American Journal of Plant Sciences
Vol.5 No.13(2014), Article
ID:47508,20
pages
DOI:10.4236/ajps.2014.513223
Phenology and Reproductive Biology of Acacia karroo Hayne (Leguminosae: Mimosoideae)
Petrus Johannes Robbertse, Elsie Sophea du Toit, John George Annandale
Department of Plant Production and Soil Science, University of Pretoria, Pretoria, South Africa
Email: hannes.robbertse@up.ac.za
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 5 May 2014; revised 4 June 2014; accepted 22 June 2014
ABSTRACT
The architectural development of Acacia karroo conforms to Troll’s model. Growth of the branches is modular and sympodial with heteroblastic leaves on all long shoots of the tree, including the seedling. Axillary buds tend to proliferate especially on flowering shoots where they form fascicles consisting of up to 10 inflorescences arranged in two parallel serial rows per leaf axil. Most axillary buds are sylleptic and basal buds which give rise to short shoots, each producing two to five cataphylls each season, but no flowers. Inflorescences are only produced on long shoots (modules) of the current season. After flowering the terminal part of the module aborts, trees are usually andromonoecious with capitate inflorescences containing 40 to 100 flowers each, with some male and some hermaphrodite. Some trees produce only male flowers. Anthesis in the same inflorescence, the same tree as well as amongst trees of the same community are synchronised and occur at intermittent intervals, each lasting three or more days at a time. Flowers are protogynous and pollen is produced in polyads, each consisting of 16 pollen grains. Ovaries contain 10 to 14 ovules each. The concave stigma has space for only one polyad which can fertilise all ovules in the ovary after a single pollination event. Fruit set is low with 0 to 10 fruits (pods) per inflorescence.
Keywords:Troll’s Model, Modular Growth, Heteroblasty, Sylleptic, Fascicles, Polyads, Protandrous, Synchronised Flowering, Andromonoecy

1. Introduction
In this contribution the generic name Acacia is used in its traditional broad sense. Acacia karroo Hayne (=Vachellia karroo (Hayne) Banfi & Galasso), commonly known as the sweet thorn or soetdoring, is one of the most abundant and widespread acacias in southern Africa. It belongs to the subgenus Acacia Vassal and like the rest of the members of in this subgenus, the flowers are borne in spherical flower heads and the stipules are straight or slightly curved spines. Other subgenera are Aculeiferum with spicate inflorescences and hooked prickles, and the Phyllodinae to which the Australian acacias (wattles) belong. Following the conservation of the name Acacia with a new type, the subgenus Phyllodinae was raised to generic level and is now being referred to as Acacia s. str. while the subgenus Acacia becomes Vachellia Wight & Arn [1] . Ross [2] in his treatment of A. karroo, refers to several morphologically distinct forms of the species. After a comprehensive comparative study of all the variations, Swartz [3] described five of them as new species (A. dyeri, A. kosiensis, A. natalitia, A. robbertsii and A. (montana) theronii). In this study the new species are excluded and the descriptions are based on the new and more narrowly defined concept of the species. Most of the work was done on trees growing in and around Pretoria, South Africa (25˚58'46''S, 28'21''E).
The reproductive biology of A. karroo is interrelated with its morphology and phenology but, despite being ecologically one of the most prominent tree species in southern Africa, very little is known about these aspects or its pollination biology. In their review on the pollination ecology of acacias, [4] stated the following: “The flowers of most acacias (including all members of the Australian acacias (subgenus Phyllodinae), offer only pollen to flowers visitors and floral nectar is limited to a minority of species in the subgenera Acacia and Aculeiferum”. They also mention that members of the subgenus Phyllodinae have long-lived, protandrous flowers while most members of the subgenera Acacia and Aculeiferum have short-lived flowers that appear to be protandrous with a daily patterning in reward provision. The main reward for pollinators is pollen that is supplied in ample quantities. Pollen of acacias is presented in polyads consisting of 4 to 32, but mostly 16 monads (grains) per polyad [5] -[8] . The function of the polyad in reproduction was reported on by Kenrick and Knox [6] , and they also showed that only one polyad fits on the small stigma and could fertilize all ovules in the ovary. Among species the number of flowers per flower head varies from a few to >200, the number of stamens per flower varies from 47 to 90. All acacias have 8 polyads per anther (Stone et al. [4] , 2003), which means that every flower head with 80 flowers and 50 stamens per flower can produce 32,200 polyads each with 16 pollen grains, amounting to 512,000 individual pollen grains (monads). According to Stone et al. [4] , floral nectar is not common in acacias although nectar was found in A. zanzibarica and A. tortilis from the subgenus Acacia in Tanzania and A. brevispica, A. mellifera and A. senegal from the subgenus Aculeiferum [9] [10] . Nectar was also reported in A. nigrescens [11] . Robbertse [12] found that the flowers of the subgenus Aculeiferum contain a cup-shaped gland (nectary) around the ovary base while it was lacking in flowers of the subgenus Acacia. A. karroo is mentioned as “An excellent source of pollen and nectar—ideal for the farmers” [13] but no real research has been done on nectar production of this species.
According to Barnes et al. [14] A. karroo is zoophilous (“zoomophilus” in publication), principally insectpollinated and they also stated that no comprehensive list of pollinating insects could be found. It was therefore necessary to address this deficiency as well.
The primary aim of this report was to study the pollination biology of A. karroo, but the phenology was included in order to understand the relationship between tree growth and flower production.
2. Materials and Methods
2.1. Phenology
Growth and development of seedlings grown from seed collected in the Faerie Glen location, Pretoria, Gauteng, were observed over a period of two years to determine the growth model of the tree. Mature trees over a wide distribution range were observed to determine the branching and modular growth.
Trees in and around Pretoria were observed by the first author over a period of 24 years from September 1990 to 2014, excluding a few years and short intervals when he was not in Pretoria during the flowering period. More detailed observations from 1990 to 2004 were made on 50 trees in each of the suburbs Meyerspark and Brummeria and from 2005 to 2011 on100 trees in the suburb Faerie Glen (25˚46'47''S, 28˚17'45''E). Every year records were kept of the dates the trees started and ended flowering. In the Faerie Glen area the time and development of the flower heads in the leaf axils were studied and photo’s taken of the different shoot types and stages of inflorescence development. During October 2013, 40 trees in Faerie Glen were marked and regularly observed to determine exact flowering cycles for each tree over the entire flowering season.
For studying the growth model, seeds were germinated and seedling development and structure were observed. For further development and structure, young as well as mature and old trees were also studied.
2.2. Inflorescence and Flower Morphology
Over the study period inflorescences (flower heads) with freshly opened flowers were collected from different trees, either from the same population or from different populations and taken to the laboratory where the inflorescences and flowers were dissected, studied and photos taken of the different parts and developmental stages. The number of male and hermaphrodite flowers per inflorescence was also determined.
2.3. Pollination and Pollinators
Observations were made in different localities and different dates as indicated in the results. Insects visiting the flowers were collected and identified up to family and/or species level. Scanning electron micrographs were also taken of the pollen and pollinators carrying pollen on their bodies. The number of ovules per ovary was determined by fixing dissected ovaries for 3 minutes in Carnoy’s fixative, rinsed in 70% ethanol, cleared in a commercial bleach, stained and mounted in decolorized aniline blue stain [15] and viewed using a fluorescence microscope. The fluorescent hypostase of ovules render them visible for counting.
Geitonogamy (same genet selfing) and xenogamy (different genet crossing) were studied by covering 10 flowering shoots (modules) on two different trees with muslin cloth bags to keep insects out. For geitonogamy inflorescences with open flowers from the same tree were used for brushing the inflorescences of five of the covered modules. For xenogamy, inflorescences from another tree were used for pollinating inflorescences of another five covered modules. Bags were removed after completion of the flowering cycle and 5 pollinated inflorescences from each covered module collected for determining fruit and seed set.
2.4. Nectar Production
As mentioned in the introduction, flowers of A. karroo do not have a cup-like gland surrounding the ovary base, but during the study of the flower parts, small glandular trichomes were observed on the basal part of the ovary. Fresh flowers as well as one-day-old flowers were used in an effort to collect nectar from the flowers using micro-pipettes but no nectar was found.
2.5. Fruit Growth Rate
On 28 December 1975, 100 inflorescences on the same tree were randomly marked during anthesis. On 14-01- 1976, 20 small fruits (pods) that had set on the marked inflorescences were randomly marked and their widths and lengths recorded. The same measurements were again recorded on 30-01-1976 and on 17-02-1976 to determine fruit growth rate. Other fruits from the same tree were collected and dissected to look at seed development.
2.6. Seed Set
During the 1990s, 100 ripe pods were randomly collected from ten different trees for determining the numbers of sound seeds, aborted seeds and parasitized seeds per pod. During March 2011, just after ripening of the pods, 30 pods each from 5 different modules were collected. All pods per module were measured and counted, the length of each pod was measured and the number of aborted and sound seeds per pod counted. Three sets of 300 healthy seeds each were weighed to get the average weight per seed.
3. Results and Discussion
3.1. Phenology
3.1.1. Tree Model
The architectural development of A. karroo conforms to Troll’s model [16] where all axes or modules are plagiotropic to ortho-plagiotropic, the architecture being build by continuous superposition of one module on top of the other. The proximal part of the module is secondarily erected as a result of contracting gelatinous fibres in the secondary xylem (Figure 1(C)). The entire tree trunk and main branches are therefore made up of the basal parts of consecutive modules while the distal parts remain branches (Figure 1(A)). The growing points of


Figure 1. Troll’s tree model. (A) Diagram of model; Green lines indicate where the trunk will develop; (B) Seedling representing Troll’s model; (C) Transverse section of the wood showing gelatinous fibres. V = Vessel; Xr = xylem ray: Arrow points to gelatinous fibre.
branches abort towards the end of the growing season or after leaf drop. New modules are formed by superposition and/or from the most distal surviving axillary bud(s) (Figure 2). Growth of the whole tree is therefore modular and sympodial. Modules of young, vigorous trees start with short internodes and reduced leaves without petiolar glands and small spinescent stipules (cataphylls), followed by longer internodes and more developed leaves (euphylls) containing petiolar glands and longer spines, while gradually terminating with reduced leaves (hypsophylls) without petiolar glands containing reduced spines (Figure 3(A)). The module ends with the abortion of the growing point. In young, vigorous trees, modules may be up to one meter long. In older, less vigorous trees, the modules become much shorter by not developing the middle part of the module with euphylls and the long spines. Older trees therefore become less spiny but more leafy due to the prevalence of leafy brachyblasts.
Axillary buds start off as single buds, but tend to proliferate (Figure 5) so that even on seedlings, series of two to three buds can be found in the axil of the first vegetative leaf following the cotyledons (Figure 4(A) and Figure 4(B)). The first-formed budis and sylleptic and sprouts close to the growing point, but mostly remain a short

Figure 2. Modular growth: End of old module and sympodial development of new module.

Figure 3. Vegetative (A) and flowering module (B). Both modules show small spines of cataphylls and longer spines of euphylls.
shoot (brachyblast) with a few reduced leaves without petiolar glands while the axillary bud primordia of the brachyblasts may develop into functional buds. Brachyblasts remain alive for many years, producing a few cataphylls every season and may become very prominent, giving the impression of pin-cushions due to the presence of persistent, minute spinescent stipules from pervious seasons’ cataphylls (Figure 6).

Figure 4. Front (A) and side (B) views of the same seedling axis showing cotyledon and stem buds. A, showing two opposite cotyledon buds (cb1 and cb2), hypocotyl (h) and proliferated second stem bud (psb). B, showing one cotyledon bud (cb2), first stem bud (sb1) second proliferated stem bud (psb) and part of hypocotyl (h).
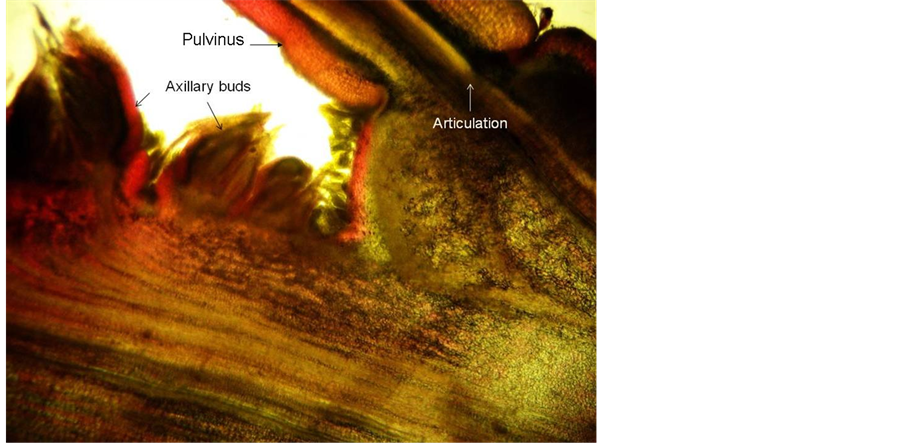
Figure 5. Longitudinal section of node showing proliferated axillary bud.
3.1.2. Flowering
During August all trees in and around Pretoria lose their leaves of the previous season. New growth on the observed and surrounding trees as well as trees outside the study area start producing new modules at the beginning of September. Short shoots produce a number of cataphylls but no inflorescences. Long shoots (new modules) are mostly produced close to the aborted apical bud (sympodially) but also from the second bud or short shoots lower down the long shoot. In trees that had reached the flowering stage, inflorescence buds are produced in the axils of leaf primordia close to the growing points of green (new) long shoot towards the middle of September. Vigorous long shoots (mostly found on younger trees) first go through a period of active vegetative growth before floral buds are produced in the axils of euphyll primordia and/or hypsophyll primordia in the apical bud of the module (Figure 3(B)). On older trees with less vigorous long shoots, floral bud production already starts lower down the module in the axils of cataphyll primordia. Inflorescence buds are formed in fasci-

Figure 6. Part of old module with short shoots (arrow), each bearing a number of cataphylls.
cles [14] of six to ten, staggered in two parallel, collateral series in each leaf primordium axil (Figure 7). As mentioned before, leaves on long shoots may contain two or more serial axillary buds. Inflorescence buds are formed by proliferation of the distal axillary bud while the proximal bud remains dormant (Figure 8). The first-formed inflorescence buds are positioned distally to the subtending leaf base and the youngest closer to the subtending leaf base, next to the serial vegetative bud. A peculiar dome-like structure was often seen between the two rows of inflorescence buds (Figure 8) that could be an indication of adnation of the ‘short shoot’ on which the floral buds are borne, to the main shoot. Long shoots continue producing new leaves/bracts and floral buds as long as favourable growing conditions prevail (Figure 9(C)) or until the apical bud is ‘exhausted’ and aborts. Heavy fruit set on the module will also terminate further growth. This phenomenon explains why trees are flowering over anextended period. In some trees new modules are formed during December/January, mostly around the tree tops. These modules extend the flowering period.
The inflorescence is a stalked, spherical capitulum, 8 to 12 mm in diameter (Figure 9) containing many (Table 1) sessile flowers, each subtended by a spoon-shaped bract. The inflorescence stalk (peduncle) consists of two internodes with involucel bracts on the node between the two internodes. One or more sterile flowers may occur in the axils of the involucel bracts (Figure 9(A)). The trees are andromonoecious with male and hermaphrodite flowers in the same inflorescence (Table 1) although some inflorescences contain male flowers only and some trees are entirely male.
The number of flowers per inflorescence varies from 33 to 107, while the average numbers on the same tree varies from 48 to 99. The ratio of male to female flowers is highly variable as illustrated in the table and some inflorescences contain only male flowers. Due to the wide variation, no attempt was made to do a statistical analysis on the results. In a similar study done in 1988, the average number of flowers per flower head varied from 72.7 ± 6.9 to 99.3 ± 3.7 and the percentage male flowers per flower head varied from 6 to 40. Similar figures were obtained during 2010 (results not shown).
3.1.3. Flower Anthesis and Morphology
Anthesis of flowers on the same inflorescence and inflorescences on the same tree is synchronized and they all open on the morning of day 1 and start wilting towards the end of day 2 of anthesis. From day 2 onwards, flow-

Figure 7. Growth point of flowering module showing how inflorescence buds are produced as a result of proliferation of an axillary bud, thus producing an inflorescence-bearing short shoot (phasicle).

Figure 8. Detail of leaf axil with inflorescence-bearing short shoot adnated to long shoot and remaining vegetative bud.
Table 1. Number and percentage male and hermaphrodite flowers per inflorescence. (a) Inflorescences collected in Roodeplaatdam Nature Reserve 1992; (b) Inflorescences collected during 1975 from three trees in different suburbs of Pretoria: Silverton (tree 1), Rissik (tree 2) and Hatfield (tree 3).
(a)
ers on more inflorescences on the same fascicle or more distal fascicles will open. Each flower consists of five fused calyx members (sepals) 1.3 to 2 mm long, five partly fused petals (2.5 to 3 mm long) and about 40 - 50 stamens extending about 3.5 mm beyond the petals and a pistil in the hermaphrodite flowers (Figure 10).

Figure 9. Details of capitate inflorescence. (A) Complete inflorescence with sterile flower in axil of involucel; (B) Peduncle with sterile involucel; (C) Tip of flowering module showing continuous production of new inflorescences.

Figure 10. Hermaphrodite (A) and male (B) flowers.
1) Anthers and pollen As in all acacias [14] anthers of A. karroo contain eight polyads each [17] and each polyad is composed of 16 grooved pollen grains (monads) (Figure 11(A) and Figure 11(B)). Most of the anthers are distally fitted with a spherical, multi-cellular “gland” about the same size as the anther, which is attached to the anther by means of a cord of cells (Figure 12). The glands are easily detached from the anther and it might serve as another means of awarding the pollinators visiting the flowers.
2) Pistil and ovules The pistil consists of a single carpel with a short stalk (gynophore), a long style and a small, concave stigma. The outer surface of the ovary is covered with numerous spherical glandular trichomes (Figure 13). In freshly
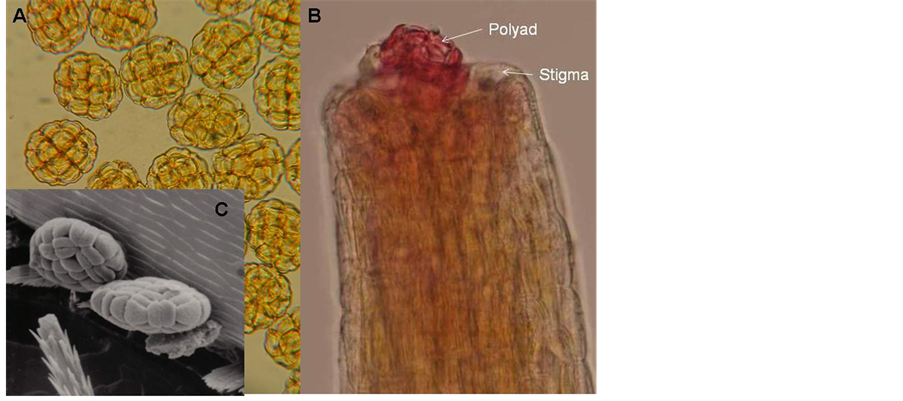
Figure 11. Light and scanning electron micrographs of polyads (A) and a pollinated pistil (B) occupying a single polyad. and a scanning electron picture of polyads on an insect, Oxythyrea rubra (C).

Figure 12. Micrograph of stamens with suspended anther glands.
opened bisexual flowers the style and pistil is hidden amongst the many stamens. Out of 100 ovaries investigated the number of ovules per ovary varied from 10 to 14 with an average of 12 ovules. The ovules are anatropous with a single vascular bundle running through the raphe from the hilum to the chalaza and from there through the antiraphe to the micropyle [18] . Each ovule contains a seven-celled, eight nucleate, polygonum type embryo sac [17] .
3.1.4. Intermittent Flowering
In Pretoria and surroundings, some trees start flowering towards the end of October while others started later in the season, but flowering amongst all trees in and around Pretoria are synchronized. Trees that start flowering later in the season link in with the rhythm of the other trees that started earlier. As depicted in Figure 3(B), Figure 9(C) and Figure 14(A)), inflorescences with the most advanced flower buds (“mature inflorescences”) are located on the most proximal end of a module. During a flowering cycle all trees with “mature” inflorescences start flowering on the same day. On the first day of a flowering cycle, two or more inflorescences per fascicle, starting from the two to five most proximal fascicles of a module, will produce open flowers (Figure 14(A)). The next day one or more additional inflorescences per fascicle will produce open flowers (flowered) while a

Figure 13. Part of ovary containing glandular trichomes.

Figure 14. Flowering module photographed on 22-12-2008 when flowers were open and again on 25-12-208 with withered (spent) flowers, illustrating intermittent flowering.
 (a)
(a) (b)
(b) (c)
(c) (d)
(d) (e)
(e)
Figure 15. Diagrams showing dates over the past 21 years when A. karroo trees were flowering in and around Pretoria during No vember (a); December (b) and January (c); D, Flowering cycles of 40 marked trees observed during 2013-2014 flowering season.
few inflorescences of adjacent, more distal fascicles will also flower. This process is repeated for another two or more days after which all inflorescences with opened flowers on the tree will wither and died (Figure 14(B)). One flowering cycle is completed when all inflorescences without set fruit have withered and abscised. Flowering cycles on the same module are repeated several times while new inflorescence buds are produced by the apical buds of the flowering modules (Figure 7 and Figure 9(C)). Intermittent flowering cycles on the same tree continue until no more inflorescence buds on the modules are left, fruit are set and the apical bud aborted. Early, intermediate as well as late flowering trees were identified. Early flowering trees started flowering during end of October to beginning November and depending on the activity of the module’s apical meristem, the trees kept on flowering until about middle to end of December. Intermediate flowering trees that started flowering towards beginning December, linked in with the flowering cycles of early flowering trees and also kept on flowering for about two months. Late flowering trees (mostly old trees) started flowering during January and some continued flowering until March. Some trees producing a new flush during December/January may extend the flowering cycles. Observations made over the past 24 years on this cyclic or intermittent flowering of trees in and around Pretoria are shown in Figures 15(A)-(C). Flowering cycles for March and April are not shown. Flowering cycles of 40 marked trees in Faerie Glen are shown in Figure 15(E).
3.2. Pollination
As a result of synchronized anthesis of the flowers on the same inflorescence, pollen shed is on the first day of anthesis while the stigmas are not yet receptive to pollen. Out of 100 pistils observed on day one of anthesis, only two contained a polyad while on the second day of anthesis, 60% of the pistils contained polyads. Stigmas therefore became receptive during the second day of anthesis which is in accordance with observations by Sedgely et al. [19] .On the first day of anthesis styles are not protruding beyond the stamens but do so during the second day (Figure 17). During the first day of anthesis the ovary has a light green colour but turns pink during the second day of anthesis (Figure 16). Flowers are therefore clearly protandrous.
The small, convex stigma can only accommodate a single polyad with its 16 pollen grains (Figure 11(B)). One polyad can therefore theoretically fertilise all (10 - 14) ovules in the ovary after a single pollination event.
In the experiment where flowering modules were covered with muslin bags at the stage just before anthesis, it was found that where geitonogamy was applied, fruit set was only 1% compared to the 4% fruit set where xenogamy was applied which indicates that the trees are mostly out crossing.
During a peak flowering cycle in 1987, insects visiting open flowers were collected from trees in Hatfield, Pretoria (28˚15'E, 25˚45'S) from 08:00 to 13:00 (Figure 17). The following different types and numbers were found.

Figure 16. Dissected hermaphrodite flowers. Ovary green on day 1 of anthesis and pink on day 2 of anthesis.

Figure 17. Honeybee visiting flowers after rain. Note the protruding styles (with receptive stigmas).
Hymenoptera Apis melifera (Apidae)-12 Hemipepsis sp. (Pompilidae)-1 Polistis sp. (Vespidae)-3 Tiphiidae–1 Ammophila (Sphecidae)-2 Scolia (Scoliidae)-3 Vespidae-4 Diptera Calliphoridae-1 Conopidae-1 Phytomia incise (Syrphidae)-3 Eristalinus taeinops (Syrphidae)-4 Musca domestica (Muscidae)-10 Coleoptera Monolepta congener (Chrysomelidae)-16 Diapromorpha sp. Chrysomelidae)-7 Rhembastus sp. (Crysomelidae)-1 Decaria sp. (Chrysomelidaae)-4 Dcapotoma sp. (Meloidae)-1 Mylaris sp. (Meloidae)-8 Lycidae-4 Pacnodasinuata (Cetoniidae, Scarbaeidae)-17 Dischita cincta (Cetoniidae, Scarbaeidae)-9 Polychaphes balteata (Cetoniidae, Scarbaeidae)-1 Oxythyrea sp. (Cetonide, Scarbaeidae)-2 Oxythyrea rubra (Ctoniidae, Scaraeidae)-4 Hemiptera Miridae-2 A similar study was made during 1990 and it was found that of all the insects visiting A. karroo flowers, 70% were Coleoptera, 15% Diptera, 10% Hymenoptera and 5% Thysanoptera. Most of the Coleoptera were from the family Chrysomelidae which agrees with the 1987 results. Figure 11(C) shows a scanning electrograph of polyads with grooved monads on Oxythyrea rubra.
3.3. Fruit Growth Rate
The fruit growth rate that was determined over a period of 51 days after anthesis during 1976 (See Materials and methods) is shown in Figure 19. The fruit had almost reached mature size (5 to 8 mm wide and 80 to 160 mm long) while the seeds were still very small (Figure 18). The pod growth rate demonstrates the bottom part of a sigmoidal curve.
3.4. Fruit and Seed Production
In a study that was made during 1990, it was found that the number of aborted seeds per pod was 0.67 (±2.1), the number of parasitized seed per pod was 0.2 (±0.5) and the number of sound seeds was 7.1 (±3.0).
During another study in 2011, the average number of pods per module was 16.8 (±9) (Figure 20) and the number of pods per single inflorescence varied from I to 10. The average length of pods was 11.3 mm (±2.6), the average number of aborted seeds per pod was 3.0 (±2.3) and the number of sound seeds per pod was 6.2 (±3.3). No insect larvae were found inside the pods although some of the aborted seeds showed signs of insect damage from the outside of the pod.
Three batches of 300 seeds each were weighed. The weight of the first batch was 9.57 g, for the second batch

Figure 18. Two months old pod valve. Pericarp has reached full size while seeds are still undeveloped.
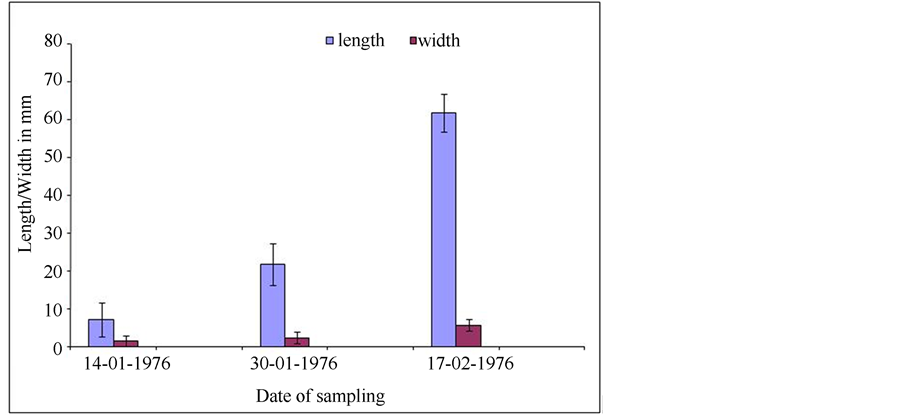
Figure 19. Diagram showing pod growth during 51 days after anthesis.
it was 10.56 g and for the third batch it was 15.04 g which gave an average of 11.7 g. The weight of a single seed was therefore about 0.39 g.
4. Discussion
4.1. Phenology
In this paper the tree architectural model of A. karroo was described for the first time. In the wider distribution of the species, many variations of this model will be found like the many-stemmed shrubs in the Karoo and Springbok Flats. As shown in Figure 4 the hypocotyl and proximal part of the tap root are swollen and serves as a storage structure. Hand sections stained with iodine (not shown) confirmed the presence of abundant amounts of starch in all parenchyma cells of the cortex, phloem, xylem and pith. In areas where night temperatures drop below 0˚C, the top part of the seedling stem can be killed by frost while the bottom part close to the ground remains alive. Due to the short internodes at the basal part of the stem (Figure 4), a number of axillary buds (>6 within the first centimetre above the hypocotyl) remain viable for sprouting during the following spring to form basal lateral shoots, each with a number of axillary buds close to ground level. This phenomenon could possibly explain how and why the growth model of shrub-like plants differs from the “typical” model. During the winter of 2010, hundreds of A. karroo seedlings in a nursery near Pretoria were seemingly killed by frost, but most of them sprouted during the ensuing spring to produce healthy plants, each with more than one stem. Story [20] mentioned that twelve months old seedlings (about 35 cm high) died back following a burn but re-sprouted from the base, each giving rise to three or four stems.
Although most authors mention that inflorescences are borne only on present season’s growth [14] [21] , the modular growth of the species has not yet been properly described as was done in this paper, also explaining how inflorescences are formed. Taegue & Walker [22] did report that the shoots are heterophyllous but did not mention that cataphylls are borne on the basal parts of modules (heteroblastic) as well as on short shoots, neither that cataphylls are associated with very short, or even inconspicuous spines, while euphylls are associated with long spines.
4.2. Flowering
Barnes et al. [14] mentioned that inflorescences are borne in fascicles in leaf axils. In this paper we show that the capitate inflorescences are produced by proliferation of the primordial axillary bud to form two serial rows of inflorescence buds (fascicles). The existence of entirely male A. karroo trees was also mentioned by Oballa [23] and Barnes et al. [14] . Parameters of the ratio of male to hermaphrodite flowers on the same inflorescence is also provided by Gordon-Grey and Ward [24] which are more or less in accordance with our figures. Intermittent flowering was noted by Van Rooyen [25] and Milton [21] mentioned that the trees flower throughout the whole season as the new shoots are produced. In this paper we supply more information about cyclic or intermittent flowering from October to March and in some years up to April. The reason why flowering amongst the trees is synchronized, remains a mystery. It was attempted to relate flowering with temperature, day length, rainfall and cycles of the moon, but no relation was found so far.
The function of the anther glands is still unknown. It was reported by Ghazoul [26] that flowers of some acacias contain a repellent to keep ants away from the flowers not to interfere with visiting pollinators. During this study, no ants were observed on the open flowers and it is quite possible that the anther glands contain such a repellent. Stone et al. [4] also mentioned, “It is possible that these glands play a role in the production of scents, either to attract pollinators, or to repel ants or both”. The glands may, however, also serve as an additional reward for some kinds of visiting insects. A study of the floral scents of some South African acacias was done by Erasmus [27] , but unfortunately she did not do a separate analysis of the anther glands.
4.3. Pollination
Sedgely & Harbard [19] found that A. karroo has protandrous flowers and the stigmas remain receptive for five days after anther dehiscence. In this study duration of stigma receptivity was not studied but the flowers withered after the third day post anthesis and inflorescences without set fruit started dropping four to five days after anthesis. The colour change of the ovary has not been reported before. The small stigma accommodating one polyad only, is a general phenomenon in acacias [6] [10] . Tybirk [28] studied flower visitors and pollination

Figure 20. Modules with aborted terminals and mature pods.
ecology of A. nilotica, A. tortilus and A. Senegal and collected 118 taxa of insects mainly from the Hymenoptera, Lepidoptera, Coleoptera and Diptera which is in accordance with insects we had found on A. karroo flowers. In Orwa et al. [29] it is stated that “It (A. karroo) exhibits a tendency towards outcrossing, as evidenced by the existence of trees that are entirely male” and members of Coleoptera, Diptera, Hymenoptera and Lepidoptera are also mentioned as pollinators.
Stone et al., [4] mentioned that nectar is not common in acacias, but that nectar is secreted by some species of the subgenus Acacia like A. zanzibarica and A. nilotica. No liquid nectar was found in A. karroo flowers during this investigation. The ovary is covered with small glandular trichomes and on different occasions, after rain, honey bees were observed “feeding” on the flowers but did not collect pollen. It is possible that the small glands do contain nectar that was dissolved during the rain, rendered it available for the bees. Flies with different mouth parts, were ‘feeding’, on the flowers, even if it was not raining. Different kinds of beetles were by far the most common visitors to the flowers.
4.4. Fruit Growth and Seed Set
Although fruit growth was not followed up to the stage of mature pods, it is clear that the growth is typically sigmoidal, reaching a maximum length of about 16 cm. Seed set is limited by the number of ovules per ovary and the number of viable pollen grains per polyad. Sap sucking insects feeding on the young pods may be responsible for the relatively high number of aborted seeds. In a study that was done during 1990, the percentage of sound seed for A. karroo varied between 6.2% and 7.1%, compared to 5.3% for A. robusta, 88% for A. galpinii, 87% for A. mellifera, 93% for A. mearnsii, 70% for A. decurrens, 91% for A. saligna and 79% for A dealbata.
5. Conclusion
In this paper the relationship between modular growth, tree model structure and intermittent flowering in A. karroo is presented for the first time. Additional information about flowering, pollination and fruit set also contributes to a better understanding of the life cycle of this important South African tree.
References
- Bouchenak-Khelladi, Y., Maurin, O., Hurter, J. and Van der Bank, M. (2010) The Evolutionary History and Biogeography of Mimosoideae (Leguminosae): An Emphasis on African Acacias. Molecular Phylogenetics and Evolution, 57, 495-508. http://dx.doi.org/10.1016/j.ympev.2010.07.019
- Ross, J.H. (1979) A Conspectus of the African Acacia Species. Memoirs of the Botanical Survey of South Africa, No. 44. Botanical Research Institute, Department of Agricultural Technical Services, Republic of South Africa.
- Swartz, P.P. (2002) Trees of Southern Africa. 3rd Edition, Struik Publishers, Cape Town, 285-295.
- Stone, G.N., Raine, N.E., Presscot, M. and Willmer, P.G. (2003) Pollination Ecology of Acacias (Fabaceae, Mimosoideae). Australian Systematic Botany, 16, 103-118. http://dx.doi.org/10.1071/SB02024
- Robbertse, P.J. (1974) A Scanning Electron Microscope Investigation of the Pollen of South African Acacia Species. Journal of South African Botany, 40, 91-99.
- Kenrick, J. and Knox, R.B. (1982) Function of the Polyad in Reproduction of Acacia. Annals of Botany, 50, 721-727.
- Kenrick, J. and Knox, R.B. (1989) Quantitative Analysis of Self-Incompatibility in Trees of Seven Species of Acacia. Journal of Heredity, 80, 240-245.
- Kenrick, J. (2003) Review of Pollen-Pistil Interaction and Their Relevance to Reproductive Biology of Acacia. Australian Systematic Botany, 16, 119-130. http://dx.doi.org/10.1071/SB02005
- Stone, G.N. and Rowe, J.A. (1998) Partitioning of Pollinators during Flowering in an African Acacia Community. Ecology, 79, 2808-2827. http://dx.doi.org/10.1890/0012-9658(1998)079[2808:POPDFI]2.0.CO;2
- Tandon, R., Shivanna, K.R. and Mohan Ram, H.Y. (2001) Pollination Biology and Breeding System of Acacia senegal. Botanical Journal of the Linnean Society, 135, 251-262. http://dx.doi.org/10.1111/j.1095-8339.2001.tb01094.x
- Flemming, P.A., Hofmeyer, S.D. and Nicolson, S.W. (2007) Role of Insects in the Pollination of Acacia nigrescens (Fabaceae). South African Journal of Botany, 73, 49-55.
- Robbertse, P.J. (1974) The Genus Acacia in South Africa II, with Special Reference to the Morphology of the Flower and Inflorescence. Phytomorphology, 24, 1-25.
- Venter, F. and Venter, J.A. (1996) Making the Most of Indigenous Trees. Briza Publications, Pretoria.
- Barnes, R.D., Filer, D.L. and Milton, S.J. (1996) Acacia Karroo: Monograph and Annotated Bibliography. Tropical Forestry Papers 32, Oxford Forestry Institute, Oxford.
- Alexander, M.P. (1987) A Method for Staining Pollen Tubes in Pistil. Stain Technology, 62, 107-112.
- Halle, F., Oldeman, R.A.A. and Tomlinson, P.B. (1978) Tropical Trees and Forests: An Architectural Analysis. Springer, Berlin. http://dx.doi.org/10.1007/978-3-642-81190-6
- Bala, B.B. (1978) A Comparative Study of Sporogenesis and Embryology of Acacia karroo and Acacia caffra. M.Sc. Thesis, University of Fort Hare, Alice.
- Robbertse, P.J. (1973) Die genus Acacia in Suid-Afrika—3 (Met spesiale verwysing na die morfologie van die saad). Tydskrif vir Natuurwetenskap, 13, 72-95.
- Sedgely, M. and Harbard, J. (1993) Pollen Storage and Breeding Systems in Relation to Controlled Pollination of Four Species of Acacia. Australian Journal of Botany, 41, 601-609. http://dx.doi.org/10.1071/BT9930601
- Story, R.A. (1952) A Botanical Survey of the Keiskammahoek District. Botanical Survey of South Africa Memoirs No. 27, Department of Agriculture, Division of Botany, Pretoria.
- Milton, S.J. (1987) Phenology of Seven Acacia Species in South Africa. South African Journal of Wildlife Research, 17, 1-6.
- Teague, W.R. and Walker, B.H. (1988) Growth Patterns and Annual Growth Cycle of Acacia karroo Hayne on Relation to Water Stress. Journal of the Grassland Society of Southern Africa, 5, 85-95. http://dx.doi.org/10.1080/02566702.1988.9648116
- Oballa, P.O. and Olng’otie, P.A.S. (1994) Chromosome Numbers in Two African Acacia Species. Kew Bulletin, 49, 107-113. http://dx.doi.org/10.2307/4110205
- Gordon-Grey, K.D. and Ward, C.J. (1975) A Contribution to the Knowledge of Floral Variation in Acacia karoo in Eastern South Adrica. Boissiera, 24a, 279-284.
- Van Rooyen, N. (1984) n Fenologiese studie van die plantegroei van die Roodeplaatdam-Natuurreservaat. PhD Thesis, University of Pretoria, Pretoria.
- Ghazoul, J. (2001) Can Floral Repellents Pre-Empt Potential Ant-Plant Conflicts? Ecology Letters, 4, 295-299. http://dx.doi.org/10.1046/j.1461-0248.2001.00229.x
- Erasmus, M.E.C. (1990) Bodamp-Gaschromatografie Met Die Dinamiese Oplosmiddeleffekinlaat: Toepassingin Die Che-Motaksnomie van Acacia. M.Sc. Thesis, University of Pretoria, Pretoria.
- Tyrbirk, K. (1992) Pollination, Breeding System and Seed Abortion in Some African Acacias. Botanical Journal of the Linnean Society, 112, 107-137. http://dx.doi.org/10.1111/j.1095-8339.1993.tb00312.x
- Orwa, C., Mutua, A., Kindt, R., Jamnada, R. and Simons, S.A. (2009) Agroforestry Database 4.0. World Agroforestry Centre, 1-5.


