Journal of Biomedical Science and Engineering
Vol. 5 No. 1 (2012) , Article ID: 16931 , 9 pages DOI:10.4236/jbise.2012.51003
Methods of N-acetylated chitosan scaffolds and its in vitro biodegradation by lysozyme
![]()
1Faculty of Chemistry, Materials and Bioengineering and HRC, Kansai University, Suita, Japan
2Department of Biotechnology, Technological University, Myitkyina, Myanmar
Email: *tamura@kansai-u.ac.jp
Received 27 December 2010; revised 5 February 2011; accepted 24 March 2011
Keywords: N-Acetylated Chitosan; Scaffold; Lysozyme; Degradation
ABSTRACT
Generally, the lysozyme degradation on chitosan (CTS) is slower than that on chitin (CT). The CTS can be fabricated in scaffold form but it is difficult to fabricate CT scaffold under mild conditions. The method for the preparation of scaffold from N-acetylated CTS (N-CTS) was investigated in this research. By using this method, the scaffolds could be fabricated chitosan to chitin with the degree of acetylation (DA) in a range of 18% - 70%. Among these scaffolds, the highest degradation of scaffold by lysozyme was observed on the N-CTS scaffold with DA 60%, which determined by examination of the reducing end contents in the degradation media and by measuring the weight loss of scaffolds. Moreover, the best condition for the degradation of N-CTS scaffold with DA 70% by lysozyme was also investigated. The maximum degradation rate of the scaffold was observed on the treatment with lysozyme 500 mg/l of acetate buffer at pH 4.5, 37˚C, 100 rpm and for 7 days.
1. INTRODUCTION
The natural biopolymers, chitin and chitosan, are made up of β(1,4)-glycosidic linked 2-amino-2-deoxy-β-D-glucose and 2-acetamino-2-deoxy-β-D-glucose residue [1]. Depending on the degree of acetylation, each chitin/chitosan has the specific effectiveness. Chitin and chitosan have been applied in many biomedical applications [2] because of these substances are biodegradable and biocompatible [3-7].
However, there have to be some considerations for biomedical applications of chitin and chitosan such as the solvent systems of chitin and in vivo degradability of chitosan. Chitin can dissolve only in strong solvent systems and may be harmful to human if it is used in medical applications. Chitosan can easily dissolve in the dilute organic acid condition that is a reliable solvent for medical applications in human [8]. Moreover, one of the considerations on the chitosan and chitin in the biomedical application is degradation of those materials such as film, membrane, fiber and scaffold being used in human body. Degradation is concerned with enzymes in our body, which degrades these chitinous materials into small molecules of residues. Lysozyme is one of enzymes present in the human body that can hydrolyze the β(1-4) linkages between N-acetylglucosamine and glucosamine in chitosan and chitin according to the distribution of N-acetyl group [9,10]. Many researchers have reported the degradation of chitosan and chitin by lysozyme [9,11-16]. This enzyme has more degradation activity on chitin than chitosan because chitin has more N-acetyl glucosamine residues. Most of the studies determined the degradation of chitin and chitosan by study on the reducing sugar unit in the degradation medium, the molecular weight of hydrolysates in the degradation medium and the weight loss of chitin and chitosan [9,17,18]. However it has not been studied yet on the optimal conditions of the degradation of specific degree of acetylation of chitosan scaffold by lysozyme in vitro. Based on the above mentioned factors, the transferring of chitosan to N-acetylated chitosan (N-CTS) might be better solution for the medical application of these materials through consideration on safety. Moreover re-acetylated chitosan can also be easily degraded by lysozyme on the β(1-4) linkage of N-acetyl glucosamine.
There have been several studied reports for N-CTS using acetic anhydride as an acetylation agent [19-23]. In this study, N-CTS has been carried out with different methods to select the best method for the preparation of different DA% of N-CTS under the mild conditions and for the preparation of N-CTS scaffold with wide range of DA%. The characteristics of prepared scaffolds such as morphology of scaffold, stability of scaffold and water absorption properties were studied. Moreover, the N-CTS with highly re-acetylated scaffold was selected and studied the best conditions for the in vitro degradation of lysozyme on the N-CTS scaffold such as the degradation medium, pH, temperature, shaking (rpm), time of degradation and lysozyme concentration. Based on these results, the possible applications of scaffold are proposed.
2. MATERIAL AND METHODS
Crab chitosan (DA 18.7%) was obtained from Koyo Chemical Co., Ltd. and other materials were of analytical grade purchased from Wako Co., Ltd., Japan. The number average molecular weight (Mn) of original CTS was re-measured by GPC method.
2.1. Methods of Acetylation of Chitosan
Chitosan was acetylated by four different methods: method A, reported by Hirano et al., 1976; method B, modified the method A by Molly. S. S. et al., 2005; and method C and D, modificated from published methods in this research [21-23] (Table 1).
Method C: The chitosan solution was reacted with acetic anhydride for overnight. The reacted solution of method C1 was dialyzed with distilled water until neutral pH and lyophilized. The precipitate of method C2 and C3 were collected by centrifugation, washed with 70% acetone for three times and with distilled water up to neutral pH and then lyophilzed.
Method D: Chitosan solution was adjusted to pH 6.5 with 20% NaOH. Then acetic anhydride was added for N-acetylation at 15˚C for 4 h. The viscosity of the solutions obtained from every step was measured by using HAAKE RheoStress 600 Rheometer, carried out at 25˚C and average shear viscosity was taken from three replicates of each sample. Finally acetylated chitosan gel solution was incubated at room temperature for over night under stirring condition and then dialyzed until pH 6.5 ~ 7 and then lyophilized. After lyophilization, the N-CTS scaffolds were obtained. All N-CTS samples were weighted after lyophilization and stored for further analysis. Each experiment was carried out three replicates (n = 3).
2.2. Observation the Morphology of N-CTS Scaffold
The dried scaffold was sliced into rectangular shape (dimension = 0.9 × 0.6 × 0.2 cm3) in surface and crosssection areas and then the sample was coated with thin layer of platinum, using a JOLE JFC-1500 sputtering device. The surface and cross-section morphology of sample was observed by using a FE-SEM JEOL JSM- 6700 scanning electron microscope with an accelerating voltage of 5 kV, 10 µA. The diameter of pores of scaffold was measured by using the JEOL-Images software. The number of pores determined on each sample was ten and the values are expressed as mean value of the diameter of 10 pores.
2.3. Study the Water Absorption Properties of the N-CTS Scaffold
The water absorption properties of the N-CTS scaffold were performed by soaking the scaffold in each buffer solution; citric buffer CIT, acetate buffer ACE, phosphate buffer PBS at pH 4.5 and 7.4. The experiment was carried out at 37˚C and 25˚C. After 48 h incubation, percentage water absorption of scaffold (Eaw) was calculated as follows Equation (1); the adsorbed buffer solution on surface of scaffold was blotted with tissue paper and the weight of the scaffold was recorded as (W1). The initial weight of dried scaffold was recorded as (W0).
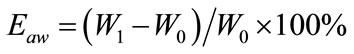 (1)
(1)
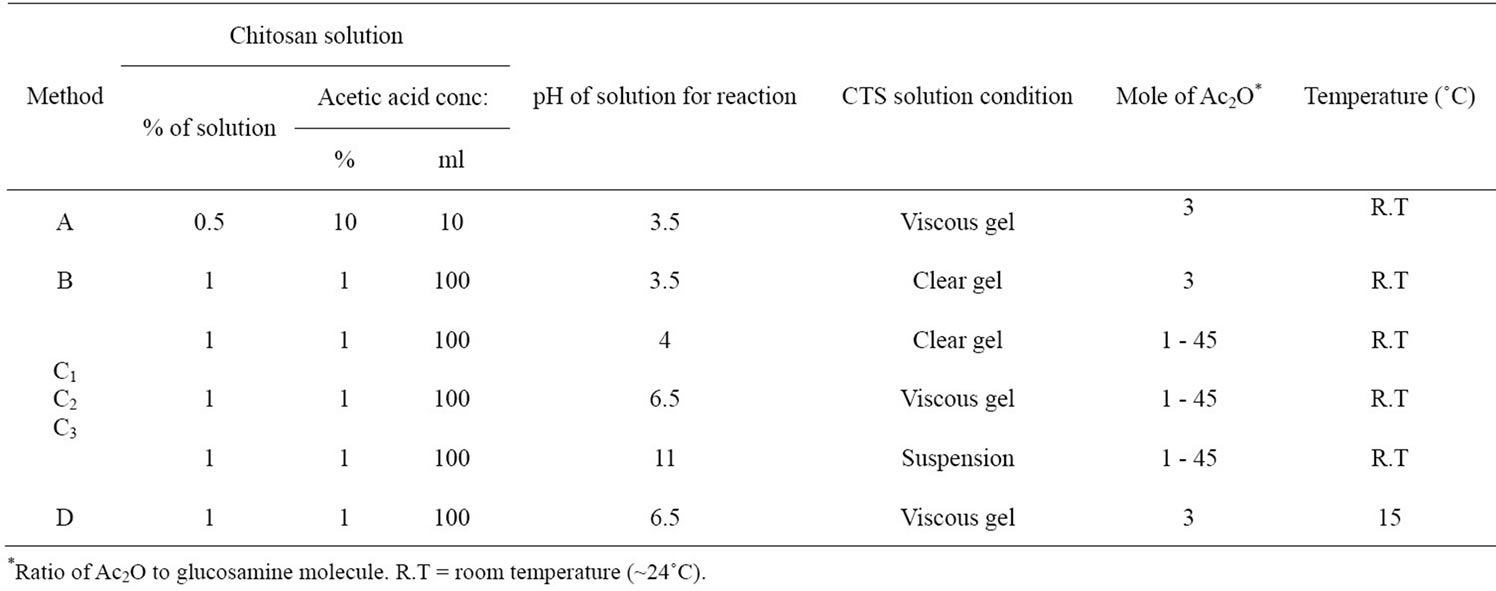
Table 1. Preparation conditions for N-acetylation of chitosan.
2.4. Study the in Vitro Degradation of the N-CTS Scaffolds by Lysozyme under Different Conditions
The eight pieces of N-CTS scaffold (50 mg (W0), one piece = (0.6 - 0.7) × (0.6 - 0.7) × (1 - 1.2) cm3) were placed in each buffer solution and incubated under different conditions. The condition of highest degradation rate on scaffold was selected to study other conditions. After degradation with lysozyme, the solution was filtered to remove the undigested scaffold pieces and collected the filtrate and then incubated in a water-bath at 95˚C for 30 min to precipitate lysozyme. The reducing sugar content in the filtrate was measured by Dygert method [24]. The amount of reducing sugar was determined by using a UV spectrophotometer (U-2010, Hitachi, Japan) and calculated using standard curve of OD 450 nm vs glucose concentration. The undigested amount of N-CTS scaffold on the filter paper was dried at 105˚C for 24 h and recorded the dried weight as (W1). The weight loss (%) of sample was determined using Equation (2). Each experiment was carried out three independent replicates.
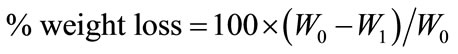 (2)
(2)
To separate between the lysozyme degradation and dissolution of scaffold, control samples (without addition of lysozyme) were carried out as the same procedure described above.
2.5. Determination of Degree of Acetylation
2.5.1. Infrared Spectrometry
Each sample 2 mg was weighted and mechanically wellblended with KBr (98 mg) and then prepared a disc [25]. IR spectrum was recorded with a Shimadzu FT-IR-2100 (Perkin-Elmer) spectrometer. The percent of the N-acetyl-glucosamine content in the sample was determined according to the Equation (3).
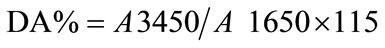 (3)
(3)
2.5.2. PUV Spectrophotometry
The DA of the N-CTS was measured by phosphoric acid ultraviolet (PUV) spectrometric analysis that was developed by Hsiao et al. and slightly modified by Hein et al. [26,27].
3. RESULTS AND DISCUSSION
3.1. Acetylation of Chitosan by Different Methods
The acetylated chitosan was prepared using crab chitosan with 4 different methods (A, B, C and D). After acetylation, the most acetylated chitosan from different methods was observed as powder form but some methods provided as scaffold form, that might be due to the nature of reacted solution (Tables 2 and 3). In method B, C2 and C3, the precipitate was obtained as soon as after completion
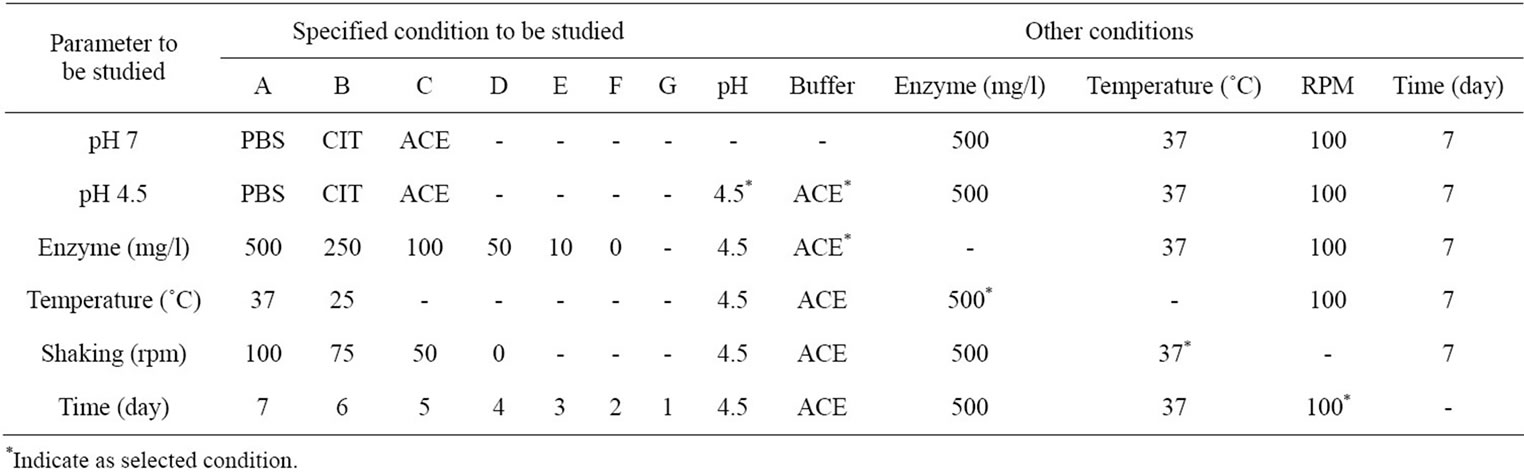
Table 2. The specific and other conditions of lysozyme degradation on N-CTS scaffold.
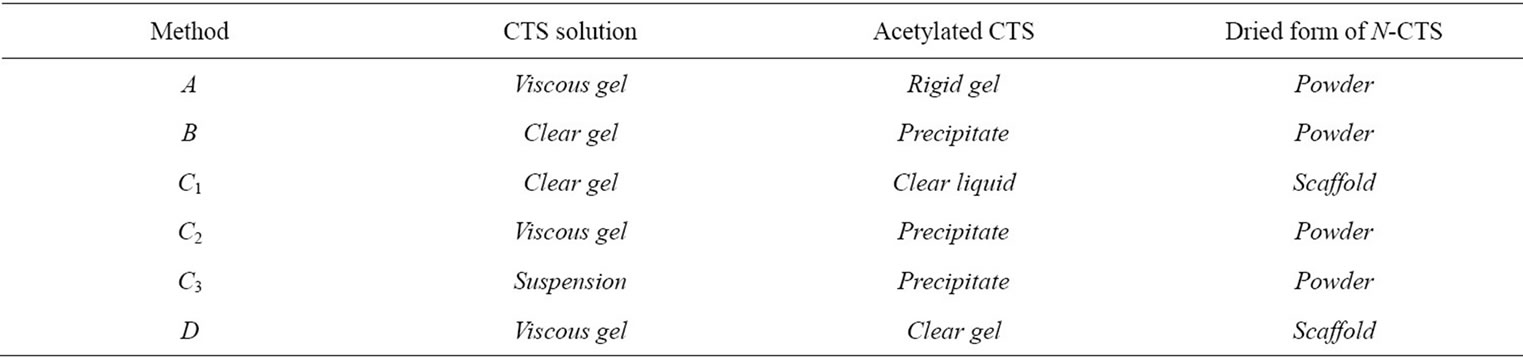
Table 3. Condition of chitosan solution and N-CTS form.
of acetylation reaction. So that these methods did not support the resultant N-CTS to fabricate as scaffold form. It can be assumed that the acetyl group of acetic anhydride were substituted to amino groups (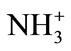 ) of chitosan molecules, then it will not become the ionic form of amine in the chitosan molecules. Method C1 and D were given the gel form of N-acetylated chitosan. However, the viscosities of two gel types were different in each chitosan solution.
) of chitosan molecules, then it will not become the ionic form of amine in the chitosan molecules. Method C1 and D were given the gel form of N-acetylated chitosan. However, the viscosities of two gel types were different in each chitosan solution.
Due to increase both chain dimensions and inter-chain repulsion through protonation of the amine groups, it has observed that the gel formation of chitosan solution at pH 6.5 before acetylation was higher than that of pH 4. The gel viscosity was slightly reduced after the acetylation. It was depended on the substitution of acetyl group to the chitosan molecules. Although the addition of amount of acetic anhydride and pH of reacted solution are the same, when the temperature is different, the substitutions of acetyl group are also different. While the acetylation reaction was happening, the increasing temperature was related to the addition of amount of acetic anyhydride, when the starting temperature is same. Normally the acetylation reaction was completed about one hour after addition of acetic anhydride to the chitosan solution. After acetylation, the resultant samples were lyophilized and observed as scaffold and powder forms. All acetylated samples increased weight (%) remarkably. However the increased weight of the N-CTS in each method was varied probably due to the variable substitution of acetyl group to the amino group of chitosan molecules.
The number average molecular weight (Mn) of acetylated CTS was increased about to 3.9 × 105 Da from that of original CTS 1.67 × 105 Da.
3.2. Determination of Solubiliy and Degree of Acetylation of the N-CTS in Powder and Scaffold Form
The resulted N-CTS samples were firstly checked their solubility in one of the chitin solvents, saturated CaCl2·2H2O: MeOH solution and one of chitosan solvents 2% HOAc solution at ambient temperature [28]. All samples were dissolved in CaCl2·2H2O: MeOH solution with time according to the degree of N-acetylation (i.e., the highest DA of sample was the fastest soluble in this solvent and longer time for lower DA of chitosan). However all samples were not dissolved in 2% HOAc solution. These tests give the preliminary results of substitution of Nacetyl group in CTS molecules (data are not shown). In order to confirm the N-acetylation in chitosan molecules, N-CTS powder and scaffolds were treated with 1M NaOH or 95% EtOH for one day and washed with water upto neutral pH. After that the samples were dissolved in 2% HOAc and all scaffold samples were decomposed in 2% HOAc. Form this observation; it was proposed that the substitution of acetyl groups in chitosan molecules of scaffold may be caused by unstable ionic bond or other molecular interaction.
To investigate the percentage degree of acetylation of N-CTSs, IR and PUV methods were used and the results were shown in Table 4.
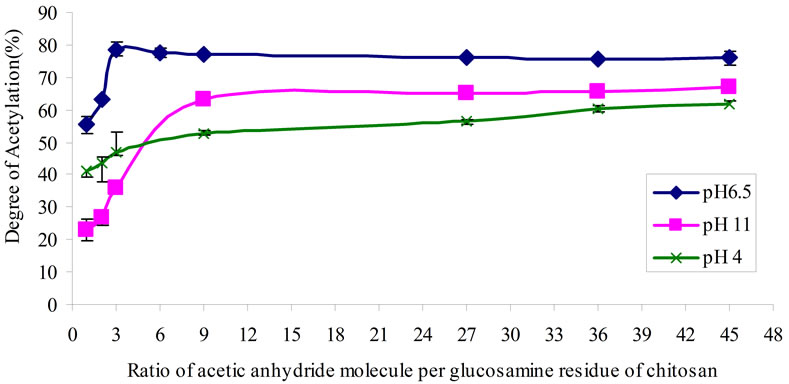
The DA of chitosan values are determined by these two methods which showed a little different in measurement of the same sample. In the subsequence discussions, the DA of the N-CTS was presented from the re sults of PUV methods. The DA of chitosan in this study obtained a range of 32% - 78%, the highest DA of NCTS was 78.67% (Figure 1(a) and Table 4).
The N-acetylation to chitosan molecule was more efficiently occurred under condition of chitosan solution at pH 6.5. The chitosan solution at pH 6.5 was increased the intra and intermolecular hydrogen bonds formation in the chitosan molecule. The free amino groups of chitosan molecule were protonated at pH 6.5 and acetyl (CH3CO–) group from acetic anhydride could be readily reacted to the ionic form of NH3+ in the chitosan molecule more than that of at pH 4 and 11. Moreover the substitution of acetyl group was increased at pH 6.5 of chitosan solution when the temperature was decreased to 15˚C. At pH 6.5 of chitosan solution, 3 mole of acetic anhydride per glucosamine residue of chitosan was observed the maximum amount of substitution of acetyl groups, see in Figure 1(a). The amino group of chitosan molecule in alkaline condition of chitosan solution was NH2 form and its substitution of acetyl group to amino group was poorer than that of acidic condition of chitosan solution. This observation can be clearly seen in method C. When an equal amount of acetic anhydride was added to the chitosan solutions at pH 4 and pH 11, the substitution of acetyl groups were observed with different values due to nature of amino group. Adding the excess amount of acetic anhydride to chitosan solution was transformed chitosan molecule to ionic form made more acetyl group substitu-
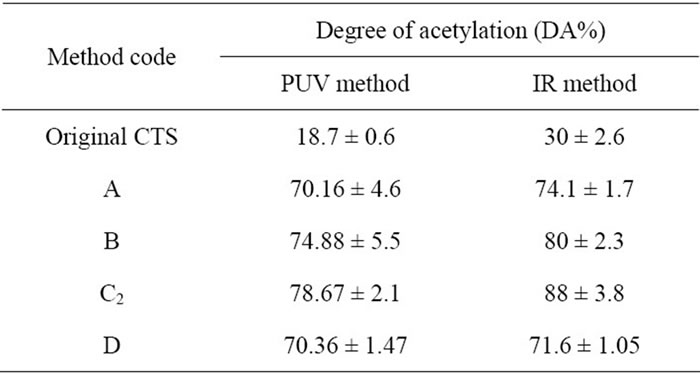
Table 4. Percentage degree of acetylation of chitosan by determination with PUV spectrophotometry and infrared (IR) spectrometry.
 (a)
(a)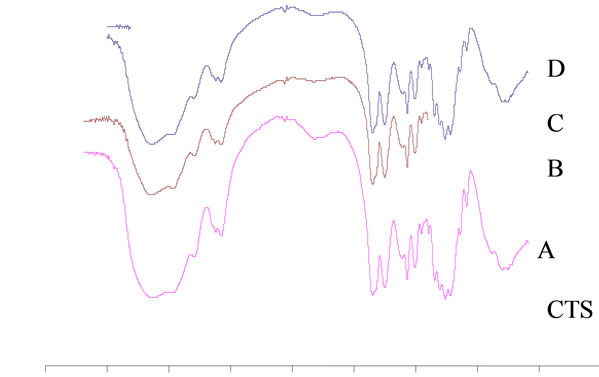 (b)
(b)
Figure 1. (a) Degree of acetylation (%) of acetylated chitosan under different mole ratio of acetic anhydride molecule to glucosamine unit of chitosan molecule at pH 4, 6.5, 11 of chitosan solutions; and (b) FT-IR spectra of CTS and N-CTS by various methods of N-acetylation (A, B, C and D), the peak changes at 1660 cm–1 (amide I) and 1560 cm–1 (amide II).
tion; this fact was seen in the addition of over 9 moles of acetic anhydride to chitosan molecule at pH 11.
Figure 1(b) shows the FT-IR spectra of CTS and NCTS resulted from different methods of N-acetylation. The spectrum of original chitosan at the peak of the 1660 cm–1 and 1560 cm–1 could be suggested that these peaks were the characteristic absorptions of the amide –C=O and –NH2 groups (amide I and amide II) respectively. The OH stretching band 3450 cm–1 was prominent and relatively isolated. The original chitosan gave DA 30% ± 2.6% by IR method. It can be clearly seen that all peaks of amide –C=O stretching vibration at 1660 cm–1 and the bending absorption of –NH2 at 1560 cm–1 are apparently stronger than these peaks of original CTS. There has not been appeared the wavelength of absorbance at 1240 cm–1 in all spectra and could be concluded that no more O-acetylation happened in the acetylation reaction of chitosan in this study.
3.3. Fabrication of the N-CTS Scaffolds and Their Characteristics
The scaffolds have been fabricated by acetylated solution of method C1 and D. The acetylated solution of method C1 was observed as N-CTS scaffolds with DA 32% - 60% and that of method D was specific for the fabrication of N-CTS scaffold with higher DA%. All resultant solutions were as gel form after acetylation and these solutions have been used to prepare N-CTS scaffold by lyophilization method.
The fabrication of scaffolds was caused by the lyophilization process with N-CTS molecules and ice crystal. The formation of hydrogen bond between the long chain polymers during freezing might be the development of N-CTS scaffold. Figures 2(a)-(c) show the SEM images of surface magnification of the N-CTS scaffolds with high DA% (60% and 70%). The pore size of this scaffold was uniformly distributed with diameter of 254 ± 61 μm and it has a polygonal structure. It was reported that the structure and size of pore could be significantly affected on the scaffold degradation of lysozyme and also affected on the absorption of water [29]. This study has been found to be the hen egg white (HEW) lysozyme could easily attach to the N-CTS scaffold and observed more degradation in scaffold than powder 100 rpm) by autoclaving at 121˚C, 30 min. But these scaffolds did not strong enough to handle in wet state after long time dipping in the neutral solutions.
When the scaffold was intended to be used in biomedical applications, it must have excellent water absorption properties and stability to maintain their shape and volume under wet condition especially. The N-CTS scaffold with high DA% was stabilized in three buffer solutions (PBS, CIT and ACE) at pH 4.5 and 7.4 even shaking at 100 rpm for several days and it could be enough to handle under wet condition. The sterilization of this scaffold was carried out by autoclaved 121˚C for 15 min; it could not observe any changes on the structure of scaffold form of N-CTS (data are not shown).
The stability of N-CTS scaffolds with 18%, 43%, 50%, 60%, DA% were observed nothing changes on configuration in PBS, CIT and ACE buffers (pH 7.4, 37˚C). The different types of buffer and pH did not affect on the absorption properties of the N-CTS scaffold with high DA% but the variation of temperature slightly affected: the temperature at 37˚C showed more absorption of buffer than that at 25˚C in three buffers. The value of absorption ratios (~4200%) were almost the same within the three buffer solutions (ACE, CIT, PBS) at 25˚C and 37˚C for 48 h. The constant absorption ratio of scaffolds occurred after one hour to 48 h absorption in each buffer.
3.4. Degradation of the N-CTS Scaffolds by Lysozyme
The in vitro degradation of N-CTS scaffold with high DA% by lysozyme was studied under different conditions to observe the conditions of the maximum degradation of scaffold. Many literatures reported that human and Hen Egg White (HEW) lysozyme are not accurately similar to amino acid residues in their structure [30-32]. Therefore HEW lysozyme was selected to study the degradation rate of the N-CTS scaffold with high DA% in this research.
The results of percentage weight loss of N-CTS scaffold and the amount of reducing ends obtained from the degradation of N-CTS scaffold with lysozyme are described in Tables 5(a) and (b). The deformation of physical nature of scaffold could be seen at first day of lysozyme treatment, all N-CTS scaffolds were destroyed to small particles after degradation process while the physical nature of scaffold maintained without lysozyme in various buffer solutions even in shaking at 100 rpm. According to the result, the degradation rate based on weight loss and reducing ends of scaffold in ACE buffer at pH 4.5 was more than that in other buffers at pH 4.5 and 7.4. So the lysozyme treatment was carried out by ACE buffer (pH 4.5) to study the further conditions of
 (a)
(a)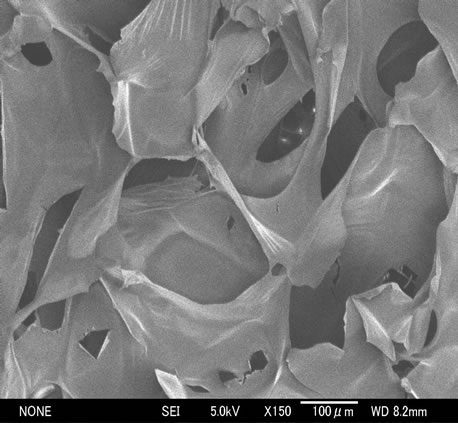 (b)
(b) (c)
(c)
Figure 2. Representative SEM images; (a) and (b) show surface magnification of DA 70% N-CTS scaffolds × 100 and 150 times, respectively; (c) shows surface magnification of DA 60% N-CTS scaffold × 100 times.
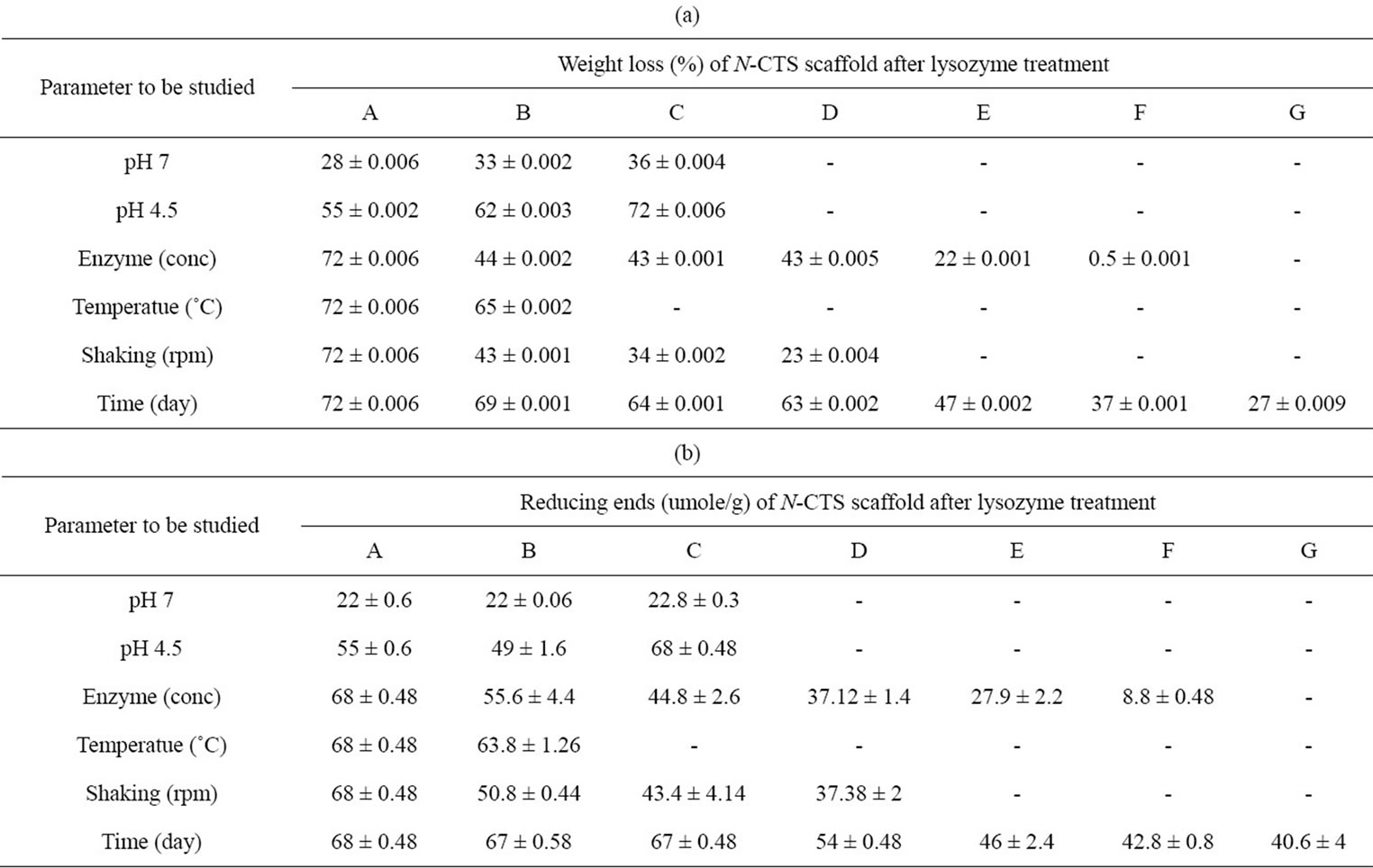
Table 5. In vitro degradation of highly DA% N-CTS scaffold with lysozyme. (a) Percentage weight loss of scaffold; (b) The amount of reducing ends content in degradation medium.
the lysozyme degradation on N-CTS scaffold with high DA%. It was a good agreement result with Nordtveit et al., who found the hen egg white lysozyme was the highest degradation activity in ACE buffer at pH 4.5 [11,12].
Thein-Han et al., 2006 [33] was reported that the lysozyme concentration of 500 mg/l on chitosan scaffold was observed the good degradation activity where as Nwe et al., 2009 [29] was studied with the lysozyme concentration of 10 mg/l and found the low degradation rate on chitosan scaffold than the use of lysozyme concentration of 500 mg/l. So that lysozyme concentration is also important factor for the degradation of chitinous materials. This study was continuously carried out on the degradation activity of scaffold with different lysozyme concentrations for the cleavage of glycosidic linkage in the β 1-4 linked GlcNAc, which can estimate the human lysozyme on N-CTS scaffold for further study. The highest degradation activity was found to be at lysozyme concentration of 500 mg/l and the degradation rate was not significantly different within the lysozyme concentration of 250 - 50 mg/l. The percentage degradation rate at lysozyme concentration of 10 mg/l was 3.5 times lower than that of 500 mg/l in the degradation medium. It can be concluded that concentration of lysozyme in the degradation medium is also effected on degradation of the N-CTS scaffold.
After that the lysozyme degradation of N-CTS scaffold was investigated on the body temperature (37˚C) and room temperature (25˚C) for 7 days. The degradation activity was found to be the higher rate at 37˚C of medium than at 25˚C. It can also be concluded that lysozyme degradation rate was affected on the temperature of degradation medium.
It was also observed that the lysozyme can degrade the N-CTS scaffold even without shaking of degradation medium and the more increasing the shaking speed, the more increasing the degradation of N-CTS scaffold by lysozyme could be. The maximum degradation was observed the shaking speed at 100 rpm.
Finally, the time effect was being found out for lysozyme degradation after observing the condition of buffer, pH, enzyme amount, temperature and shaking. All experiments were carried out for 1 to 7 days and the highest rate for lysozyme degradation of this scaffold was observed on the 7th day. The N-CTS scaffolds under specific conditions (i.e. the conditions showed the highest degradation of scaffold by lysozyme) without lysozyme were observed that the scaffolds were remained its original structure until 7 days of incubation. According to the experimental data compared with the weight loss of scaffold and reducing ends in the supernatant of medium, the maximum degradation conditions for the in vitro degradation of N-CTS scaffold was found to be lysozyme concentration of 500 mg/l in acetate buffer at pH 4.5, 37˚C, 100 rpm for 7 days.
3.5. Comparison of Lysozyme Degradation on N-CTS Scaffolds with Different DA%
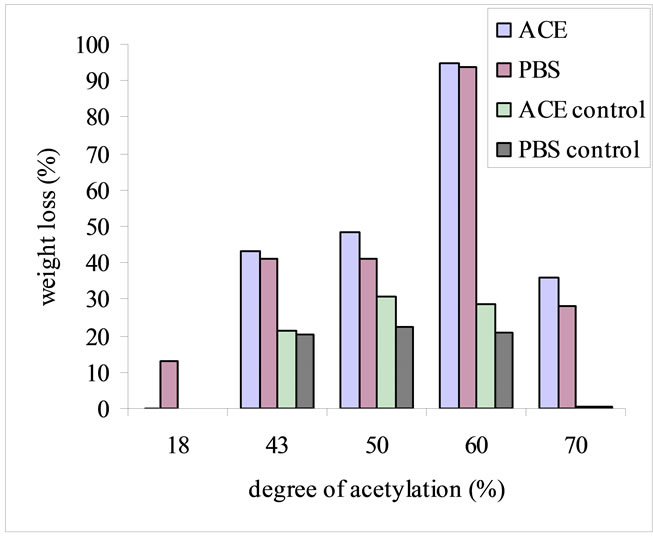
The scaffolds with DA 18% and 43% were dissolved in ACE buffer at pH 4.5. Due to this fact, the lysozyme degradation activity on different N-CTS scaffolds was performed on the conditions of PBS and ACE buffers pH 7.4, rpm 100, lysozyme concentration of 500 mg/l at 37˚C for 7 days, which was based on specific conditions of N-CTS scaffold with DA 70%. During incubation with lysozyme, N-CTS scaffold with DA 60% was broken into small pieces within 24 h. The degradation rate was increased after two days of incubation and then after three days only the small particles were found in the incubated solution. On the fifth day, there was no particle in the incubation medium. Based on the data of weight loss of scaffold and reducing sugar ends, among DA 18%, 43%, 50%, 60%, 70% of N-CTS scaffolds, the N-CTS scaffold with DA 60% was observed the highest in vitro degradation by lysozyme in this study, Figures 3(a) and (b). Normally, lysozyme degrades the N-CTS by hydrolyzing of β 1-4 glycosidic bonds which linked to the N-acetyl glucosamine sequence and its biodegradability is increased with increased N-acetylated glucosamine units in the substrate [9,34]. The degradation rate of N-CTS scaffold with DA 60% was significantly greater than that of the other scaffolds; see Figure 3(b). The N-CTS scaffold with DA 18% to less than 60% without lysozyme treatment were observed as the deformation of scaffolds and some weight loss %, see Figure 3(a). The character of degradation rate of the N-CTS scaffold with DA 18% to less than 60% can be explained by the solubility of chitosan in neutral aqueous environment. This fact has been reported that the chitosan with DA close to 50% is water-soluble [35,36]. With solubility character and DA% had been affected on the degradation of the N-CTS scaffold with DA 60%, resulting the highest in vitro degradation activity by lysozyme among other scaffolds. However the N-CTS scaffolds with DA 60% had poor stability in wet condition than N-CTS scaffold with DA 70%. So DA 70% N-CTS scaffold is proposed to use as scaffold in biomedical applications.
4. CONCLUSION
The amount of substitution of acetyl group depended on pH, the temperature of reaction solution and molar ratio of acetic anhydride to amine groups of chitosan molecules. The whole range of N-acetyl groups in the chitosan molecules could be measured with good accuracy value by PVU method. The different degree of N-CTS could be obtained using different methods with same molar ratio of acetic anhydride and temperature. The
 (a)
(a)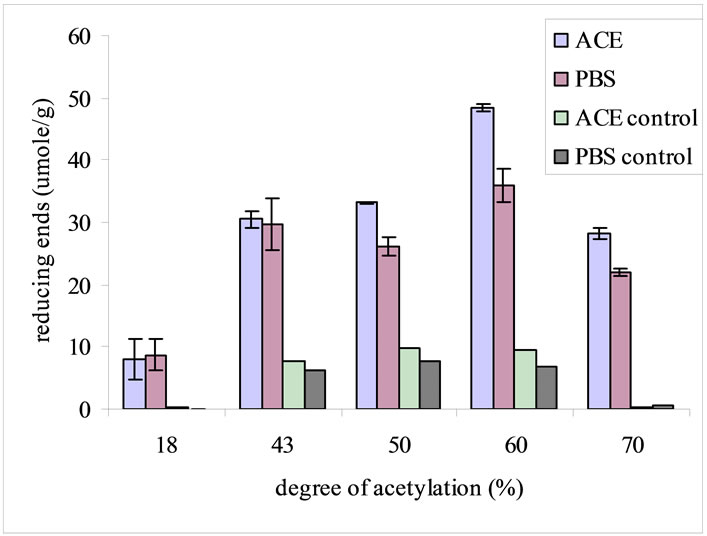 (b)
(b)
Figure 3. In vitro degradation of different DA% of N-CTS scaffolds with lysozyme concentration of 500 mg/l in PBS and ACE buffers at pH 7.4, 37˚C, 100 rpm, for 7 days. The degradation was determined based on percentage weight loss of the scaffold (a) and reducing ends in the degradation media (b). Data are shown as mean ± standard deviation (n = 3).
acetylated CTS gel could be fabricated into N-CTS scaffold with different DA%. Method D was found to be the best method for fabrication of N-CTS scaffold. The maximum degradation rate by lysozyme on N-CTS scaffold with high DA% was observed in ACE buffer at pH 4.5, lysozyme concentration of 500 mg/l, 37˚C, shaking speed 100 rpm for 7 days. According to the described results of in vitro degradation, stable in various conditions and water absorption of N-CTS scaffold with high DA% is proposed to use as a hemostat and other biomedical applications.
5. ACKNOWLEDGEMENTS
The authors would like to extend their thanks to “High Technology Research Center” Project for Private Universities; matching fund subsidy from MEXT (Ministry of Education, Culture, Sports, Science and Technology, Japan), 2008-2009 for providing funds for this research and Koyo. Co., Ltd. for supplying chitosan.
![]()
![]()
REFERENCES
- Christe, B.L. (2009) Introduction to biomedical instrumentation: The technology of patient care. Cambridge University Press, Cambridge.
- Cutnell, J.D. and Johnson, K.W. (1998) Physics. 4th Edition, John Wiley & Sons Ltd., New York. doi:10.1016/S0142-9612(03)00026-7
- Kim, S., Jeon, Y., Byun, H. and Park, P. (2001) Subacute oral toxicity of chitosan oligosaccharide on Sprague Dawley rats. Proceedings of the 8th International Chitin and Chitosan Conference and 4th Asia Pacific Chitin and Chitosan Symposium, Kodansha Scientific Ltd., Tokyo, 276.
- Ivashov, S.I., Razevig, V.V., Sheyko, A.P. and Vasilyev, I.A. (2004) Detection of human breathing and heartbeat by remote radar. Progress in Electromagnetic Research Symposium, 28-31 March 2004, Pisa, 663-666.
- Sung, P.T., Chien, Y.H., Chung, Y.H., Da, M.W., Ling, H.L., Juin, Y.L. and Hsyue, J.H. (2007) Preparation and cell compatibility evaluation of chitosan/collagen composite scaffolds using amino acids as crosslinking bridges. Journal of Applied Polymer Science, 105, 1774-1785. doi:10.1002/app.26157
- Tokura, S., Nishi, N., Itoyama, K., Shirai, A., Nishimura, S., Saiki, I. and Azuma, I. (1994) Chitin derivatives with biomedical functions. In: Karnicki, Z.S., Brzeski, M.M., Bykowski, P.J. and Wojtasz-Pajak, A., Eds., Wirtschaftsverlag NW: Verlag Für Neue Wissenschaft GmbH, Bremerhaven, Germany, 287.
- Vande Vord, P.J., Matthew, H.W.T., De Silva, S.P., Mayton, L., Wu, B. and Wooley, P.H. (2002) Evaluation of the biocompatibility of a chitosan scaffold in mice. Journal of Biomedical Materials Research, 59, 585-590. doi:10.1002/jbm.1270
- Hirano, S., Shigemasa, R. and Minami, S. (1995) Applications of chitin and chitosan for biomaterials. Biotechnology and Genetic Engineering Reviews, 13, 383-420.
- Abia, S. (1992) Studies on chitosan: Lysozymic hydrolysis of partially N-acetylated chitosans. International Journal of Biological Macromolecules, 14, 225-228. doi:10.1016/S0141-8130(05)80032-7
- Edmund Optics, Linear Position Sensor Module (NT57- 058). http://www.edmundoptics.com/products/displayproduct.cfm?productid=2476
- Nordtveit, R.J., Varum, K.M. and Smidsord, O. (1994) Degradation of fully water-soluble, partially N-acetylated chitosan with lysozyme. Carbohydrate Polymer, 23, 253-260. doi:10.1016/0144-8617(94)90187-2
- Nordtveit, R.J., Varum, K.M. and Smidsord, O. (1996) Degradation of partially acetylated chitosans with hen egg white and human lysozyme. Carbohydrate Polymer, 29, 163-167. doi:10.1016/0144-8617(96)00003-3
- Rothamel, D., Schwarz, F., Sculean, A., Herten, M., Scherbaum, W. and Becker, J. (2004) Biocompatibility of various collagen membranes in cultures of human PDL fibroblasts and human osteoblasts-like cells. Clinical Oral Implants Research, 15, 443-449. doi:10.1111/j.1600-0501.2004.01039.x
- Varum, K.M., Myhr, M.M., Hjerde, R.J.N. and Smidsod, O. (1997) In vitro degradation rates of partially N-acetylated chitosan in human serum. Carbohydrate Research, 299, 99-101. doi:10.1016/S0008-6215(96)00332-1
- Woodard, J.R., Hilldore, A.J., Lan, S.K., Park, C.J., Morgan, A.W., Eurell, J.A.C., Clark, S.G., Wheeler, M.B., Jamison, R.D. and Johnson, A.J.W. (2007) The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials, 28, 45-54. doi:10.1016/j.biomaterials.2006.08.021
- Yang, Y., Hu, W., Wang, X. and Gu, X. (2007) The controlling biodegradation of chitosan fibers by N-acetylation in vitro and in vivo. Journal of Materials Science: Materials in Medicine, 18, 2117- 2121. doi:10.1007/s10856-007-3013-x
- Hirano, S. and Midorikawa, T. (1998) A novel method for the preparation of N-acylchitosan fibre and N-acylchitosan-cellulose fibre. Biomaterials, 19, 293-297. doi:10.1016/S0142-9612(97)00216-0
- Hirano, S. and Yagi, Y. (1989) The effects of N-substitution of chitosan and the physical form of the products on the rate of hydrolysis by chitinase form Streptomyces griseus. Carbohydrate Research, 83, 103-108. doi:10.1016/S0008-6215(00)85369-0
- Aiba, S. (1994) Preparation of N-acetylchitooligosachrides from lysozymic hydrolysates of partially N-acetylated chitosans. Carbohydrate Research, 261, 297-306. doi:10.1016/0008-6215(94)84025-3
- Kurita, K., Koyama, Y., Nishimura, S. and Kamiya, M. (1989) Facile preparation of water soluble from chitosan. Chemistry letters, 18, 1597-1598. doi:10.1246/cl.1989.1597
- Hirano, S., Ohe, Y. and Ono, H. (1976) Selective N-acylation of chitosan. Carbohydrate Research, 47, 315-320. doi:10.1016/S0008-6215(00)84198-1
- Kurita, K., Chikaoka, S., Kamiya, M. and Koyama, Y. (1988) Studies on Chitin 14. N-acetylation behavior of chitosan with acetylchloride and acetic anhydride in a highly swelled state. Bulletin of the Chemical Society of Japan, 61, 927-930.
- Molly, S.S., Thomas, F., Hui, S.K. and Karineh, K. (2005) Controlling cell adhesion and degradation of chitosan films by N-acetylation. Biomaterials, 26, 5872-5878. doi:10.1016/j.biomaterials.2005.02.033
- Dygert, S., Li, L.G., Floride, D. and Thomas, J.A. (1965) Determination of reducing sugar with improved precision. Analytical Biochemistry, 13, 367. doi:10.1016/0003-2697(65)90327-1
- Sabnis, S. and Block, L.H. (1997) Improved infrared spectroscopic method for the analysis of degree of N-acetylation of chitosan. Polymer Bulletin, 39, 67-71. doi:10.1007/s002890050121
- Hsiao, H.Y., Tsai, C.C., Chen, S., Hsieh, B.C. and Chen, R.L.C. (2004) Spectrophotometric determination of deacetylation degree of chitinous materials dissolved in phosphoric acid. Macromolecular Bioscience, 4, 919-921. doi:10.1002/mabi.200400084
- Hein, S., Chuen-How, N., Stevens, W.F. and Wang, K. (2008) Selection of a practical assay for the determination of the entire range of acetyl content in chitin and chitosan: UV spectrophotometry with phosphoric acid as solvent. Journal of Applied Biomaterials, 86B, 558-586.
- Tamura, H., Nagahama, H. and Tokura, S. (2006) Preparation of chitin hydrogel under mild conditions. Cellulose, 13, 357-364. doi:10.1007/s10570-006-9058-z
- Nwe, N., Furuike, T. and Tamura, H. (2009) The mechanical and biological properties of chitosan scaffolds for tissue regeneration templates are significantly enhanced by chitosan from Gongronella butleri. Materials, 2, 379-398. doi:10.3390/ma2020374
- Cohen, J.S. (1969) Proton magnetic resonance studies of human lysozyme. Nature, 223, 43-46. doi:10.1038/223043a0
- Banjard, S.H., Blake, C.C.F. and Swan, I.D.A. (1974) In lysozyme, Osserman, E.F., Canfield R.E. and Beychok, S., Eds., Academic Press Inc., New York, 71-79.
- Ren, D., Yi, H., Wang, W. and Ma, X. (2005) The enzymatic degradation and swelling properties of chitosan matrices with different degrees of N-acetylation. Carbohydrate Research, 340, 2403-2410. doi:10.1016/j.carres.2005.07.022
- Thein-Han, W.W. and Kitiyanant, Y. (2006) Chitosan scaffold for in vitro buffalo embryonic stem-like cell culture: An approach to tissue engineering. Journal of Biomedical Materials Research, 80B, 92-101. doi:10.1002/jbm.b.30573
- Amaral, I.F., Sampaio, P. and Barbosa, M.A. (2006) Three-dimensional culture of human osteoblastic cells in chitosan sponges: The effect of the degree of acetylation. Journal of Biomedical Materials Research, 76A, 335-346. doi:10.1002/jbm.a.30522
- Hirano, S., Yamaguchi, Y. and Kamiya, M. (2003) Water-solube N-(n-fatty acyl) chitosans. Macromolecular Bioscience, 3, 629-631. doi:10.1002/mabi.200350029
- Sannan, T., Kurita, K. and Iwakura, Y. (1976) Studies on chitin. 2. Effect of deacetylation on solubility. Makromolekular Chemistry, 177, 3589-3600. doi:10.1002/macp.1976.021771210
NOTES
*Corresponding author.

