 Vol.2, No.11, 1335-1344 (2010) doi:10.4236/health.2010.211199 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/ Health Openly accessible at Quality nutrition through pigeonpea—a review Kul Bhushan Saxena, Ravikoti Vijaya Kumar*, Rafat Sultana International Crops Research Institute for the Semi-Arid Tropics, Patancheru, India; Corresponding Author: R.VIJAYKUMAR@CGIAR.ORG Received 2 April 2010; revised 23 April 2010; accepted 24 May 2010. ABSTRACT Protein mal-nutrition is widespread among poor of developing and under developed countries. Since animal protein is beyond the reach of this group, their primary protein supply comes from plant based products. Amongst these, pigeon- pea or red gram (Cajanus cajan (L.) Millspaugh) is an important food legume that can be grown under rainfed conditions with least inputs. Pi- geonpea is rich in starch, protein, calcium, man- ganese, crude fiber, fat, trace elements, and mi- nerals. Besides its high nutritional value, pi- geonpea is also used as traditional folk medi- cine in India, China, Philippines and some other nations. Literature on this aspect show that pi- geonpea is capable to prevent and cure a num- ber of human ailments such as bronchitis, cou- ghs, pneumonia, respiratory infections, dysen- tery, menstrual disorders, sores, wounds, ab- dominal tumors, tooth ache, and diabetes. Keywords: Protein; Pigeonpea; Cajanus Cajan; Nutrition; Folk Medicine 1. INTRODUCTION Protein availability in developing countries at present is about one-third of its normal requirements and with ever growing human population; various nutritional de- velopment programs are facing a tough challenge to meet the targeted protein demand. Legumes in the de- veloping world are known to offer food proteins that are generally grown under risk-prone marginal lands with low inputs. Among the legumes pigeonpea or red gram (Cajanus cajan (L.) Millspaugh) occupies an important place in rainfed agriculture. Globally, it is cultivated on 4.79 M ha in 22 countries [1] but with only a few major producers. In Asia, India (3.58 M ha), Myanmar (560,000 ha), and Nepal (20,703 ha) are important pi- geonpea producing countries. In African continent, Kenya (196,261 ha), Malawi (123,000 ha), Uganda (86,000 ha), Mozambique (85,000 ha), and Tanzania (68,000 ha) pro- duce considerable amounts of pigeonpea. The Caribbean islands and some South American countries also have reasonable areas under pigeonpea cultivation. The presence of high genetic diversity made [2-4], to believe that India is the primary center of origin of cul- tivated pigeonpea from where it spread to Africa about 4000 years ago. There are several local names of Ca- janus cajan in different parts of the world [5]. Among these “pigeonpea” is the globally popular name that was coined by [6] in Barbados, where the crop was grown in barren lands for feeding its seeds to pigeons. In India it is popularly known as red gram, tur, or arhar. Pigeonpea is a perennial plant (Figure 1) and it can survive up to 3-4 years [5]. It is a short-day plant with a high sensitiv- ity to photo-periodic changes. There are two major growth patterns in pigeonpea; the determinate types which pro- duce pods in clusters at the top of the canopy and its further growth ceases after flowering that results in more or less uniform maturity. The other and most common growth habit is non-determinate type, where the pods are borne in axillary clusters. In general, the later types can tolerate major biotic and abiotic stresses due to their inherent capacity to regenerate. The traditional pigeon- pea cultivars and most landraces are tall and take about 180-280 days to mature. However some early maturing Figure 1. A commercial crop of pigeonpea.  K. B. Saxena et al. / HEALTH 2 (2010) 1335-1344 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/ 1336 Openly accessible at cultivars are also now available [5]. In India, de-hulled split cotyledons (Figure 2(a)) of pigeonpea seeds are cooked to make dal (thick soup) for eating with bread and rice; while in southern and eastern Africa, and South America its whole dry seeds (Figure 2(b)) are used in a porridge like recipe. The fully grown seeds of pigeonpea when, harvested green (Figure 2(c)) before loosing their green color, are used as fresh, frozen, or canned vegetable. Its broken seeds, skin, and pod walls are fed to domestic animals; and the dry stems are used as domestic fuel wood. In tropics and sub tropics pigeonpea is considered a life line of subsistence agri- culture. Pigeonpea plant is known to provide several benefits to soil such as fixing atmospheric nitrogen, add- ing organic matter and micro nutrients, and breaking hard plough pan with its long tap roots and, thereby sometimes referred as “biological plough”. Pigeonpea can be grown successfully in a wide range of soil types and is capable of producing reasonable quantities of nu- tritive food even in the degraded soils and with mini- mum external inputs. 2. NUTRITIONAL QUALITY Pal [7] published the first information on biological value, net protein content, essential amino acids, and digestibility of different pulses. In this study pigeonpea was rated the best as far as its biological value was con- cerned; and it was recommended that for a balanced vegetarian diet pigeonpea should be eaten with rice. Re- ferences [8-10] reported large variability for various chemical constituents and nutritive value of pigeonpea. It is to be noted that besides inherent genotypic differ- ences, some degree of variation in protein content can also arise due to differences in environmental conditions where the crop was grown, methods of sampling and analyses, and seed storage conditions and its duration. 2.1. Nutritional Value of Dry Seeds Pigeonpea seeds are made up of 85% cotyledons, 14% seed coat, and about 1% embryo, and contain a variety of dietary nutrients [11]. The cotyledons are rich in car- bohydrates (66.7%) while a major proportion (about 50%) of seed protein is located in embryo. About one-third of seed coat is made up of fiber. The quantities of important sulfur-containing amino acids such as me- thionine and cystine range around 1% and they are pre- sent in cotyledons and embryo; while calcium is pre- dominantly present in seed coat and embryo. Singh [12] found that the globulins constitute about 65% of total proteins. In general, pigeonpea is not rated superior for sulfur-containing amino acids [13], but it is not linked (a) (b) (c) Figure 2. (a) Dehulled split cotyledons (dal); (b) Whole dry seeds of pigeonpea; (c) Vegetable pigeonpea seeds. with low methionine content [14]. In comparison to other protein fractions, globulin is rather inferior in sulfur- containing amino acids while albumin has high amino acid content. In pigeonpea seed the proportion of prola- min is low while sugars such as stachyose and verbas- cose are high [15]. 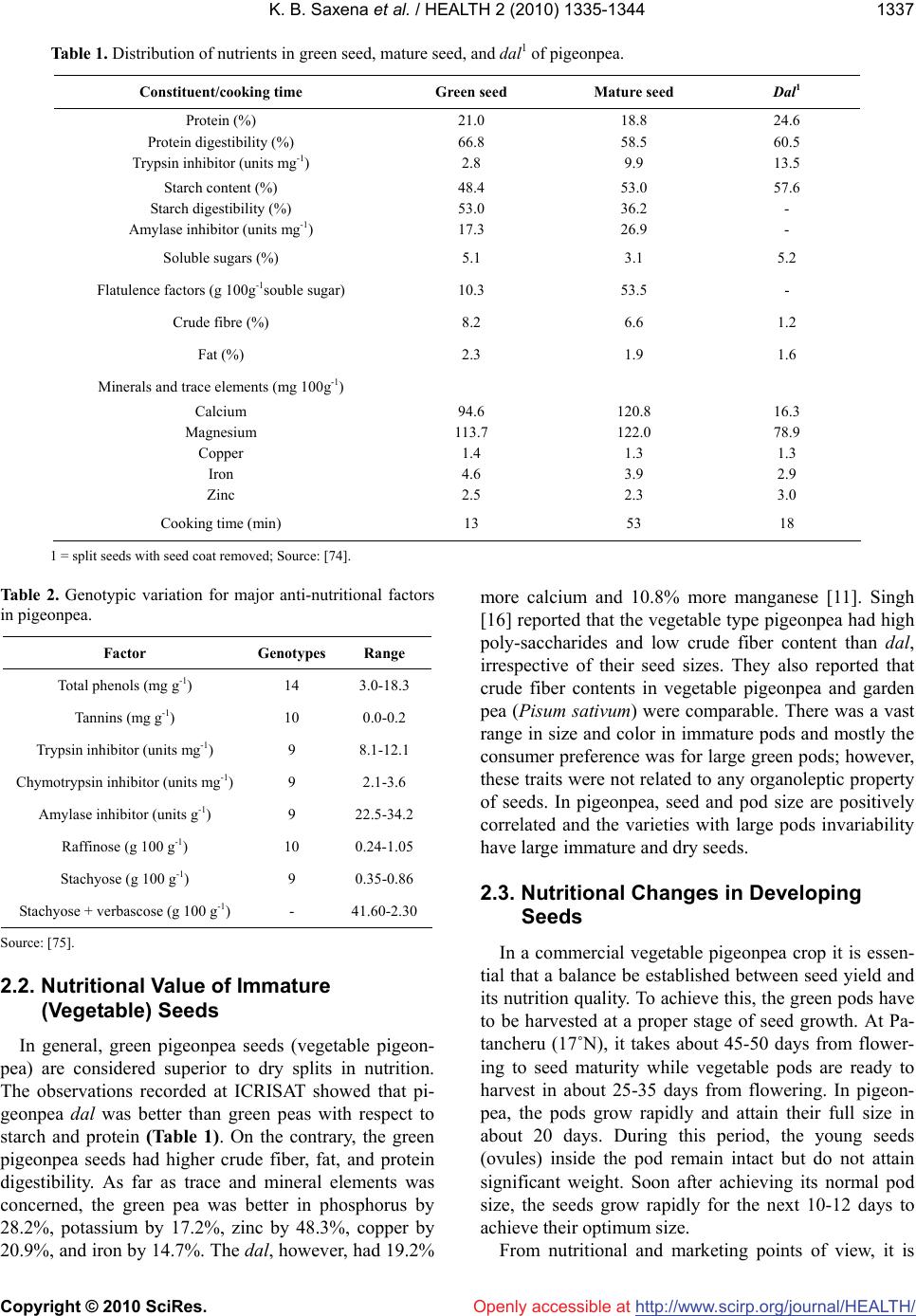 K. B. Saxena et al. / HEALTH 2 (2010) 1335-1344 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/Openly accessible at 1337 Table 1. Distribution of nutrients in green seed, mature seed, and dal1 of pigeonpea. Constituent/cooking time Green seed Mature seed Dal1 Protein (%) Protein digestibility (%) Trypsin inhibitor (units mg-1) 21.0 66.8 2.8 18.8 58.5 9.9 24.6 60.5 13.5 Starch content (%) Starch digestibility (%) Amylase inhibitor (units mg-1) 48.4 53.0 17.3 53.0 36.2 26.9 57.6 - - Soluble sugars (%) 5.1 3.1 5.2 Flatulence factors (g 100g-1souble sugar) 10.3 53.5 - Crude fibre (%) 8.2 6.6 1.2 Fat (%) 2.3 1.9 1.6 Minerals and trace elements (mg 100g-1) Calcium Magnesium Copper Iron Zinc 94.6 113.7 1.4 4.6 2.5 120.8 122.0 1.3 3.9 2.3 16.3 78.9 1.3 2.9 3.0 Cooking time (min) 13 53 18 1 = split seeds with seed coat removed; Source: [74]. Table 2. Genotypic variation for major anti-nutritional factors in pigeonpea. Factor Genotypes Range Total phenols (mg g-1) 14 3.0-18.3 Tannins (mg g-1) 10 0.0-0.2 Trypsin inhibitor (units mg-1) 9 8.1-12.1 Chymotrypsin inhibitor (units mg-1)9 2.1-3.6 Amylase inhibitor (units g-1) 9 22.5-34.2 Raffinose (g 100 g-1) 10 0.24-1.05 Stachyose (g 100 g-1) 9 0.35-0.86 Stachyose + verbascose (g 100 g-1) - 41.60-2.30 Source: [75]. 2.2. Nutritional Value of Immature (Vegetable) Seeds In general, green pigeonpea seeds (vegetable pigeon- pea) are considered superior to dry splits in nutrition. The observations recorded at ICRISAT showed that pi- geonpea dal was better than green peas with respect to starch and protein (Table 1). On the contrary, the green pigeonpea seeds had higher crude fiber, fat, and protein digestibility. As far as trace and mineral elements was concerned, the green pea was better in phosphorus by 28.2%, potassium by 17.2%, zinc by 48.3%, copper by 20.9%, and iron by 14.7%. The dal, however, had 19.2% more calcium and 10.8% more manganese [11]. Singh [16] reported that the vegetable type pigeonpea had high poly-saccharides and low crude fiber content than dal, irrespective of their seed sizes. They also reported that crude fiber contents in vegetable pigeonpea and garden pea (Pisum sativum) were comparable. There was a vast range in size and color in immature pods and mostly the consumer preference was for large green pods; however, these traits were not related to any organoleptic property of seeds. In pigeonpea, seed and pod size are positively correlated and the varieties with large pods invariability have large immature and dry seeds. 2.3. Nutritional Changes in Developing Seeds In a commercial vegetable pigeonpea crop it is essen- tial that a balance be established between seed yield and its nutrition quality. To achieve this, the green pods have to be harvested at a proper stage of seed growth. At Pa- tancheru (17˚N), it takes about 45-50 days from flower- ing to seed maturity while vegetable pods are ready to harvest in about 25-35 days from flowering. In pigeon- pea, the pods grow rapidly and attain their full size in about 20 days. During this period, the young seeds (ovules) inside the pod remain intact but do not attain significant weight. Soon after achieving its normal pod size, the seeds grow rapidly for the next 10-12 days to achieve their optimum size. From nutritional and marketing points of view, it is  K. B. Saxena et al. / HEALTH 2 (2010) 1335-1344 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/Openly accessible at 1338 essential that the green pods are picked at a proper stage to reap maximum seed yield with highest nutritional quality. In this context, Singh [17] observed that in grow- ing seeds the starch content was negatively associated with their protein and sugar contents. The amount of cru- de fiber content in the growing seeds increased slowly with maturation. The soluble sugars and proteins de- creased in the developing seeds but the starch content increased rapidly between 24 and 32 days after flower- ing. Meiners [18] showed that the minerals and trace ele- ments such as calcium, iron, zinc, magnesium, and cop- per remained more or less constant and did not change markedly during seed development in pigeonpea. 2.4. Nutritional Value of Split Peas (DAL) Carbohydrates (67%) and protein (22%) are main con- stituents of pigeonpea seeds [11]. Hulse [19] reported that the seed protein among cultivars ranged around 22%. The quality of protein is determined by its quantity and digestibility, and amino acid contents. In pigeonpea the amino acid such as lysine and threonines are in good proportions, while methionine and cystine are deficient [12]. Pigeonpea cotyledons are also rich in calcium and iron. Saxena [20] used wild relatives of pigeonpea as high protein donor parents and demonstrated that seed protein content in cultivated types can be enhanced through conventional breeding. Singh [21] assessed these high protein lines for their chemical composition. They re- ported large differences between the levels of protein in high-protein (28.7 to 31.1%) lines and control cultivars (23.1 to 24.8%). As expected, the starch component in high-protein lines was relatively less (54.3 to 55.6 %) than that of controls (58.7 to 59.3%). Also the high-pro- tein lines were marginally lowers (2.5 to 2.6%) in fat content when compared with control cultivars (2.9 to 3.1%). The differences in the major protein fractions of the high and normal-protein lines were also large. In comparison to controls (60.3 to 60.5%), the globulin fraction was higher (63.5 to 66.2%) in the high-protein lines and the reverse was true for glutelin. 2.5. Anti-Nutritional Factors Like other legumes, pigeonpea seeds also contain some anti-nutritional factors (Table 2). These include oligo-sac- charides (raffinose and verbascose), polyphenols (phenols and tannins), phytolectins, and enzyme inhibitors (tryp- sin, chymotrypsin, and amylase). According to Kamath [22], pigeonpea seeds also have some amounts of un- available carbohydrates which adversely affect the bio- availability of certain vital nutrients. Some of the anti- nutritional factors such as phytolectins are heat sensitive and are destroyed during cooking. Godbole [23] reported protease inhibitors in seven- day old seeds; while Ambekar [24] found that such in- hibitors are either not synthesized or inactive up to 28 days of the seed development. No other plant part except seed exhibited trypsin or chymotrypsin inhibitors [25]. The white seeded pigeonpea cultivars contain relatively less amounts of polyphenols. Such cultivars are prefer- red in many countries where de-hulling facilities are not available and whole seeds are consumed. In comparison to the white seeded cultivars the red seeded types con- tain three times greater quantity of polyphenols [26]. Similarly, the enzyme inhibition activity was also greater in the colored seeds of pigeonpea. Since in India almost entire pigeonpea production (3.2 m tones) are de-hulled and converted into dal for consumption, the tannins pre- sent in the colored seed coat pose no nutritional problem. 3. COOKING QUALITY Pigeonpea seeds in the form of either dry, green, or split peas are invariably consumed after cooking. There- fore, besides various nutritional aspects the cooking time and other related parameters assume importance. Con- sumers always prefer a dal that cooks fast and produces more volume upon cooking with high consistency and flavor. Cooking time of dal is independent of taste and flavor [9]. Jambunathan [27] studied various physico- chemical characters of pigeonpea, and reported that quick cooking trait of dal was associated with large seed size, high solid dispersal, more water absorption, and high nitrogen solubility. Narasimha [28] and Pal [7] reported a positive association of cooking time of pigeonpea seeds with their calcium and magnesium contents. According to Salunkhe [29], cooking of pigeonpea improved the bio-availability of nutrients and at the same time de- storyed some anti-nutritional factors. Heat treatment of pigeonpea seeds is also known to enhance their starch digestibility. The lines, which take long time to cook, generally face the danger of loosing important vitamins from food. Cooking pigeonpea seed after germination enhances their starch digestibility [30] but reduces the levels of oligo-saccharides [31]. The fermentation of seeds helps in reducing inhibitory activity of digestive enzymes [32]. Geervani [33] reported that thiamine and riboflavine were destroyed by heat but niacin content was unaltered during boiling, pressure cooking, and roa- sting of pigeonpea seeds. She further found that the avai- lability of lysine and methionine decreased on roasting but the available methionine increased on boiling and pressure-cooking. 4. NUTRIENT LOSSES DURING STORAGE AND PROCESSING Pigeonpea is predominantly cultivated by small holder  K. B. Saxena et al. / HEALTH 2 (2010) 1335-1344 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/Openly accessible at 1339 farmers and they generally store the whole seeds for over 6-12 months round-the-year for consumption. In ru- ral areas the farmers’ process small quantities of grain as and when needed, and they do de-hulling by hand-oper- ated traditional grinding stones called chakki or quern. Since in pigeonpea the cotyledons are attached tightly with seed coat by various gums, the de-hulling involves the process of dissolving the gum layers by soaking whole seeds in water, heat treatment, or adding oil after surface scarification. This is followed by drying, de-husk- ing, and splitting of cotyledons. During this process the losses of certain proportions of cotyledons are inevitable, and it is estimated that by using advanced processing technology about 15%-17% of grain mass is lost; while with chakki such losses may shoot up to 20%-25%. In rural areas, the seeds are generally stored in gunny bags or bins made of mud and husk. According to Daniel [34], food grains containing more than 10 mg uric acid per 100 g of food are unfit for human consumption. Pi- geonpea seeds when stored for eight months turned unfit for consumption as their total uric acid content crossed the safe prescribed limit [35]. Cooking time of pigeon- pea in general increased with storage time [36]. Daniel [34] reported that lysine, threonine, and protein effi- ciency ratios were adversely affected in pigeonpea when the seeds were stored in jute bags. The storage of pi- geonpea seeds also resulted in the loss of vitamins. Such losses were less (10%-26%) in the protected seed and high (32%-49%) in the infested seeds [37]. Thiamine and niacin contents also registered decline during storage. Factors such as moisture, temperature, relative humidity, and seed hardness determined the extent of quality losses during storage [38]. Reddy [39] reported that in comparison to inner layers of cotyledons the outer layers are rich in protein. From nutrition point of view, this is a matter of concern since de-hulling not only removes protein-rich germ but also some proportion of the outer layers of the cotyledons. Singh [40] further observed that de-hulling also removes about 20% calcium and 30% iron. According to Kurien [41], the dal yield under controlled conditions achieves an efficiency of 80%-84% but at commercial level the recovery remains around 70%. Therefore, with a combi- nation of a superior variety and efficient processing tech- nology, the nutrient availability can be maximized. 5. ENVIRONMENT AND PROTEIN The growth and development of pigeonpea plants are known to be highly influenced by environmental factors such as temperature, photoperiod, available moisture, soil nutrition etc. The large variation observed for protein content of pure lines over locations or years is a good example of the role that environment can play in the ex- pression of this trait. Sham [42] reported significant ef- fects of location and fertilizer application on pigeonpea seed protein. Oke [43] found that incorporation of 20 ppm sulfur alone or in combination with phosphorus in- creased methionine content of pigeonpea. Saxena [44] observed significant differences for pro- tein content across locations and months. The same vari- ety, when grown in different months at a particular loca- tion showed significant variation in its protein content. For example, in cultivar “Prabhat” planted at Patancheru (17˚N) over 12 months, the seed protein content ranged from 21.6% to 25.2%; similarly at Pantnagar (29˚N), in 10 plantings within a year, the protein content of cultivar Prabhat ranged between 24.5% and 27.9%. 6. MEDICINAL USES Plant kingdom had been considered from a long time a reservoir of folk medicines. The herbal medicines, also called as phyto-medicines, refer to using a plant or its part such as leaves, flowers, fruits, bark, or seeds for me- dicinal purposes. Since ancient periods and long before any recorded history China, India, and Egypt were the leaders in folk medicines. The ancient Chinese and Egy- ptian papyrus writings described various medicinal uses of some plant species. The native Africans and Ameri- cans also used different herbs in a number of healing rituals. The herbal medicine system has a long tradition of usage outside the boundaries of synthetic medicine system. With the advent of improved chemical analytical methods along with quality control technologies and advances in clinical research, the value of herbal medi- cines increased in treating and preventing of some hu- man diseases. Subsequently, effective traditional medical systems such as “Ayurveda” in India and “Traditional Chinese Medicine” in China were developed with a fairly good recognition. Slowly the people in different parts of the world also started using the common herbal plants for medicinal purposes. In the early 19th century, when the first chemical ana- lysis became available, the scientists began to extract the active ingredients from selected plant species for phar- maceutical usage and some chemists started synthesizing targeted plant compounds in their laboratories. Gradually, the usage of herbal medicines declined in favor of syn- thetic drugs. However, the World Health Organization estimated that 80% of people worldwide, particularly from developing and under-developed countries, still rely on herbal medicines for some part of their primary health care. In Germany, about 600-700 plant-based me- dicines are still prescribed by 70% of the Physicians. Recently, some of the developed countries have also shown interest in the natural or organic remedies [45].  K. B. Saxena et al. / HEALTH 2 (2010) 1335-1344 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/Openly accessible at 1340 6.1. Herbal Properties Pigeonpea is being used as an integral part of tradi- tional folk medicine in India, China, and some other de- veloping countries [46]. The importance of pigeonpea plant in ethnical folk medicine is well known in the pre- vention and cure of certain human ailments and its brief account is given below: Flowers: Pigeonpea floral decoctions are traditionally used for treating ailments such as bronchitis, coughs, and pneumonia. Pigeonpea flowers are also prepared into a “tea” for treating upper respiratory infections and pain. The flowers, when prepared in an infusion, are used for treating dysentery and menstrual disorders. Seeds: Scorched seeds, when added to coffee alleviate headache and vertigo. Fresh seeds are believed to help incontinence of urine in males, while immature seeds are recommended for treatment of kidney ailments [47]. Pi- geonpea seeds are infused to make a diuretic “tea” for inflammation and blood disorders. In South America pi- geonpea seeds are used for febrifuge, stabilization of the menstrual period and dysentery [48]; in Africa pigeon- pea seeds are used for treating hepatitis and measles [49]. In Mexico, pigeonpea seeds are used as styptic drug and laxative; while in China these are used to arrest bleeding, relieve pain, kill worms, and as an expectorant and seda- tive drug [50,51]. Some herbal researchers are of the opi- nion that pigeonpea diminishes swelling of internal or- gans such as liver, intestines etc. Clinical studies have also shown that the seed extract of pigeonpea helps in inhibiting sickling of red blood cells and therefore, has potential to treat the person suffering from sickle cell anemia [52]. Roots: Dried roots of pigeonpea are sometimes used as an alexeritic, anthelminthic, expectorant, sedative, and vulnerary. Leaves: In India pigeonpea leaves are used for curing sores, wounds, abdominal tumors, and diabetes [53,54]. The leaves of the pigeonpea are decocted in Argentina for treating acute bronchial problems and genital and skin irritations. The young leaves of pigeonpea can be chew- ed for treating cough, diarrhea [55], traumatism, burnt infection, bedsore, toothache, mouthwash, sore gums, child-delivery, and dysentery [56,57]. It was also found that pigeonpea leaves have notable anti-inflammatory and antibiotic effects; and also inhibit capillary perme- ability [58]. In China, pigeonpea is considered as an ex- cellent “Traditional Chinese Medicine” for therapy of ischemic necrosis of femoral head. The leaves are pre- pared in an infusion for overcoming anemia, hepatitis, urinary infections, yellow fever, and ulcers. In Brazil, pigeonpea leaves are infused for coughs, fevers, and ulcers. 6.2. Chemical Constituents of Leaves In order to know the major chemical constituents of pigeonpea leaves, efforts were made to isolate and iden- tify various active chemical compounds. The research efforts revealed that some polyphenols, especially fla- vonoids, play an important role in curing certain human ailments [59-62]. The four major flavonoids identified in the extracts of pigeonpea leaves are quercetin, luteolin, apigenin, and isorhamnetin. These compounds are known for their important pharmacological activities [56,63,64]. Flavonoids are polyphenolic compounds, which are widely found in plant kingdom. As intrinsic components of fruits, vegetables, and beverages many of the 4000 different flavonoids known to-date, are present in a common regular diet [65]. Pigeonpea leaves also contain other compounds such as hordenine, juliflorine, betulinic acid, stigmasterol, beta-sitosterol, etc. In recent years, exten- sive research is being carried on various antibacterial, anti-fungal, anti-viral, anti-cancer, and anti-inflammatory properties of these flavonoids [66-68]. 7. GENETIC ENHANCEMENT OF PROTEIN CONTENT In most third world countries supply of staple food has failed to keep pace with population growth and, there- fore, breeding for high quality foods never received a priority and the resources were always diverted for en- hancing yield of major food crops. Considering the state of protein malnutrition in most developing and under- developed countries, the genetic enhancement of seed protein in pigeonpea was initiated at ICRISAT. To start a high protein breeding program, information on the genetic control of protein content is essential. Saxena [69] reported the role of both additive and non- additive gene actions in determining protein content and it was found to be controlled by 3-4 genes [70] that were recessive in nature [71]. Since the genetic variation for protein in primary (cultivated) gene pool was limited, the high-protein sources available in the secondary (wild) gene pool such as Cajanus sericeus, C. lineatus, and C. scarabaeoides were used in breeding. This program su- ccessfully developed some high protein (26.9%-27.4%) lines with good seed size (9.6-10.5 g 100-1 seeds), and high seed yield (1660-2096 kg ha-1). The evaluation of these high-protein lines revealed that from one hectare of field 350-450 kg crude protein can be harvested, reflect- ing an additional harvest of 80-100 kg protein ha-1. Cul- tivation of such lines will markedly improve availability of protein to farmers without sacrificing seed yield. The results of rat feeding trials of these high-protein lines showed that boiled dal was comparable to the con- trol in true protein digestibility, biological value, and net  K. B. Saxena et al. / HEALTH 2 (2010) 1335-1344 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/Openly accessible at 1341 protein utilization [21]. They further concluded that the high-protein lines were nutritionally superior to normal- protein cultivars as the former contain quantitatively more utilizable protein and sulfur containing amino acids. 8. CULTIVATION OF PIGEONPEA Early maturing varieties are short in stature and re- quire high (about 300,000 plants ha-1) density in contrast to late maturing types which need 40,000-50,000 plants ha-1 for optimum yields. For backyard and bund cultiva- tion the pigeonpea plants are maintained for more than one year and they attain a height of over 3 m. The pods are picked for house-hold use, as and when required. No specific agronomic practices are followed in this system. For local market relatively large populations are grown on field bunds, mainly around paddy fields. In this sys- tem, 3-4 seeds are sown in a single hill and the plants produce a large number of branches on either side of the bund. For peri-urban commercial crop, a field with known history of good soil fertility and drainage should be se- lected. The sowings on raised beds with appropriate slo- pe is ideal for pigeonpea. Application of 100 kg ha-1 of di-ammonium phosphate and other soil amendments for the known soil deficiencies are advisable before sowing. Sowing should be undertaken at the onset of rainy sea- son with row-to-row and plant-to-plant spacing of 100 cm and 50 cm, respectively. The seeds are placed about 5 cm deep and covered firmly with soil. For weed con- trol spraying of a pre-emergence herbicide such as Ba- salin @ 1.5 L ha-1, followed by two hand weeding are enough to control rainy season weeds. Irrigation is gen- erally not recommended if the crop is grown for domes- tic consumption on deep Vertisols. In pigeonpea insect control is very vital. Pod borers (Helicoverpa armigera and Maruca vitrata), pod fly (Melanagromyza obtusa), and blister beetle (Mylabris pustulata) are major pi- geonpea insects. These may cause severe damage to seed yield and quality. To control these insects appropriate chemical control is essential. Fusarium wilt and sterility mosaic are two major pigeonpea diseases. Wilt is caused by a soil-borne fungus Fusarium udum Butler while ste- rility mosaic is caused by virus. At present a number of disease resistant cultivars are available and the losses can be minimized by using such cultivars. 9. PIGEONPEA IN RURAL DIETS In pigeonpea methionine, cystine, tryptophan and thre- onine are the limiting essential amino acids, whereas in rice and wheat lysine is the limiting amino acid. A food combining both cereals and pulses provide a balanced diet because they complement the amino acid profiles of each other. The mutual quality compensation is closest to the ideal value when the ratio by weight of cereals to legume is roughly 70:30 [19]. In southern and eastern Africa this ratio is 90:10, reflecting shortage of protein in the diet. Daniel [72] studied supplementation of cereal diets with various proportions of pigeonpea and reported that supplementation of ration with pigeonpea signifi- cantly enhanced the nutritive value of diet. Supplemen- tation of rice diet with 8.5% and 16.7% pigeonpea dal markedly improved the quality of diet. Similarly, Kurien [41] demonstrated that a supplement of pigeonpea in maize diet significantly improved the quality of food. Bidinger [73] observed that pigeonpea was by far the most preferred pulse crop in Indian villages and its con- sumption patterns differed widely by age group, farm size, and the village. The consumption rate was found linear with small farmers consuming the least amount and the large farmers the most. National Institute of Nu- trition in India recommends cereal: pulse ratio of 3:1 for very young children, 5:1 for women, and 6:1 for men. In most of the cases, rural diets standards could not be met. Bidinger [73] further reported that 10% of protein and 5% of energy in the village diets came from pigeonpea. The maximum lysine provided from the diet was 21.7%. These values are low and reflect low consumption of legumes. 10. SUMMARY Legumes are a rich source of food proteins that are ge- nerally grown under risk—prone marginal lands. Amongst various food legumes, pigeonpea occupies an important place and has been rated the best as far as its biological value is concerned. It has been recommended for a bal- anced diet with cereals, especially to fill in the nutri- tional gap for proteins amongst the poorer section in developing economies that cannot afford a non-vegeta- rian diet. At present, the protein availability in develop- ing countries is about one-third of normal requirements and with ever growing population; various nutritional development programs are facing a tough challenge to meet the protein demand. In general, pigeonpea can be grown both as annual crop or perennial plants in homestead and is consumed either as decorticated splits or green seeds as vegetables. It has been found that vegetable pigeonpea are consi- dered superior to dry splits in crude fiber, fat, protein digestibility as well as trace elements and minerals. Be- sides its nutritional value, pigeonpea also possesses va- rious medicinal properties due to presence of a number of polyphenols and flavonoides. It is an integral part of traditional folk medicine in India, China, and some other nations. Pigeonpea is known to prevent and cure human ailments like bronchitis, coughs, pneumonia, respiratory  K. B. Saxena et al. / HEALTH 2 (2010) 1335-1344 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/ 1342 Openly accessible at infections, pain, dysentery, menstrual disorders, curing sores, wounds, abdominal tumors, and diabetes in tradi- tional folk medicine. REFERENCES [1] FAO (2008) http://faostat.fao.org/ [2] Vavilov, N.I. (1939) The new systematic of cultivated plants. In: Huxley, J. Ed., The New Systematic, Oxford University Press, London, 549-566. [3] De, D.N. (1974) Pigeonpea. In: Hutchinson, J. Ed., Evo- lutionary Studies in World Crops, Cambridge Press, Lon- don, 79-87. [4] van der Maesen, L.J.G. (1980) India is the native home of the pigeonpea. In: Arends, J.C., Boelema, G., Groot, C.T. and Leeuwenberg, A.J.M. Eds., Liber Gratulatorius in nonerem H.C.D. de Wit, Agricultural University, Wa- geningen, 257-262. [5] Saxena, K.B. (2008) Genetic improvement of pigeonpea - A review. Tropical Plant Biology, 1, 159-178. [6] Plukenet, L. (1692) Phytographia, 3, table 213, figure 3. [7] Pal, R.K. (1939) A review of the literature on the nutri- tive value of pulses. Indian Journal of Agricultural Sci- ence, 9, 133-137. [8] Sharma, Y.K., Tiwari, A.S., Rao, K.C. and Mishra, A. (1977) Studies on chemical constituents and their influ- ence on cooking ability in pigeonpea. Journal of Food Science and Technology, 14, 38-40. [9] Manimekalai, G., Neelakantan, S. and Annapan, R.S. (1979) Chemical composition and cooking quality of some im- proved varieties of red gram dal. Madras Agriculture Journal, 66, 812-816. [10] Singh, U., Jain, K.C., Jambunathan, R. and Faris, D.G. (1984) Nutritional quality of vegetable pigeonpeas [Ca- janus cajan (L.) Millspaugh]: Dry matter accumulation, carbohydrates and proteins. Journal of Food Science, 49, 799-802. [11] Faris, D.G. and Singh, U. (1990) Pigeonpea: Nutrition and Products. In: Nene, Y.L., Hall, S.D. and Sheila, V.K. Eds., The Pigeonpea, CAB International, Wallingford, 401-434. [12] Singh, U. and Jambunathan, R. (1982) Distribution of seed protein fractions and amino acids in different ana- tomical parts of chickpea (Cicer arietinum L.) and pi- geonpea (Cajanus cajan L.). Qualitas Plantarum Plant Foods Human Nutrition, 31, 347-354. [13] Eggum, B.O. and Beames, R.M. (1983) The nutritive value of seed proteins. In: Gottschalk, W. and Muller, H.P. Eds., Seed Protein: Biochemistry, Genetics, and Nutritive Value, Martinus Nijhoff/W. Junk Publishers, the Hague, 499-531. [14] Singh, U. and Eggum, B.O. (1984) Factors affecting the protein quality of pigeonpea (Cajanus cajan L.). Qualitas Plantarum Plant Foods Human Nutrition, 34, 273-283. [15] Nigam, V.N. and Giri, K.V. (1961) Sugar in pulses. Ca- nadian Journal of Biochemistry and Physiology, 39, 1847-1853. [16] Singh, L., Singh, N., Srivastava, M.P. and Gupta, A.K. (1977) Characteristics and utilization of vegetable types of pigeonpea (Cajanus cajan (L.) Millspaugh). Indian Journal of Nutrition and Dietetics, 14, 8-10. [17] Singh, U., Jambunathan, R., Saxena, K.B. and Singh, L. (1991) Chemical changes at different stages of seed de- velopment in vegetable pigeonpea (Cajanus cajan). Jour- nal of the Science of Food and Agriculture, 57, 49-54. [18] Meiners, C.R., Derise, N.L., Lau, H.C., Ritchey, S.J. and Murphy, E.W. (1976) The content of nine mineral and elements in raw and cooked mature dry legume. Journal of Food Chemistry, 24, 1126-1130. [19] Hulse, J.H. (1977) Problems of nutritional quality of pi- geonpea and chickpea and prospects of research. In: Hulse, J.H., Rachie, K.O. and Billingsley, L.W. Eds., Nu- tritional Standards and Methods of Evaluation for Food Legume Breeders, International Development Research Center, Ottawa, 88-100. [20] Saxena, K.B. (2000) Pigeonpea theory and techniques, In: Gupta, S.K. Ed., Agroloios (India), Jodhpur, 82-112. [21] Singh, U., Jambunathan, R., Saxena, K.B. and Subrama- niam. N. (1990) Nutritional quality evaluation of newly developed high protein genotypes of pigeonpea (Cajanus cajan L.). Journal of the Science of Food and Agriculture, 50, 201-209. [22] Kamath, M.V. and Belavady, B. (1980) Unavailable car- bohydrates of commonly consumed Indian foods. Jour- nal of Science of Food and Agriculture, 31, 194-202. [23] Godbole, S.A., Krishna, T.G. and Bhatia, C.R. (1994) Changes in protease inhibitory activity from pigeonpea (Cajanus cajan (L.) Millspaugh) during seed develop- ment and germination. Journal of Science Food and Ag- riculture, 66, 497-501. [24] Ambekar, S.S., Patil, S.C., Giri, A.P. and Kachole, M.S. (1996) Trypsin and amylase inhibitors in pigeonpea seeds. International Chickpea and Pigeonpea Newsletter, 3, 106- 107. [25] Mutimani, V.H. and Paramjyothi, S. (1995) Protease in- hibitors in some pigeonpea lines. International Chickpea and Pigeonpea Newsletter, 2, 79-81. [26] Singh, U. (1984) The inhibition of digestive enzymes by polyphenols of chickpea (Cicer arietinum L.) and pi- geonpea [Cajanus cajan (L). Millspaugh]. Nutrition Re- port International, 29, 745-753. [27] Jambunathan, R. and Singh, U. (1981) Grain quality of Pigeonpea. Proceedings of International Workshop on Pigeonpeas, Patancheru, 15-19 December 1980, 1, 351- 356. [28] Narasimha, H.V. and Desikachar, H.S.R. (1978) Objec- tive methods for studying cooking ability of tur pulse (Cajanus cajan) and factors affecting varietal differences in cooking. Journal of Food Science and Technology, 15, 47-50. [29] Salunkhe, D.K. (1982) Legumes in human nutrition: Cu- rrent status and future research needs. Current Science, 51, 387-394. [30] Jyothi, E. and Reddy, P.R. (1981) The effect of germina- tion and cooking on the in vitro digestibility of starch in some legumes. Nutritional Report International, 23, 799- 804. [31] Iyengar, A.K. and Kulkarni, P.R. (1977) Oligosaccharide levels of processed legumes. Journal Food Science and Technology, 14, 222-223. [32] Rajalakshmi, R. and Vanaja, K. (1967) Chemical and bio- logical evaluation of the effects of fermentation on the  K. B. Saxena et al. / HEALTH 2 (2010) 1335-1344 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/Openly accessible at 1343 nutritive value of foods prepared from rice and legumes. British Journal of Nutrition, 21, 467-473. [33] Geervani, P. (1981) Nutritional Evaluation of Pigeonpea (Variety Hyderabad 3A) processed by traditional meth- ods. Proceedings of International Workshop on Pigeon- peas. Patancheru, 15-19 December, 1980, 2, 427-434. [34] Daniel, V.A., Rajan, P., Sanjeevarayappa, K.V., Sriniva- san, K.S. and Swaminathan, M. (1977) Effect of insect infestation on the chemical composition and the protein efficiency ratio of the proteins of bengal gram and red gram. Indian Journal of Nutrition and Dietetics, 14, 70- 74. [35] Vimala, V. and Pushpamma, P. (1983) Storage quality of pulses stored in three agro-climatic regions of Andhra Pradesh-1-Quantitative changes. Bulletin Grain Techno- logy, 21, 54-62. [36] Vimala, V. and Pushpamma, P. (1985) Effect of improved storage methods on cook ability of pulses stored for one year in different containers. Journal of Food Science and Technology, 22, 327-329. [37] Uma, R.M. and Pushpamma, P. (1981) Effect of insect infestation and storage on the nutritional quality of dif- ferent varieties of pigeonpea. Proceedings of Interna- tional Workshop on Pigeonpeas, Patancheru, 15-19 De- cember 1980, 2, 443-451. [38] Squire, F.A. (1933) Guiva Department of Agriculture. Rice Bulletine, 1, 51-57. [39] Reddy, L.J., Green, J.M., Singh, U., Bisen, S.S. and Jambunathan, R. (1979) Seed protein studies on Cajanus cajan, Atylosia spp. and some hybrid derivatives. In: Seed Protein Improvement. Cereals and Grain Legumes, International Atomic Energy Agency, Vienna, 2, 105-117. [40] Singh, U. and Jambunathan, R. (1990) Pigeonpea: Post- harvest technology. In: Nene, Y.L., Hall, S.D. and Sheila, V.K. Eds., Pigeonpea, CAB International, Wallingford, 435-455. [41] Kurien, P.P. (1981) Advances in milling technology of pigeonpea. Proceedings of International Workshop on Pigeonpeas, Patancheru, 15-19 December 1980, 1, 321- 328. [42] Sham, N.L. (1976) Effect of nitrogen, phosphorus and sulphur on protein content of arhar Cajanus cajan (L.). Seed Farming, 2, 37-39. [43] Oke, O.L. (1969) Sulphur nutrition of legumes. Experi- mental Agriculture, 5, 111-116. [44] Saxena, K.B., Faris, D.G. and Kumar, R.V. (1984) Breed- ing for special traits. Pigeonpea Breeding Annual Report, ICRISAT, Patancheru, 99. [45] Steven, D. and Ehrlich, N.M.D. (2009) Solutions Acu- puncture, a private practice specializing in complemen- tary and alternative medicine, Phoenix, AZ. Review pro- vided by VeriMed Healthcare Network. [46] Morton, J.F. (1976) The pigeonpea (Cajanus cajan (L) Millspaugh), a high protein tropical bush legume. Horti- culture Science, 11, 11-19. [47] Duke, J.A. (1981) Hand book of legumes of world eco- nomic importance. Plenum Press, New York. [48] Duke, J.A. and Vasquez, R. (1994) Amazonian ethno- botanical dictionary. CRC Press, Boca Raton. [49] Abbiw, D.K. (1990) Useful plants of Ghana; Richmond intermediate technology publications and royal botanic gardens: Kew, London, UK. [50] Yuan, H., Yao, L.L. and Chen, L.K. (1984) Chinese Journal of Integrated Traditional and Western Medicine, 4, 352-354. Cited from Zu, Y.G., Fu, Y.J., Liu, W., Hou, C.L. and Kong, Y. (2006) Simultaneous Determination of Four Flavonoids in Pigeonpea [Cajanus cajan (L.) Millsp.] Leaves Using RP-LC-DAD, Chromatographia, DOI: 10. 1365/s10337-006-0784-z. [51] Tang, Y., Wang, B. and Zhou, X.J. (1999) Effect of ex- ternal application of herbal cajani preparation on the fi- bronection content during healing process of open wound. Journal of Guangzhou University of Traditional Chinese Medicine, 16, 302-304. [52] Prema, L. and Kurup, P.A. (1973) Hypolipidaemic activ- ity of the protein isolated from Cajanus cajan in high fat-cholesterol diet fed rats. Indian Journal of Biochem- istry and Biophysics, 10, 293-296. [53] Amalraj, T. and Ignacimuthu S. (1998) Evaluation of the hypoglycemic effect of Cajanus cajan (seeds) in mice. Indian Journal of Experimental Biology, 36, 1032-1033. [54] Grover, J.K., Yadav, S. and Vats, V.J. (2002) Medicinal plants of India with anti-diabetic potential. Ethnophar- macol, 81, 81-100. [55] Edbordo, C.R. (1978) Medicinal plant of the Philippines, Katha Publishing Company Inc, Quezon City. [56] Chen, D.H., Li, H.Y. and Lin, H. (1985) Studies on chemical constituents in pigeonpea leaves. Chinese Tra- ditional Herbal Drugs, 16, 134-136. [57] Li, Z.H., Zhou, C.H., Gu, Y. and Zhang, J.Y. (2001) The present status of study and utilization of pigeonpea in China and its prospects. Forest Research, 14, 674-681. [58] Sun, S.M., Song, Y.M. and Liu, J. (1995) Studies on the pharmacology of Cajanin preparation. Chinese Tradi- tional and Herbal Drugs, 26, 147-148. [59] Liu, H.Y., Qiu, N.X., Ding, H.H. and Yao, R.Q. (2008) Polyphenols contents and antioxidant capacity of 68 Chinese herbals suitable for medical or food uses. Food Research International, 41, 363-370. [60] Yuan, J., Zhang, J., Lin, J. and Xiong, Y.H. (2004) Tradi- tional Chinese Drug Research & Clinical Pharmacology, 1, 429-431. Cited from Zu, Y.G., Fu, Y.J., Liu, W., Hou, C.L. and Kong, Y. (2006) Simultaneous Determination of Four Flavonoids in Pigeonpea [Cajanus cajan (L.) Millsp.] Leaves Using RP-LC-DAD, Chromatographia, DOI: 10. 1365/s10337-006-0784-z. [61] Fan, Y.G., Xu, C.Y. and He, W. (2002) Chinese Journal of Basic Medicine in Traditional Chinese Medicine, 8, 35-37. Cited from Zu, Y.G., Fu, Y.J., Liu, W., Hou, C.L. and Kong, Y. (2006) Simultaneous Determination of Four Flavonoids in Pigeonpea [Cajanus cajan (L.) Millsp.] Leaves Using RP-LC-DAD, Chromatographia, DOI: 10. 1365/s10337-006-0784-z. [62] Yuan, H., Li, X. and He, W. (1999) Chinese Journal of Traditional Medicine Trauma and Orthopedic, 17, 4-8. Cited from Zu, Y.G., Fu, Y.J., Liu, W., Hou, C.L. and Kong, Y. (2006) Simultaneous Determination of Four Flavonoids in Pigeonpea [Cajanus cajan (L.) Millsp.] Leaves Using RP-LC-DAD, Chromatographia, DOI: 10. 1365/s10337-006-0784-z. [63] Paul, W.C, Philip, C.S. and Monique, S.J. (2003) Pheno- lic compounds on the pod-surface of pigeonpea, Cajanus cajan, mediate feeding behavior of Helicoverpa armigera larvae. Journal Chemical Ecology, 29, 811-821.  K. B. Saxena et al. / HEALTH 2 (2010) 1335-1344 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/Openly accessible at 1344 [64] Lin, L., Xie, N. and Cheng, Z.H. (1999) Flavonoids from Cajanus cajan L. Journal China PharmaceuticalsUni- versity, 30, 21-23. [65] Crozier, A., Jensen, E., Lean, M.E.J. and McDonald, M.S. (1997) Quantitative analysis of flavonoids by reversed- phase high-performance liquid chromatography. Journal of Chromatography, 761, 315-321. [66] Matsuda, H., Morikawa, T., Ando, S., Toguchida, I. and Yoshikawa, M. (2003) Structural requirements of flavon- oids for nitric oxide production inhibitory activity and mechanism of action. Bio-Organic and Medicinal Chem- istry, 11, 1995-2000. [67] Srinivas, K.V.N., Rao, S.K., Mahender, Y.I., Das, B., Krishna, K.V.S.R., Kishore, K.H. and Murty, U.S.N. (2003) Flavonoids from Caesalpinia pulcherrima. Phytochemis- try, 63, 789-793. [68] Kim, H.K., Cheon, B.S., Kim, Y.H., Kim, S.Y. and Kim, H.P. (1999) Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure-activity relationships. Bioche- mical Pharmacology, 58, 759-765. [69] Saxena, K.B. and Sharma, D. (1990) Pigeonpea Genetics. In: Nene, Y.L., Hall, S.D. and Shiela, V.K. Eds., The Pi- geonpea, CAB International, Wallingford, 137-157. [70] Dahiya, B.S., Brar, J.S. and Bhullar, B.S. (1977) Inheri- tance of protein content and its correlation with grain yield in pigeonpea (Cajanus cajan (L.) Millspaugh). Qualitas Plantarum Plant Food and Human Nutrition, 27, 327-334. [71] Durga, B.K. (1989) Genetic studies on protein content and nitrogen accumulation in pigeonpea. Ph.D. Thesis, Osmania University, Hyderabad. [72] Daniel, V.A., Narayanaswamy, D., Desai, B.L.M., Kurien, S., Swaminathan, M. and Parpia, H.A.B. (1970) Supple- mentary value of varying levels of red gram (Cajanus cajan) to poor diets based on rice and ragi. Indian Jour- nal of Nutrition and Dietetics, 7, 358-362. [73] Bidinger, P.D. and Nag, B. (1981) The role of pigeonpeas in village diets. Proceeding of International Workshop on Pigeonpeas, Patancheru, 15-19 December 1980, 1, 357-364. [74] Faris, D.G., Saxena, K.B., Mazumdar, S. and Singh, U. (1987) Vegetable Pigeonpea: A promising crop for India. ICRISAT, Patancheru. [75] Singh, U. (1988) Anti nutritional factors of chickpea and pigeonpea and their removal by processing. Plant Foods for Human Nutrition, 38, 251-261.
|