Journal of Water Resource and Protection
Vol. 3 No. 6 (2011) , Article ID: 5733 , 7 pages DOI:10.4236/jwarp.2011.36046
Cell Size versus Taxonomic Composition as Determinants of As (III & V) Sensitivity in the Estuarine Diatom Communities
Department of Marine Science, Calcutta University, Kolkata, India
E-mail: tkjana@hotmail.com
Received March 15, 2011; revised April 17, 2011; accepted May 20, 2011
Keywords: Diatoms, Cell Size, Arsenic (III&V), Mangrove
ABSTRACT
Despite scarce studies have analyzed the relative growth inhibition of As (III) and As (V) to diatom, clear pattern of interspecies difference have been shown, identifying cell size as a key property determining the sensitivity of diatom to As. Evidence from cultures suggests that cell size is a key factor in determining the extent of arsenic (III) & (V) stress of diatom, with relatively lesser effects of As (V) than As (III) on small cells. Cent percent growth inhibition was observed for large size group (Coscinodiscus radiatus, Surirella, Amphipleura, Thalassiothrix, Cyclotella and Thalassiosira decipiens) relative to smaller size group (Skeletonema cf. costatum, Navicula rhombica, Amphora hyalina, Nitzschia longissima except Thalassisira). Interspecies differences in As tolerance by diatom in the mangrove ecosystem indicates cell size could be only one factor contributing to these differences. The results show that 81.7% of total arsenic was uptaken from culture media originally amended with arsenic. Looking to the extreme tolerance and arsenic removal efficiency, application of the species with smaller cell size relative to the other tested diatom for bioremediation purpose can be envisaged.
1. Introduction
Arsenic, widely distributed contaminants of different water bodies is one of the many environmental known carcinogenic agents and mutagens. The chemical form and oxidation state of arsenic govern its biological availability and physiological and toxicological effects of arsenic. Toxicity of arsenic is reduced due to its conversion from inorganic to organic form, and is increased by its reduction from arsenate to arsenite for most biological system [1]. Trivalent arsenic has a higher affinity for thiol groups relative to pentavalent state, as it readily forms kinetically stable bonds to sulfur and induces enzyme inactivation [2]. However, organisms may develop arsenic resistance depending on both endogenous and exogenous factors i.e. changing membrane transport and secretion. Transport systems of phytoplankton incorporating the elements necessary for cell growth is not specific and their size and cell surface to volume ratio are important factors for sensitivity towards nutrients and toxic metals in the ambient medium [3-5]. Toxic ions chemically similar to nutrients when present in excessive concentrations may interfere with normal biological processes, producing inhibitory effects. A variety of evidence in the literature indicates that the sensitivity of phytoplanktonic cells to inhibition by arsenic may be function of species composition and cell size [6,7]. Abundance of arsenic in organic form is more pronounced during spring phytoplankton bloom [8,9] and could be a mechanism to avoid toxicity by eliminating intercellular arsenate which is taken up inadvertently along with phosphate resulting inhibition of cellular oxidative phosphorylation and growth [10,11]. In the eutrophic ecosystem, like nutrient rich mangroves with occurrence of rapid growth and species succession [12], arsenic resistance could be more pronounced in the smaller species, microflagellates and/ or pinnate diatoms.
Occurrence of elevated level of arsenic (several hundred mg·L–1) in ground and pore water sourced from alluvial aquifers in the Bengal delta plains in West Bengal and Bangladesh relative to that of the Sundarban mangrove water (4.67 - 38 nm, [9]) have received significant attention during the last decade [13-16]. Any shift in size structure of phytoplankton communities that might occur in response to arsenic contamination has wide-ranging implications for pelagic food web processes and for interactions with the benthos via sedimentation [17]. Edmonds and his co-workers [18] suggested that marine unicellular algae could carry out biotransformation and detoxify inorganic arsenic at arsenate concentration several fold ambient levels and could be promising to harness for remediation of arsenic contamination. Evidence from cultures suggests that cell size is a key factor in determining the extent of arsenic (III) & (V) stress of diatom, with relatively lesser effects of As (V) than As (III) on small cells [6]. This hypothesis was investigated in mixed culture of estuarine diatom by measuring size-dependent growth under arsenic (III) & (V) concentration gradient.
The aim of this paper is to evaluate effect of trivalent and pentavalent arsenic species on the growth of euryhaline phytoplankton communities in vitro and to evaluate the hypothesis that cell size dominates over taxonomic differences in controlling the degree of arsenic toxicity inhibiting primary production.
2. Material and Methods
Natural phytoplankton Navicula rhombica, Amphora hyalina, Coscinodiscus radiatus, Nitzschia longissima, Skeletonema cf costatum, Cyclotella, Thalassiosira decipiens, Thalassiothrix , Amphipleura, Surirella sp., were isolated from water of the Sundarban mangroves (21˚32' - 22˚40'N and 88˚05' - 89˚E), North East Coast of India. Two experiments were performed, one each in monsoon (September) and spring (January). The isolates were grown and maintained in f/2 medium [19] at 18˚C and 3200 lux of illumination. The algal species in f/2 medium were spiked with 0, 200, 400, 800, 1200, 1600 mg·L–1 As (V) (Arsenate) and As (III) (Arsenite) in two sets of culture vessels. Every day, changes in phytoplankton number and their size were measured in triplicate for 6 days and values reported here are mean of the experimental data. Nutrient concentration in the medium were not limiting during the course of experiment.
Phytoplankton were filtered from each flask through Millipore (0.45 mm) filter paper after 6 days of culture. Phytoplankton residue were refluxed with acid mixture (H2SO4 and HNO3 1:10V/V) [20] and arsenic in each sample was determined after hydride generation using Atomic Absorption Spectrometry [9]. The change of chemical form of As from inorganic to organic in the medium was studied by the analysis of filtrate for As before and after alkaline persulphate oxidation [21] using spectrophotometric method modified by Cummings [22]. 1 ml of sample from each flask was taken in Sedgwick Rafter counting chamber for enumeration and cell volume was determined by using the simplest geometric configuration that best fit the shape of the cell [12].
Growth rate of phytoplankton is expressed in terms of doubling time (d) in days and specific growth constant (µ·day–1) is expressed by:
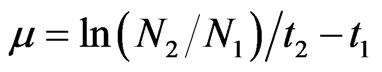 (1)
(1)
where N2 and N1 are the phytoplankton number produced during time t1 and t2. The effect of nutrient concentration (N) on the growth constant, µ is described by the Michaelis-Menten equation:
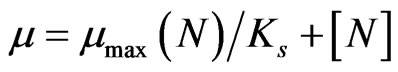 (2)
(2)
where µmax is the maximum growth of the phytoplankton, and Ks is the constant for nutrient uptake. The full inhibition model [23], after linear transformation, was obtained by multiplying both terms in the right hand side of the Equation (2) by the inhibition term (1 + [As]/Ki, where [As] is the concentration of inhibitor and Ki is the inhibition constant ) to give the following equation after setting [N] to infinity:
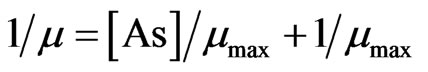 (3)
(3)
3. Results
A typical example of inhibition of growth of phytoplankton collected in the monsoon and effectuated by the increased order of arsenic is shown in Figure 1. Background Arsenic concentrations averaged 1.5 µg·L–1, with a range of 0.94 to 2.75 µg·L–1. Phytoplankton growth rates (µ) were 0.8433 - 1.0033 d–1 and cell density ranged between 1.1 and 25 × 105 cells·L–1 in the control. In two experiments both As (V) & (III) exhibited growth inhibition with increasing order of arsenic dosing and percentage of decreases of growth rates (µ) and cell densities relative to control were 70.0% - 57.23% and 116% - 52%, respectively for As (V), and 49.7% - 38.7% and 48.8% - 26%, respectively for As (III) in the concentration range between 200 and 1600 µg·L–1. Representative graphs for the changes in cell density (Number·L–1) over time in control and arsenic treated cultures of the dominant diatom species with different cell size for monsoon sample are given in Figure 2 for As (V) and in Figure 3 for As (III). A similar pattern was observed for spring diatom samples.
Skeletonema cf. costatum dominated for the entire experiment in control and arsenic treated culture, comprising an average of 40 and 66.8% of total cell density, respectively. In all arsenic-treated (V & III) culture media, regardless of dose Thalassiothrix, Amphipleura and Surirella declined from 4.8%, 5.6% and 2.5% of total cell density, respectively in control to zero within 1st day of the start. But Coscinodiscus radiatus, Cyclotella and Thalassiosira decipiens comprising an average of 7.2%, 4.0% and 12.0% of total cell density, respectively declined to insignificant densities within 5 days of the start of As (V) and As (III) treatment (Figures 2-4). ConverselyFigure caption:
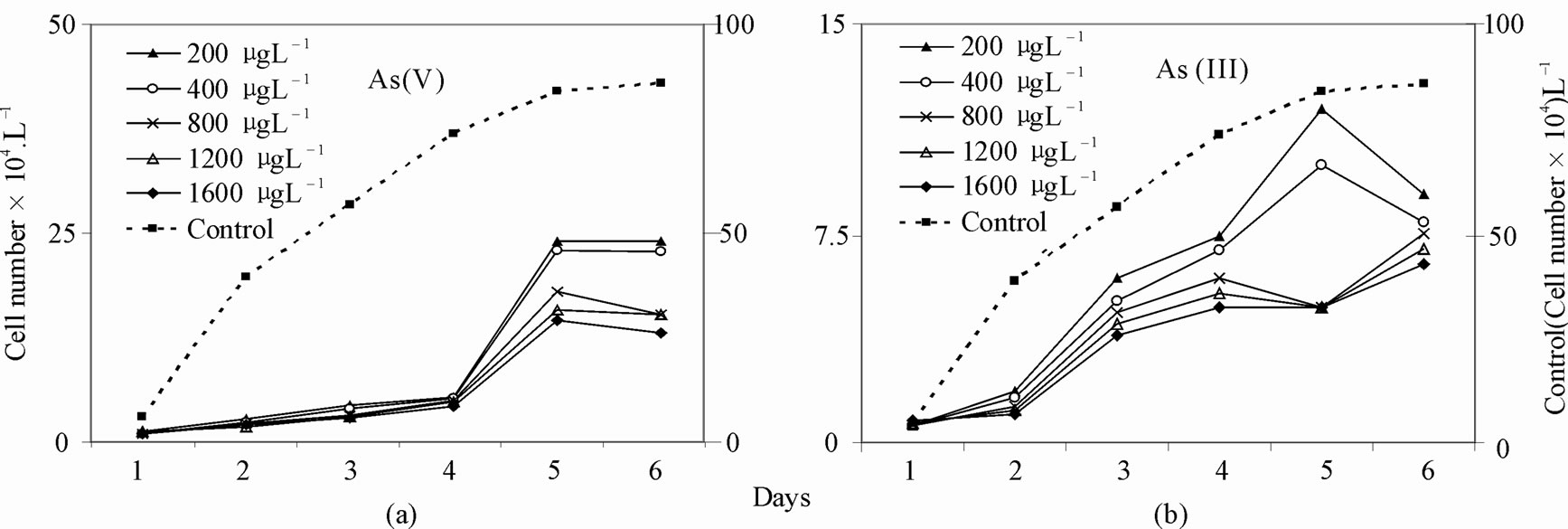
Figure 1. Change of total density (Cell Number·L–1) of test diatoms in control and arsenic-treated media a. As (V), b. As (III).
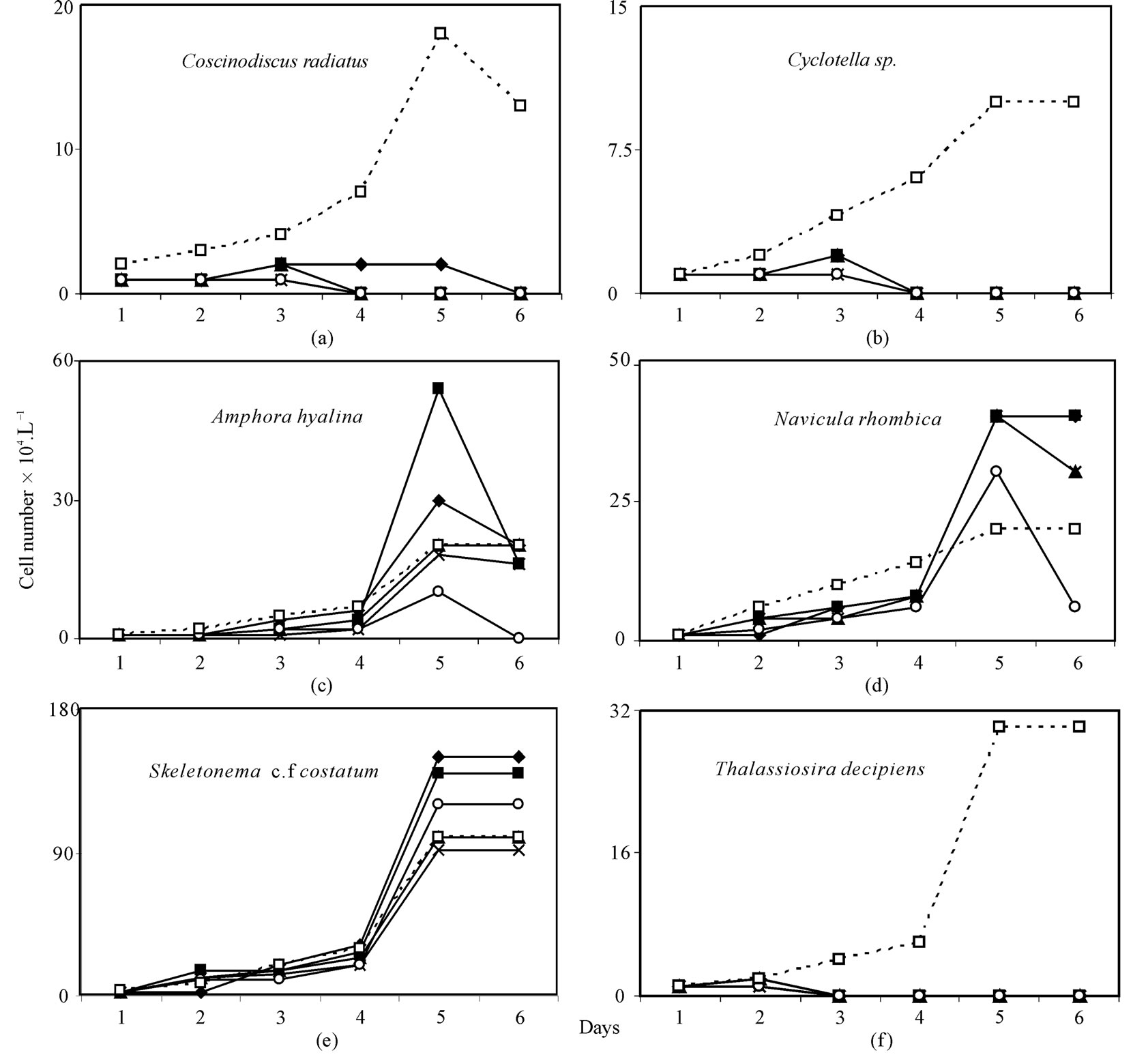
Figure 2. Response of diatoms (a) Coscinodiscus radiatus, (b) Cyclotella sp., (c) Amphora hyalina, (d) Navicula rhombica, (e) Skeletonema cf. costatum and (f) Thalassiosira decipiens to different concentration of As (V) ( 200 μg·L–1,
200 μg·L–1,  400 μg·L–1,
400 μg·L–1,  800 μg·L–1,
800 μg·L–1,  1200 μg·L–1,
1200 μg·L–1, 1600 μg·L–1,
1600 μg·L–1,  control).
control).
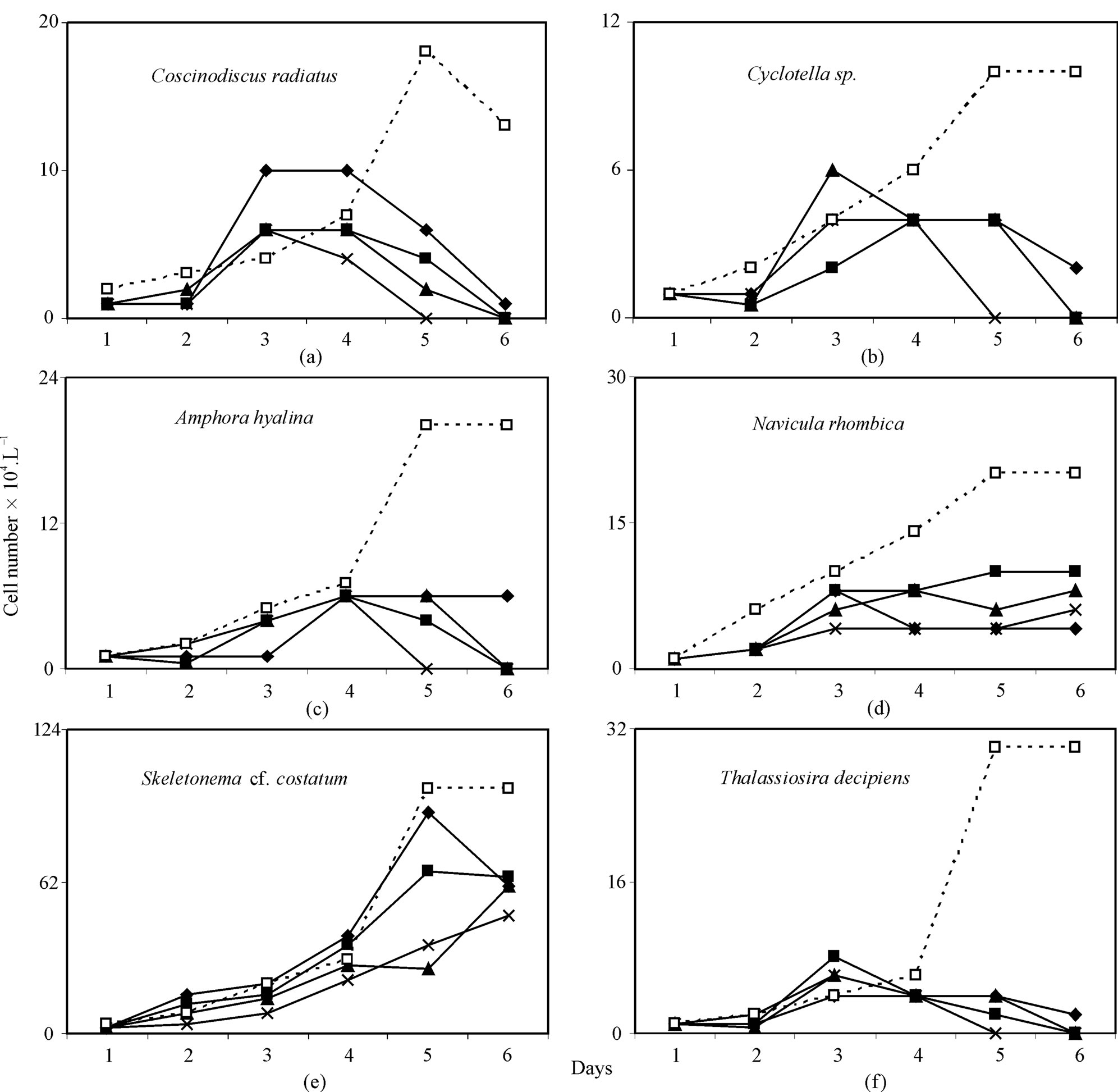
Figure 3. Response of diatoms (a) Coscinodiscus radiatus, (b) Cyclotella sp., (c) Amphora hyalina, (d) Navicula rhombica, (e) Skeletonema cf. costatum and (f) Thalassiosira decipiens to different concentration of As (III)  200 μg·L–1,
200 μg·L–1,  400 μg·L–1,
400 μg·L–1,  800 μg·L–1,
800 μg·L–1,  1600 μg·L–1,
1600 μg·L–1,  control).
control).
Amphora hyalina, Navivula rhombica, Skeletonema cf. costatum dominated arsenic-treated culture media to a much greater extent than control, reaching densities ranging 0% - 11.5%, 3.3% - 11.5%, 50% - 85.7% of total cells, respectively in As (III) treated assemblages and ranging 0% - 11.7%, 4.6% - 19.7%, 51.7% - 92.3% of total cells, respectively in As (V) treated assemblages vs. 8.0%, 8.0%, 40.0% of total density, respectively in control assemblages. Predominant species with prominent arsenic resistance were found to be Skeletonema cf. costatum, Navicula rhombica and Amphora hyalina for As (V) and Skeletonema cf. costatum for As(III) (Figure 4).
All parameters in the equation 3 (µmax and Ki) were estimated by linear regression between 1/ µ vs As(III & V). The Ki and µmax were found 0.7986 – 1.5162 × 104 and 0.6261 – 0.6595 d–1 (R2 = 54.21 – 97.99%, p < 0.001) for As (V), and 0.2376 – 0.0.464 × 104 and 0.5264 – 0.5387 d–1 (R2 = 96.72 - 98.84%, p < 0.001) for As (III). Extent of growth inhibition by As (III) was greater relative to As (V).
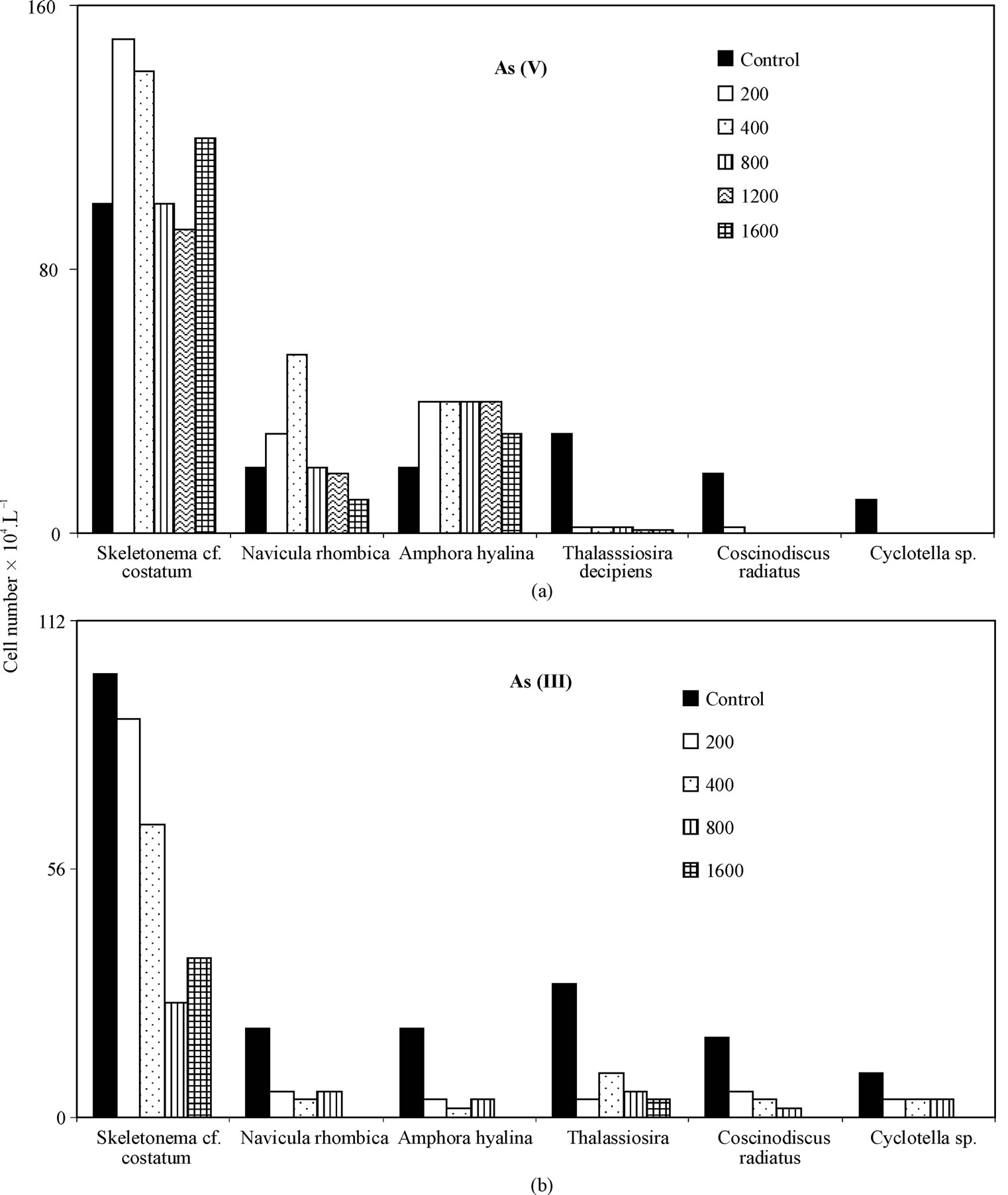
Figure 4. Densities of test diatoms (cell number.L-1) in control and As-treated culture media a. As (V) b. As (III) after 5th day of start.
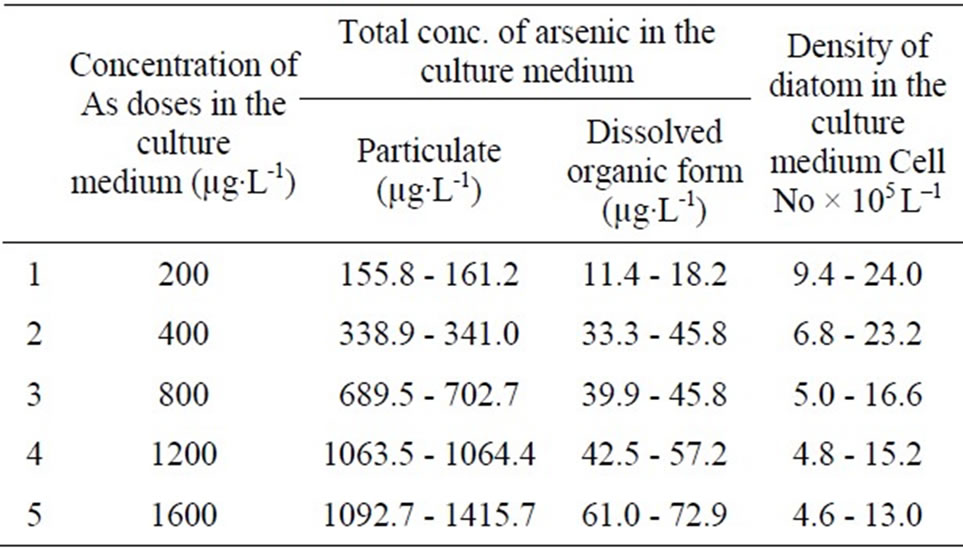
Table 1. Mean concentration of particulate, dissolved inorganic and organic arsenic in the test diatom culture medium treated with arsenic.
Due to the difference in size of Skeletonema cf. costatum, Nitzschia longissima, Coscinodiscus radiatus, Cyclotella between spring and monsoon sample (8.18, 10.46, 10883.6, 104.6 versus 38.89, 97.22, 47732.14, 449.15 µm3cell–1) total biovolume of diatom sample used in the experiment was greater in the monsoon than in the spring (105.5 × 106 versus 29.2 × 106 µm3·L–1). In both instances, the lowest As (V & III) dose was sufficient to cause a significant deviation in growth rates, though, the results of μmax and Ki were different between spring and monsoon experiment (0.6261 d–1, 0.7986 × 104 for As (V) and 0.5264 d–1, 0.2376 × 104 for As (III) in the spring versus 0.6595 d–1, 1.5162 × 104 for As (V) and 0.5387 d–1, 0.464 × 104 for As (III) in the monsoon), and relative growth of small size diatom were greater than larger species. As (V) and As (III) acted differently for inhibiting growth of phytoplankton species, for example, the effect was more pronounced in relatively large centric diatoms, Coscinodiscus radiatus (μmax = 0.7489 - 1.1917 d–1, Ki = 159 - 1250 for As (V), and μmax = 0.9787 - 1.0808 d–1, Ki = 578 - 1459 for As (III)) than in Skeletonema cf. costatum (μmax = 1.0881 - 1.3089 d–1 and Ki = 1910 - 3063 for As (V), and μmax = 0.8326 - 0.9363 d–1, Ki = 3003 - 5342 for As (III)).
In the culture media, dissolved inorganic arsenic and dissolved organic arsenic and particulate form of arsenic (phytoplanktonic) were determined and results are given in the Table 1. Arsenate was up taken by the test diatoms from the culture media and subsequently was converted to particulate (81.7%) and dissolve organic form (6.1%) in the medium.
4. Discussion
The response of phytoplankton species composition to both As (III) & As (V) was significant, and deviation in growth rates were observed for increasing arsenic dose. The ANOVA for the data showed overall significant differences of population between control and As (III) & (V) treated samples (For 200 µg·L–1, F = 0.54, P = 0.596, DF = 2, 15; for 400 µg·L–1, F = 0.77, P = 0.481, DF = 2, 15; For 800 µg·L–1, F = 1.35, P = 0.29, DF = 2, 15; for 1600 µg·L–1, F = 2.08, P = 0.159, DF = 2, 15).
Inhibition of growth by arsenic doses varied greatly with respect to size and shape. Decline in growth rate of relatively larger diatoms than small species in As-treated culture could be of important ecological significances. The smaller species not only contain smaller quantities of carbon leading to decline of carbon sequestration but also could lead to an increase in grazing by microzooplankton and perhaps altered trophic structure in the nutrient rich mangrove ecosystem. In this study, speciation of arsenic changed significantly, and more toxic inorganic form was converted to less toxic particulate and organic form, in consistent with the highly productive coastal water where interconversion of arsenic to organic form (methyl arsonate/ dimethyl arsenate was reported [24-27]).
There were significant negative relationships between Ki (inhibition constant) and V (biovolume, µm3cell–1) (r = –0.5, n = 10, p = 0.004 for As (V) and r = –0.5, n = 10, p = 0.012 for As (III)). Small size fraction of diatom (Skeletonema cf. costatum, Navicula rhombica and Amphora hyalina) treated with As (V) and As (III) showed least growth inhibition relative to larger counterpart (Coscinodiscus radiatus, Surirella, Thalassiothrix, Amphipleura). However, the spring sample of Thalassiosira decipiens showed As tolerance in contrast to the monsoon sample. Interspecific differences in As tolerance by diatom in the mangrove ecosystem indicated cell size could be only one factor contributing to these differences, DNA base content, sequence and their damage could play a significant role.
5. Conclusions
Based on this experimental study, it can be concluded that the test diatoms with smaller in cell size exhibit remarkable tolerance towards arsenic relative to those with large cell size and they have a potential to remove arsenic from the medium under laboratory conditions and are the promising candidate to be effectively exploited for bioremediation of the arsenic-contaminated sites.
6. Acknowledgments
The University Grant Commission, New Delhi; is gratefully acknowledged for financial support sanctioned under Major project.
REFERENCES
- M. Neff, “Ecotoxicology of Arsenic in Marine Environment,” Environmental Toxicology and Chemistry, Vol. 16, 1997, pp. 917-927.
- “Arsenic in Drinking Water,” National Research Council, National Academies Press, Washington, 1999.
- R. A. Armstrong, “Nutrient Uptake as a Function of Cell Size and Surface Transfer Density: A Michaelis Like Approximation to the Model of Pasciak and Gavis,” Deep Sea Research, Part I, Vol. 55, No. 10, 2008, pp. 1311- 1317. doi:10.1016/j.dsr.2008.05.004
- D. L. Aksnes and J. K. Egge, “A Theoretical Model for Nutrient Uptake in Phytoplankton,” Marine Ecological Progress Series, Vol. 70, 1991, pp. 65-72. doi:10.3354/meps070065
- A. J. Miao, W. X. Wang and P. Juneau, “Comparison of Cd, Cu, and Zn Toxic Effects on Four Marine Phytoplankton by Pulse-Amplitude-Modulated Fluorometry,” Environmental Toxicology and Chemistry, Vol. 24, No. 10, 2005, pp. 2603-2611. doi:10.1897/05-009R.1
- G. F. Riedel and J. G Sanders, “The Interrelationships among Trace Elements Cycling, Nutrient Loading, and System Complexity in Estuaries: A Mesocosm Study,” Estuaries, Vol. 26, No. 2A, 2003, pp. 339-351. doi:10.1007/BF02695972
- J. G. Sanders and G. F. Riedel, “Trace Element Transformation during the Development of an Estuarine Algal Bloom,” Estuaries, Vol. 16, No. 3A, 1993, pp. 521-532. doi:10.2307/1352599
- P. Michel, B. Boutier, H. Herbland, B. Averty, L. F. Artigas, D. Anger and E. Charttier, “Behavior of Arsenic on the Continental Shelf of the Gironde Estuary: Role of Phytoplankton in Vertical Fluxes during Spring Bloom Condition,” Oceanologica Acta, Vol. 21, No. 2, 1997, pp. 325-333. doi:10.1016/S0399-1784(98)80019-4
- S. K. Mandal, M. Dey, D. Ganguly, S. Sen and T. K. Jana, “Biogeochemical Controls of Arsenic Occurrence and Motility in the Indian Sundarban Mangrove Ecosystem,” Marine Pollution Bulletin, Vol. 58, No. 5, 2009, pp. 652-657. doi:10.1016/j.marpolbul.2009.01.010
- Y. Gao and A. Mucci, “Individual and Competitive Adsorption of Phosphate and Arsenate of Goethite in Artificial Sea Water,” Chemical Geology, Vol. 199, No. 1-2, 2003, pp. 91-109. doi:10.1016/S0009-2541(03)00119-0
- K. Knauer and H. Hemond, “Accumulation and Reduction of Arsenate by the Fresh Water Green Alga Chlorella sp. (Chlorophyta),” Journal of Phycology, Vol. 36, No. 3, 2000, pp. 506-509. doi:10.1046/j.1529-8817.2000.99056.x
- H. Biswas, M. Dey, D. Ganguly, T. K. De, S. Ghosh, and T. K. Jana, “Comparative Analysis of Phytoplankton Composition and Abundance over a Two-decade Period at the Land-ocean Boundary of a Tropical Mangrove Ecosystem,” Estuaries and Coasts, Vol. 33, No. 2, 2010, pp. 384-394. doi:10.1007/s12237-009-9193-5
- S. K Acharya, S. Lahiri, B. C. Kaymahashay and A. Bhowmik, “Arsenic Toxicity in Ground Water in Parts of Bengal Basin in India and Bangladesh: Role of Quarternary Stratigraphy and Holocene Sea Level Fluctuation,” Environmental Geology, Vol. 39, No. 10, 2000, pp. 1127- 1137.doi:10.1007/s002540000107
- D. Das, G. Samnta, B. K. Mondal, R. T. Chowdhury, C. R. Chanda, P. P. Chowdhury, G. K. Basu and D. Chakraborti, “Arsenic in Ground Water in Six Districts of West Bengal, India,” Environmental Geochemistry and Health, Vol. 18, No. 1, 1996, pp. 5-15. doi:10.1007/BF01757214
- R. Nickson, J. McArthur, N. Burgess, K. M. Ahmed, A. P. Ravenscroff, and M. Rahaman, “Arsenic Poisoning of Bangladesh Ground Water,” Nature, Vol. 395, 1998, p. 338. doi:10.1038/26387
- J. Metral, L. Charlet, S. Burean, S. Basu Mullik, S. Chakraborty, K. M. Ahmed, M. W. Rahman, Z. Cheng and A. Vangeen, “Comparison of Dissolved and Particulate Arsenic Distribution in Shallow Aquifers of Chakdaha, India and Araihazar, Bangladesh,” Geochemical Transactions, Vol. 9, 2008, p. 1. Udoi:10.1186, 1467-4866-9-1.
- T. Kiorboe “Turbulance, Phytoplankton Cell Size and Structure of Pelagic Food Webs,” Advances in Marine Biology, Vol. 29, 1993, pp. 1-72. doi:10.1016/S0065-2881(08)60129-7
- J. S. Edmonds, Y. Shibata, K. A. Francesconi, R. J. Rip pington and M. Morita, “Arsenic Transformations in Short Marine Food Chain Studied by HPLC-ICP/MS,” Applied Organometalic Chemistry, Vol. 11, No. 4, 1997, pp. 281- 287. doi:10.1002/(SICI)1099-0739(199704)11:4<281::AID-AOC581>3.0.CO;2-S
- A. Skovgaard and S. M Deuer, “Long-Term Exposure of Dinoflagellates to 14carbon Effects on Growth Rate and Measurements of Carbon Content,” Journal of Plankton Research, Vol. 25, No. 8, 2003, pp. 100-109. doi:10.1093/plankt/25.8.1005
- A. Al-Tisan, Ibrahim and C. P. Joseph, “Distribution of Heavy Metals in Plankton Collected during the Umikata Maru Cruise (II) in the Ropme Sea Area,” Proceeding of the Umitaka Maru Symposium, Tokyo, 1995.
- A. Aminot and R. Keroue, “An automated Photo-Oxidation Method for the Determination of Dissolved Organic Phosphorus in Marine and Fresh Water,” Marine Chemistry, Vol. 76, No. 1-2, 2001, pp. 113-126. doi:10.1016/S0304-4203(01)00052-4
- D. E. Cummings, F. Caccavo Jr, S. Fendorf and R. F. Rosenzweig, “Arsenic Mobilization by the Dissimilarity Fe (III) Reducing Bacterium Shewanella alga Bry,” Environmental Science and Technology, Vol. 33, 1999, pp. 723-729. doi:10.1021/es980541c
- J. H. Han and J. D. Floros, “Modeling the Growth Inhibition Kinetics of Baker’s Yeast by Potassium Sorbate using Statistical Approaches,” Journal of Food Science, Vol. 63, No. 1, 1998, pp. 12-14. doi:10.1111/j.1365-2621.1998.tb15664.x
- W. R, Cullen, L. G. Harrison and H. L. G. Hewitt, “Bioaccumulation and Excretion of Arsenic Compounds by a Marine Unicellular Alga, Polyphysa peniculus,” Applied Organometallic Chemistry, Vol. 8, No. 4, 1994, pp. 313- 324. doi:10.1002/aoc.590080406
- D. A. Bright, M. Dodd and K. J. Reimer, “Arsenic in subArctic Lake Influenced by Gold Mine Effluent: The Occurrence of Organoarsenicals and ‘Hidden’ Arsenic,” The Science of the Total Environment, Vol. 180, No. 2, 1996, pp. 165-182. doi:10.1016/0048-9697(95)04940-1
- Y. Sohrin, M. Matsui, M. Kawashima, M. Hojo and H. Hasegawa. “Arsenic Biogeochemistry Altered by Eutrophication in Lake Biwas,” Japan Environmental Science and Technology, Vol. 31, No. 10, 1997, pp. 2712-2720. doi:10.1021/es960846w
- K. A. Francesconi and J. S. Edmonds “Arsenic and Marine Organisms,” Advances in Organic Chemistry, Vol. 44, 1997, pp. 147-189.

