Journal of Water Resource and Protection
Vol.08 No.04(2016), Article ID:65731,11 pages
10.4236/jwarp.2016.84037
The Synthesis of Nano TiO2 and Its Use for Removal of Lead Ions from Aqueous Solution
Afshin Shokati Poursani1*, Abdolreza Nilchi2, Amirhessam Hassani1, Seyed Mahmood Shariat1, Jafar Nouri1
1Department Environmental Pollution, Faculty of Energy and Environment, Science and Research Branch, Islamic Azad University, Tehran, Iran
2Nuclear Science and Technology Research Institute, Tehran, Iran

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 21 August 2015; accepted 18 April 2016; published 21 April 2016
ABSTRACT
In this study, nano-TiO2 particles were synthesized by sol-gel method. The synthesized nanoparticles were characterized by Fourier Transform Infrared (FT-IR), X-ray diffraction (XRD), Transmission electron microscope (TEM) and Brunauer-Emmett-Teller (BET). The results showed that the average size of TiO2 nanoparticles and their specific surface area were 21.1 nanometer and 55.35 m2/gr, respectively. The effects of several variables such as adsorbent weight, pH and contact time on lead ions adsorption were studied in batch experiments and finally the optimum conditions for lead ions adsorption by synthesized nano-TiO2 were obtained. The results showed that the synthesized nano TiO2 had a good capacity to adsorb lead ion. The kinetic data were described by pseudo-first and second-order models. Freundlich and Langmuir isotherm models were used for the analysis of equilibrium data, and results showed that the Langmuir model was suitable for describing the equilibrium data of lead ion adsorption by nano TiO2. Using the Langmuir isotherm, the maximum sorption capacity of Pb2+ was estimated to be 7.41 (mg/g) at 25˚C.
Keywords:
TiO2, Lead, Adsorption, Kinetic Studies

1. Introduction
The increase of pollutant concentrations in water resources is one of the most serious environmental problems worldwide. Heavy metals are one of the major chemical pollutants that cause acute toxicity [1] and have a long-term accumulation in the environment [2] . The presence of heavy metals, such as lead, in water has been a public concern during the last decade [3] . The wastewater from different industries including electroplating, metallurgical, nuclear, wood and paper, painting, and dyeing contains a large amount of lead ions [3] . However, the wastewater from battery manufacturing is one of the main sources for dispersion of lead and other heavy metal ions in water resources [3] . The adverse effect of lead and heavy metals on environment and human health is obvious to everyone [4] . Elimination of heavy metal ions from the industrial wastewaters is the best way to control them and ensure the environmental and human health [5] . Many different methods such as chemical precipitation, ion exchange, adsorption, membrane filtration, electrochemical and electrical coagulation technologies have been used for heavy metal removal from aqueous solutions [6] . Most of these methods are not economically viable and require either high energy or large amounts of chemicals especially when heavy metals concentration is low [7] . In this situation, adsorption seems to be better than other methods because of its efficiency, flexibility, simplicity, and low waste production [2] .
Among the different adsorbent materials used for heavy metals removal, nano-sized materials are being widely used due to their properties [8] . Carbon nanotubes, nano-metal oxides, nano-zeolite composites, polymers, and polymer-metal oxides are some of the adsorbents used for this purpose [5] . Nano-metal oxides are a group of nanomaterials whose application for adsorption purposes is increasing due to their unique properties [2] . Among the nano-metal oxides, TiO2 has unique physical and chemical properties such as non-toxicity, large surface area and photocatalysis [9] .
The conducted studies have showed that TiO2 nanoparticles and composites containing TiO2 have a good capability to adsorb heavy metals from aqueous solutions. For example, adsorption of lead ion by bauxite containing 3.12% TiO2 was examined by Wang et al. (2008) [10] . In a study by Ozlem Kocabas-Atakland Yurum (2013), TiO2 nanoparticles were synthesized and used as an adsorbent for the removal of lead ions from water [11] . Moreover, Samadi et al. (2014) synthesized the Cu-TiO2/chitosan nanocomposite and used it for the removal of lead ion [12] . Sreekantan et al. (2014), also, synthesized copper-incorporated titania nanotubes (TNTs) and used them to eliminate lead ions from water [13] . Li et al. (2015) investigated adsorption of lead ion by commercial TiO2 nanoparticles and TiO2/cellulose fibers composite and finally compared the adsorption efficiencies [14] . All of these experimental works confirmed the capability of TiO2 and its composite to adsorb lead ions.
In this study, the sol-gel method was applied to synthesize TiO2 nanoparticles. In this method, the nanoparticles were uniform both in size and shape and the synthesis procedure was simple to use. The aim of this study was to use synthesized nano TiO2 for the adsorption of lead from aqueous solution under batch conditions. The structure of the synthesized nanoparticles was characterized using TEM, XRD, BET and FT-IR. Moreover, the effects of pH, contact time and adsorbent weight on adsorption process were investigated along with studying the lead ions desorption. All the experiments were carried out in the laboratory of the Faculty of Environment and Energy, Science and Research Branch of Tehran Islamic Azad University.
2. Experimental
2.1. Materials and Methods
All the selected reagents were of analytical grade and purchased from Merck. The stock solutions for preparation of lead solution were prepared by dissolving Pb(CH3OO)2∙3H2O in deionized water.TiCl4 and NH4 were used to synthesize TiO2 nanoparticles by the sol-gel method. For adjusting pH, 1 M HNO3, NaOH and NH3 solutions as well as a Metrohm pH meter model 744 were used. Sartorius Electrical Balans Model BP 221S, Laboren oven, mixer HT Infors AG model CH-4103-BOT Tmingen, Centrifuge model MSE ministral1000 were used to conduct the experiments, and analysis of heavy metals was carried out using Inductivity Coupled Plasma (ICP) model Optima 2000 DV.
2.2. Synthesis and Preparation of TiO2
TiCl4 (30 mL) was added to deionized water (1L) under vigorous stirring (1000 rpm). pH of the solution was adjusted by adding NH3 (drop wise) to reach 7.8, and the mixing was continued until gel was formed. The gel was left for 7 days until colloidal sediment of TiOH2 was formed. Then, TiOH2 sediment was separated by filtration. The reaction was performed as presented by Equation (1).
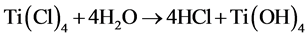 (1)
(1)
The separated sediment was placed in oven for 24 hours at 70˚C. Then, calcination was performed at 400˚C for 4 hours.
2.3. Batch Adsorption Studies
Adsorption experiments were performed by adding 0.15 g of adsorbent to 50 mL of solution with the initial Pb2+ ions concentration of 25 mg/L in a flask. The effect of pH on sorption ions was studied in the range of 3 - 6.5, at the temperature of 25˚C and contact time of 4 h. The effect of contact time was investigated by varying the time from 10 to 240 min, at a temperature of 25˚C, with the obtained pH values. The effect of adsorbent weight on sorption metal ions was studied in the range of 1 to 4 g/L (0.05, 0.10, 0.15 and 0.20 g of adsorbent in 50 mL of metal ions solution) at the contact time of 4 h, temperature of 25˚C and the obtained pH values.
The concentration of lead ions before and after equilibrium sorption was determined using ICP. The uptake percentages of the lead ions were calculated according to Equation (2) [5] :
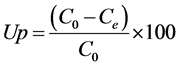 (2)
(2)
where, C0 and Ce are initial and equilibrium concentrations of ions (mg/L), respectively.
3. Results and Discussion
3.1. Adsorbent Characterization by X-Ray Diffraction
Characterization of crystalline size of the adsorbent was determined by XRD (model STV_MP STOE Company, Germany).For this purpose, Cu radiation (λCu = 1.5405 A) was used and the sample was scanned in a 2θ range of 8 - 108.5˚ at a scanning rate of 0.015˚/S. The crystalline size was determined from the characteristic peak at 2θ = 25.326˚ (corresponding to the 440 plane) using Scherrer formula crystalline size, nm = Kλ/W cosθ, where K is shape factor = 0.9, λ is wavelength of the X-ray used (1.5405), and W is (Wb-Ws, width of peak at half-height at 2θ = 25.326) the difference of broadened profile width of the experimental sample and the standard width of reference TiO2 sample(reference code 01-073-1764, pdf2-2003) [15] . Comparison of the graphs shows that all peaks are in good agreement with the standard spectrum [16] . The mean crystalline size was obtained to be 21.1 nm. Figure 1 shows the XRD graph of synthesized nano TiO2 sample (Figure 1(a)) in comparison of reference sample of TiO2 (Figure 1(b)).
3.2. FT-IR Analysis
Figure 2 shows the FT-IR (model Vector 22 Brucker Company, USA) analysis of nano TiO2. All the adsorption bands were at 3446, 1635, 1031 and 455 cm−1. The strong IR band at 3446 cm−1 was assigned to the stretching bands of adsorbed water [15] . The bands around the peak of 1635 cm−1 were assigned to Hydroxyl bond. The strong bands around 1031 cm−1 were related to the titanium, oxygen and nitrogen bonds (Ti-O-N). Broad band around the peak of 455 cm−1 was related to the vibration of titanium and oxygen bonds and anatase form of TiO2 [17] .
3.3. TEM Images
The TEM images (taken by PHILIPS, EM 208) of synthesized nano TiO2 are illustrated in Figure 3. It can be observed that the nanoparticles are very aggregate, with a mean diameter of about 21.1 nm. In the present context, the discrepancy between the X-ray crystallite size and that measured in the TEM can be interpreted as being attributed to lattice distortions in the prepared powder. As a result of a sub-structure much smaller than the nano-crystalline, the grain size measured in the TEM is in good agreement with the findings of Chandramouli et al. [17] .
3.4. Surface Areas and Pore Volumes
Specific surface area was determined through nitrogen adsorption isotherms method. Using the BET (model


Figure 1. XRD graph of nano TiO2, (a) synthesized sample of TiO2 in comparison with (b) reference TiO2 sample.
Figure 2. FT-IR graph of synthesized nano TiO2.
Figure 3. TEM images of TiO2 nanoparticles, magnification of 100 nm.
Quantachrome NOVA 2200e) method, the surface area of the sample was calculated to be 55.35 m2/g. Also, the pore size distribution was attained by Barrett-Joyner-Halenda (BJH method revealed the mesoporosity) [18] . The pore size obtained by this method was 19.21 nm.
3.5. Adsorption Properties of TiO2
3.5.1. Effect of pH
Optimization of the initial pH value of the adsorption is an important parameter that allows for obtaining a high adsorption capacity. The effect of pH on lead ions adsorption is shown in Figure 4. The pH range in this study was selected to be 3 to 6.5 based on previous studies [15] [19] . A very low adsorption rate was observed at pH <3 [20] . At low pH values, the sorbent surface would be closely associated with H3O+ which binds the access of metal ions to the adsorbent surface. At pH6, the amount of lead ions sorption onto the adsorbent (nano-structure of TiO2) increased with the increase of pH, since the competition between hydrogen ion and metal ions decreased. As can be seen in Figure 4, the maximum adsorption capacity of lead ions was obtained at pH6. However, the sorption capacity decreased with further increase of pH values, since the lead adsorption was optimum at pH 6. Therefore, this pH was chosen for the subsequent experiments.
3.5.2. Effect of Contact Time
The effect of contact time on ions adsorption by nano-structured TiO2 was studied. Lead ions adsorption from aqueous solution, which had been adjusted to the nano-structured TiO2 (0.15 g in 50 mL) at optimum pH, was studied at different shaking times in the range of 10 - 240 min (Figure 5). The lead ions removal efficiency reached the maximum value after 4 h. This could be attributed to the fact that initially all the adsorbent sites were vacant and the solute concentration gradient was high. Therefore, based on the results, a contact time of 4 h was selected in subsequent studies. The results indicated that within 4h of contact the lead ions were mostly removed from aqueous solution [20] .
3.5.3. Effect of Adsorbent Amount
The effect of adsorbent amount on adsorption rate was examined by a series of experiments performed using different amounts of nano TiO2 (0.05, 0.10, 0.15 and 0.20 g) (Figure 6). The maximum uptake attained after using 0.2 g of adsorbent was 90% for lead.
Figure 4. Effect of pH value on adsorption of Pb2+ onto TiO2 nanoparticles. Conditions: adsorbent weight 3 g/L, adsorption time 4 h.
Figure 5. Effect of time on adsorption of Pb2+ onto TiO2, Conditions: adsorbent weight of 3 g/L at pH 6.
Figure 6. Effect of mass adsorbent value on adsorption of Pb2+ onto TiO2, Conditions: adsorption time of 4 h at pH 6.
3.5.4. High Adsorption Efficiency
The results showed the high adsorption efficiency of nano TiO2 for lead ions. This could be ascribed to the uniformity of size and shapes of nanoparticles.
3.5.5. Desorption Studies
The aqueous solutions (50 mL) containing the lead ions (25 mg/L), kept under the optimum experimental condition, were stirred with 0.15 g of the adsorbent for 240 min at 25˚C. Then, desorption studies were carried out using 50 mL of 1 M HNO3 during 1-hour mixing time. It was found that the adsorbed ions could be quantitatively stripped by contacting nitric acid (Table 1). Finally, lead ions desorption from the surface of adsorbent (nano TiO2) was done, and the results showed the high efficiency of desorption rate.
3.6. Kinetic Study
Figure 5 indicates the variation in amount of lead ions adsorbed at different time intervals for a fixed initial ion concentration of 25 mg/L. The data revealed that the amount of adsorbed ions studied increased with the increase of contact time. To describe changes of metal ions sorption with time, two simple kinetic models were tested. The experimental kinetic data for lead ions adsorption from aqueous solutions on the TiO2 nano particles were modeled using pseudo-first and second-order kinetic models. In order to investigate the accuracy of the models in predicting the lead ions adsorption behavior, the correlation coefficient (R) of each model, which is an important factor, was used.Success of the models in predicting the kinetics of adsorbate sorption was described by a relatively high R value [5] . The rate constant of lead ion removal from the solution by nano TiO2 was also determined using pseudo-first and second-order rate models. The Lagergren’s pseudo first-order expression is given by Equation (3) [5] [21] :
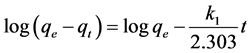 (3)
(3)
where, qe and qt are amounts of the lead adsorbed onto the sorbent (mg/g) at equilibrium and at time t respectively, and k1 is the rate constant of the first-order adsorption (min−1). The straight line plots of 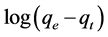 against t were used to determine the rate constant, k1 and correlation coefficient; R2 values of the lead were calculated from this plot. The calculated correlation coefficient for pseudo-first-order and the values of constants are shown in Table 2. The pseudo second-order rate model is expressed by Equation (4) [5] [22] :
against t were used to determine the rate constant, k1 and correlation coefficient; R2 values of the lead were calculated from this plot. The calculated correlation coefficient for pseudo-first-order and the values of constants are shown in Table 2. The pseudo second-order rate model is expressed by Equation (4) [5] [22] :
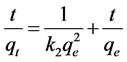 (4)
(4)
where, K2 is the rate constant of adsorption (gr∙mg−1∙min−1), qe is the amount adsorbed at equilibrium, and qt is the amount adsorbed at any time. The equilibrium adsorption amount (qe) and the pseudo-second-order rate parameters (K2) can be calculated from the slope and intercept of t/qt plotted versus t. The values of constants and calculated correlation coefficients for pseudo-second-order are presented in Table 2.
Table 1. Desorption studies of the studied ions from surface TiO2 nanoparticles.
Table 2. Calculated parameters of the pseudo-first-order and pseudo second- order kinetic models for Pb2+ Ions adsorbed onto nano TiO2.
In adsorption of lead ions, correlation coefficient of the pseudo-second-order equation was larger than that of the pseudo-first-order equation, indicating that lead ion adsorption onto the TiO2 nanoparticles followed the pseudo-second-order kinetic model. It was observed that the predicted qe value for the pseudo-second-order model well agreed with the experimental value. Therefore, the pseudo-second-order kinetic model was found to be more suitable for predicting the kinetic sorption process of lead ion onto the TiO2 nanoparticles. The kinetic sorption fitted plots are illustrated in Figure 7.
3.7. Adsorption Isotherms
Adsorption equilibrium is usually described by an isotherm equation whose parameters express the surface properties and affinity of the sorbent at a fixed temperature and pH. An adsorption isotherm describes the relationship between the amount of adsorbate on the adsorbent and the concentration of dissolved adsorbate in the liquid at equilibrium. Having this in mind, the adsorption isotherms for the removal of lead ions from aqueous solution by nano TiO2 were determined. Figure 8 shows the experimental and isotherm data fitted by Langmuir and Freundlich isotherm models at 298 K.
3.7.1. Langmuir Isotherm Model
Langmuir sorption isotherm models the monolayer coverage of the sorption surfaces and assumes that sorption occurs on a structurally homogeneous adsorbent and all the sorption sites are energetically identical [5] [23] .


Figure 7. (a) Pseudo-first and (b) second-order kinetic plots for Pb2+ sorption ions adsorbed onto nano TiO2.


Figure 8. (a) Langmuir and (b) Freundlich adsorption isotherm plots for the sorption of Pb2+ ions.
The linearized form of the Langmuir equation is given by Equation (5) [5] [24] :

where, qmax is the maximum sorption capacity (mg/g), and b is a constant related to binding energy of the sorption system (l/mg). The graphic presentations of (Ce/qe) versus Ce give those straight lines that the numerical values of constants qmax and b have evaluated form the slope and intercept of plots (Table 3).
3.7.2. Freundlich Isotherm
Freundlich equation is derived to model the multilayer sorption and for the sorption on heterogeneous surfaces. The logarithmic form of Freundlich equation can be described by Equation (6) [5] [24] :

where, Kf is a constant indicative of the relative sorption capacity of nano TiO2 (mg/g), and 1/n is a constant indicative of the intensity of sorption process. The numerical values of the constants 1/n and Kf are computed from the slope and the intercepts of log qe versus logCe curve. The correlation coefficient and other parameters
Table 3. Parameters of Langmuir and Freundlich isotherms for the studied ions sorption onto nano-TiO2.
obtained for the adsorbent are given in Table 3 [5] [24] .
Comparison of R2 values presented in Table 3 results in selecting the appropriate adsorption isotherm model describing the adsorption process of the studied ion by nano TiO2. R2 value close to 1 is indicative of the suitability of this model for describing the experimental data [5] [25] . This comparison reveals that the adsorption process isotherms of lead ion (R2 = 0.97) can be more suitably described by the Langmuir model (Figure 8). The experimental results showed that lead ions were adsorbed onto TiO2 in greater amounts as compared to conventional and commercial TiO2 nanoparticles [11] [26] .
The results of Qmax and Kf values in Langmuir and Freundlich isotherms show the capability of sorption on an adsorbent. Comparing the quantities presented in Table 3, adsorption rate of lead ions onto TiO2 was found to be 7.41 mg/g.
The Langmuir isotherm fitted well to the experimental data, probably because of the homogeneous distribution of active sites on nano-structure of TiO2 adsorbent. Based on the Langmuir model assumptions, adsorption energies are uniform and independent of surface coverage, and complete coverage of surface by amonolayer of adsorbate indicates the maximum adsorption.
4. Conclusion
The results indicated that nano-structured TiO2 synthesized by sol-gel method could be an effective adsorbent for the adsorption of Pb2+ ions from aqueous solutions under optimized conditions of pH 6, adsorbent weight of 3 g/L, contact time of 4 h and at room temperature (25˚C). All kinetic results suggested that sorption of Pb2+ by nano-structured TiO2 followed the second-order kinetics model relying on an assumption that sorption might be a rate-limiting step involving valence forces through sharing or exchange of electrons between adsorbent and sorbent. The adsorption isotherms for Pb2+ fitted well to the Langmuir adsorption isotherm equations. The maximum capacity of adsorbent was 7.41 mg∙gr−1 for Pb2+. Comparison of the results from this study and those from similar studies shows that lead ions removal by synthesized nano TiO2 is favorable and the synthesized adsorbent would be reusable with high efficiency after desorption process. The nano-structure of TiO2 exhibited a good capability to be used in water and wastewater treatment for the removal of lead ions.
Cite this paper
Afshin Shokati Poursani,Abdolreza Nilchi,Amirhessam Hassani,Seyed Mahmood Shariat,Jafar Nouri, (2016) The Synthesis of Nano TiO2 and Its Use for Removal of Lead Ions from Aqueous Solution. Journal of Water Resource and Protection,08,438-448. doi: 10.4236/jwarp.2016.84037
References
- 1. Sounthararajah, D. P., Loganathan, P., Kandasamy, J. and Vigneswaran, S. (2015) Adsorptive Removal of Heavy Metals from Water Using Sodium Titanate Nanofibres Loaded onto GAC in Fixed-Bed Columns. Journal of Hazardous Materials, 287, 306-316.
http://dx.doi.org/10.1016/j.jhazmat.2015.01.067 - 2. Zhang, D., Zhang, C.L. and Zhou, P. (2011) Preparation of Porous Nano-Calcium Titanate Microspheres and Its Adsorption Behavior for Heavy Metal Ion in Water. Journal of Hazardous Materials, 186, 971-977.
http://dx.doi.org/10.1016/j.jhazmat.2010.11.096 - 3. Razzaz, A., Ghorban, S., Hosayni, L., Irani, M. and Aliabadi, M. (2016) Chitosan Nanofibers Functionalized by TiO2 Nanoparticles for the Removal of Heavy Metal Ions. Journal of the Taiwan Institute of Chemical Engineers, 58, 333-343.
http://dx.doi.org/10.1016/j.jtice.2015.06.003 - 4. Rahmani, A., Mousavi, H.Z. and Fazli, M. (2010) Effect of Nanostructure Alumina on Adsorption of Heavy Metals. Desalination, 253, 94-100.
http://dx.doi.org/10.1016/j.desal.2009.11.027 - 5. Poursani, A. S., Nilchi, A., Hassani, A.H., Shariat, M. and Nouri, J. (2015) A Novel Method for Synthesis of Nano-γ-Al2O3: Study of Adsorption Behavior of Chromium, Nickel, Cadmium and Lead Ions. International Journal of Environmental Science and Technology, 12, 2003-2014.
http://dx.doi.org/10.1007/s13762-014-0740-7 - 6. Mahapatra, A., Mishra, B.G. and Hota, G. (2013) Electrospun Fe2O3-Al2O3 Nanocomposite Fibers as Efficient Adsorbent for Removal of Heavy Metal Ions from Aqueous Solution. Journal of hazardous Materials, 258-259, 116-123.
http://dx.doi.org/10.1016/j.jhazmat.2013.04.045 - 7. Nilchi, A., Dehaghan, T.S. and Garmarodi, S.R. (2013) Kinetics, Isotherm and Thermodynamics for Uranium and Thorium Ions Adsorption from Aqueous Solutions by Crystalline tin Oxide Nanoparticles. Desalination, 321, 67-71.
http://dx.doi.org/10.1016/j.desal.2012.06.022 - 8. Bailey, S.E., Olin, T. J., Bricka, R.M. and Adrian, D.D. (1999) A Review of Potentially Low-Cost Sorbents for Heavy Metals. Water Research, 33, 2469-2479.
http://dx.doi.org/10.1016/S0043-1354(98)00475-8 - 9. Pourreza, N., Rastegarzadeh, S. and Larki, A. (2014) Simultaneous Preconcentration of Cd(II), Cu(II) and Pb(II) on Nano-TiO2 Modified with 2-Mercaptobenzothiazole Prior to Flame Atomic Absorption Spectrometric Determination. Journal of Industrial and Engineering Chemistry, 20, 2680-2686.
http://dx.doi.org/10.1016/j.jiec.2013.10.055 - 10. Wang, Y.H., Lan, Y. and Huang, C. B. (2008) Adsorption Behavior of Pb and Cd Ions on Bauxite Flotation Tailings. Journal of Central South University of Technology, 2, 183-187.
http://dx.doi.org/10.1007/s11771-008-0035-6 - 11. Kocabas-Atakli, Z.Ö. and Yürüm, Y. (2013) Synthesis and Characterization of Anatase Nanoadsorbent and Application in Removal of Lead, Copper and Arsenic from Water. Chemical Engineering Journal, 225, 625-635.
http://dx.doi.org/10.1016/j.cej.2013.03.106 - 12. Samadi, S., Khalilian, F. and Tabatabaee, A. (2014) Synthesis, Characterization and Application of Cu-TiO2/Chitosan Nanocomposite Thin Film for the Removal of Some Heavy Metals from Aquatic Media. Journal of Nanostructure in Chemistry, 4, 1-8.
http://dx.doi.org/10.1007/s40097-014-0084-3 - 13. Sreekantan, S., Zaki, S.M., Lai, C.W. and Tzu, T.W. (2014) Copper-Incorporated Titania Nanotubes for Effective Lead Ion Removal. Materials Science in Semiconductor Processing, 26, 620-631.
http://dx.doi.org/10.1016/j.mssp.2014.05.034 - 14. Li, Y., Cao, L., Li, L. and Yang, C. (2015) In Situ Growing Directional Spindle TiO2 Nanocrystals on Cellulose Fibers for Enhanced Pb2+ Adsorption from Water. Journal of Hazardous Materials, 289, 140-148.
http://dx.doi.org/10.1016/j.jhazmat.2015.02.051 - 15. Li, X., Liu, W. and Ni, J. (2015) Short-Cut Synthesis of Tri-Titanate Nanotubes Using Nano-Anatase: Mechanism and Application as an Excellent Adsorbent. Microporous and Mesoporous Materials, 213, 40-47.
http://dx.doi.org/10.1016/j.micromeso.2015.04.018 - 16. Chandramouli, V., Anthonysamy, S., Rao, P.V., Divakar, R. and Sundararaman, D. (1996) PVA Aided Microwave Synthesis: A Novel Route for the Production of Nanocrystalline Thoria Powder. Journal of Nuclear Materials, 231, 213-220.
- 17. Chandramouli, V., Anthonysamy, S., Rao, P.V., Divakar, R. and Sundararaman, D. (1996) PVA Aided Microwave Synthesis: A Novel Route for the Production of Nanocrystalline Thoria Powder. Journal of Nuclear Materials, 231, 213-220.
http://dx.doi.org/10.1016/0022-3115(96)00368-6 - 18. Villarroel-Rocha, J., Barrera, D. and Sapag, K. (2014) Introducing a Self-Consistent Test and the Corresponding Modification in the Barrett, Joyner and Halenda Method for Pore-Size Determination. Microporous and Mesoporous Materials, 200, 68-78.
http://dx.doi.org/10.1016/j.micromeso.2014.08.017 - 19. Asencios, Y.J. and Sun-Kou, M.R. (2012) Synthesis of High-Surface-Area γ-Al2O3 from Aluminum Scrap and Its Use for the Adsorption of Metals: Pb (II), Cd (II) and Zn (II). Applied Surface Science, 258, 10002-10011.
http://dx.doi.org/10.1016/j.apsusc.2012.06.063 - 20. Sen, T.K. and Sarzali, M.V. (2008) Removal of Cadmium Metal Ion (Cd2+) from Its Aqueous Solution by Aluminium Oxide (Al2O3): A Kinetic and Equilibrium Study. Chemical Engineering Journal, 142, 256-262.
http://dx.doi.org/10.1016/j.cej.2007.12.001 - 21. Li, Y., Cao, L., Li, L. and Yang, C. (2015) In Situ Growing Directional Spindle TiO2 Nanocrystals on Cellulose Fibers for Enhanced Pb2+ Adsorption from Water. Journal of Hazardous Materials, 289, 140-148.
http://dx.doi.org/10.1016/j.jhazmat.2015.02.051 - 22. Aksu, Z. (2002) Determination of the Equilibrium, Kinetic and Thermodynamic Parameters of the Batch Biosorption of Nickel (II) Ions onto Chlorella vulgaris. Process Biochemistry, 38, 89-99.
http://dx.doi.org/10.1016/S0032-9592(02)00051-1 - 23. Langmuir, I. (1916) Theconstitution and Fundamental Properties of Solids and Liquids. PART I. SOLIDS. Journal of the American Chemical Society, 38, 2221-2295. http://dx.doi.org/10.1021/ja02268a002
- 24. El-Kamash, A.M. (2008) Evaluation of Zeolite A for the Sorptive Removal of Cs+ and Sr2+ Ions from Aqueous Solutions Using Batch and Fixed Bed Column Operations. Journal of Hazardous Materials, 151, 432-445.
http://dx.doi.org/10.1016/j.jhazmat.2007.06.009 - 25. Shiri-Yekta, Z., Yaftian, M.R. and Nilchi, A. (2013) Silica Nanoparticles Modified with a Schiff Base Ligand: An Efficient Adsorbent for Th(IV), U (VI) and Eu(III) Ions. Korean Journal of Chemical Engineering, 30, 1644-1651.
http://dx.doi.org/10.1007/s11814-013-0077-9 - 26. Sis, H. and Uysal, T. (2014) Removal of Heavy Metal Ions from Aqueous Medium Using Kuluncak (Malatya) Vermiculites and Effect of Precipitation on Removal. Applied Clay Science, 95, 1-8.
http://dx.doi.org/10.1016/j.clay.2014.03.018
NOTES
*Corresponding author.








