9] Chahen, L., Therrien, B. and Suss-Fink, G. (2007) Square-planar diacetatopalladium complexes with trans- configured secondary amine ligands that avoid orthome- talation: ligand synthesis, coordination, molecular struc- ture and catalytic potential for suzuki cross-coupling re- actions. European Journal of Inorganic Chemistry, 2007, 5045-5051. [10] Zalevskaya, O.A., Vorob’eva, E.G., Dvornikova, I.A. and Kuchin, A.V. (2008) Palladium complexes based on op- tically active terpene derivatives of ethylenediamine. Russian Journal of Coordination Chemistry, 34 , 855- 857. [11] Overman, L.E. and Remarchuk, T.P. (2000) Catalytic asymmetric intramolecular aminopalladation: enantiose- lective synthesis of vinyl-substituted 2-oxazoli dinones, 2-imidazolidinones, and 2-pyrrolidinones. Journal of American Chemical Society, 124, 12-13. [12] Kirsch, S.F. and Overman, L.E. (2005) Catalytic asym- metric synthesis of chiral allylic esters. Journal of American Chemical Society, 127, 2866-2867. [13] Levrat, F., Stoeckli-Evans, H. and Engel, N. (2002) Enantiomeric excess determination of α-amino acids by 19F NMR spectroscopy of their N,N-dimethyl-(2,2,2- trifluoro-1-phenylethyl)amine-C,N)palladium complexes. Tetrahedron: Asymmetry, 13, 2335-2344. [14] Dunina, V. V., Kuz'mina, L. G., Kazakova, M.Y., Grishin, Y.K., Veits, Y.A. and Kazakova, E.I. (1997) Or- tho-palladated α-phenylalkylamines for enantiomeric pu- rity determination of monodentate P*-chiral phosphines, Tetrahedron: Asymmetry, 8, 2537-2545. [15] Albert, J., Granell, J., Muller, G., Sainz, D., Font-Bardia, M. and Solans, X. (1995) Chiral Cyclopalladated com- pounds for enantiomeric purities of functionalized phosphines by means of multinuclear NMR. Tetrahedron: Asymmetry, 6, 325. [16] Ding, Y., Chiang, M., Pullarkat, S.A., Li, Y. and Leung, P.H. (2009) Synthesis, coordination characteristics, con- formational behavior and bond reactivity studies of a novel chiral phosphapalladacycle complex. Organome- tallics, 28, 4358-4370. [17] Albert, J., Cadena, J.M., Delgado, S. and Granell, J. (2000) Synthesis and resolution of a new P-chiral hydroxy phosphine. The Journal of Organometallic Chemistry, 603, 235-239. [18] Dunina, V.V., Kuz'mina, L.G., Rubina, M.Y., Grishin, Y.K., Veits, Y.A. and Kazakova, E.I. (1999) A resolution of the monodentate P*-chiral phosphine PBU(t)C6H4Br-4 and its NMR-deduced absolute configuration. Tetrahe- dron: Asymmetry, 10, 1483-1497. [19] Gur'eva, Y.A., Zalevskaya, O.A., Frolova, L.L., Alexeev, I.N. and Kutchin, A.V. Chiral imines and amines on the basis of 2α-hydroxypinan-3-one. Chemistry of Natural Compounds, in press. [20] Cope, A.C. and Siekman, R.W. (1965) Formation of co- valent bonds from platinum or palladium to carbon by direct substitution. Journal of American Chemical Soci- ety, 87, 3272-3273.
|