Natural Science
Vol.4 No.6(2012), Article ID:19965,6 pages DOI:10.4236/ns.2012.46050
Levels of trace metals in three fish species Decapterus macrellus, Decapterus macrosoms and Decapterus russelli of the family carangidae from the Gulf of Aqaba, Red Sea, Jordan
![]()
1Faculty of Marine Sciences, University of Jordan, Aqaba, Jordan; m.khalaf@ju.edu.jo
2Chemistry Department, University of Jordan, Amman, Jordan
3Biology Department, University of Jordan, Amman, Jordan
Received 3 April 2012; revised 10 May 2012; accepted 28 May 2012
Keywords: Trace Metals; Decapterus Fish; Gulf of Aqaba; Jordan
ABSTRACT
Fishes of the family Carangidae, Decapterus macarellus, Decapterus macrosoma, and Decapterus russelli, were collected from the Jordanian Aqaba coast during 2008-2009 for the determination of their metal concentrations in various organs (muscle, liver, kidney, gonads, gills, and stomach food contents) using flame atomic absorption spectrometry (AAS). The results showed significant differences in metal concentration between species. The present study indicated significant differences of heavy metal elements among different organs of the same species. The results did not reveal any significant differences between male and female organs, and the only significant difference were found for Pb in Decapterus macorsoma and for Cu in Decapterus russelli. The low metal concentrations found in muscle tissue (in all studied species) has implications for human health.
1. INTRODUCTION
Fish is widely consumed by many people of the world because it has high protein content and low saturated fatty acids such as omega fatty acids, which support good health [1]. Decapterus macarellus, Decapterus macrosoma, and Decapterus russelli of the family Carangidae were popular among commercial fishes, representing approximately 14.5% of the marine Jordanian catch [2].
Human activities such as metal-related industries have greatly increased the input of heavy metals into the aquatic systems, where these metals are accumulated by aqauatic organisms and may be further transferred up to top trophic levels [3]. Many plant and animal species have been suggested as bioindicators for monitoring a variety of contaminants in the marine ecosystem [4,5]. Fish are often at the top of the aquatic food chain and may concentrate large amounts of some metals from the water [6]. Fish is constantly exposed to pollutants through water and food, which can result in bioaccumulation and biomagnifications occurring in different fish organs. Fish have been found to be good indicator for heavy metal contamination in aquatic systems [7,8].
Several studies on metal concentrations in fish have been reported from the Jordanian coast of the Gulf of Aqaba [9-15]. The aim of this work was to determine the concentrations of Cu, Ni, Mg, Pb, Zn, Cd and Fe in the muscle, liver, kidney, gonad and gills of the fishes Decapterus macarellus, Decapterus macrosoma, and Decapterus russelli collected from the Gulf of Aqaba.
2. MATERIAL AND METHODS
2.1. Study Area
The Gulf of Aqaba is a partially enclosed water body that constitutes the eastern segment of the V-shaped northern extension of the Red Sea (Figure 1). It is located in a sub-tropical arid area between longitude 34˚25'E to 35˚00'E and latitude 28˚00'N to 29˚33'N. The Gulf of Aqaba is 180 km long and has a maximum width of 25 km, which decreases at the northern tip to about 5 km. It is connected to the Red Sea through the Straits of Tiran, which has a depth of about 252 m [16]. The present study area lies within the Jordanian portion of the Gulf of Aqaba, which is situated at the northern tip of the Gulf and extends south for about 27 km to the Saudi Arabia boarder.
Figure 1. Study area and sampling sites in the northern Gulf of Aqaba.
2.2. Fish Species
The fish species which were under investigation in this study are Decapterus macarellus, commonly known as Mackerel Scad, and D. macrosoma commonly known as Shortfin Scad usually form large schools mostly in open water, feeding primarily on zooplankton, zoobenthos and other planktonic invertebrates and D. russelli known as Indian Scad, form large schools in deep water from middle to benthic zones, feeding mainly on zooplankton, zoobenthos, benthic crustaceans and nektons [17,18].
2.3. Sampling Design and Sampling Processing
The fishes were collected during 2008 and 2009 in the northern Gulf of Aqaba. All species were caught by gillnet. The fishing gear was set up with a local fisherman using a small boat of 7 m length provided with a 40- horse-power engine approximately 2000 m from the northern beach. Immediately after capture, the fishes were kept in an ice-box and transferred to the laboratory of the Marine Science Station in Aqaba. The specimens were identified to species level. After measuring total length, each fish was dissected using a plastic knife. The sex of each specimens was determined, and samples of muscle, liver, kidney, gonads, gills, and stomach food contents were taken, rinsed with distilled water, and oven dried at 85˚C to constant weight. Sub-samples of each organ were homogenized, and a weight of 0.5 to 1.0 g was taken in a porcelain crucible and ash dried at 540˚C. Samples were mixed with 5 ml of 2M HCL, heated in the crucible on a hot plate at 100˚C for half hour, and cooled to room temperature, after which the sample solution was filtered through a Whatman No. 43 filter paper into a 25 ml volumetric flask and filled to the mark with deionized water. Final metal concentrations of (Cu, Ni, Mg, Pb, Zn, Cd and Fe) were measured using Flame Atomic Absorption Spectrometer available at the University of Jordan/ Chemistry Department. One-way analysis of variance using [19] was performed to test the effect of species, sex and organs on the final metal concentration.
The AAS-instrument was calibrated using standard solutions prepared from commercially available standard chemicals (Merck, Germany). Analytical blanks were run in the same way as the samples and the metal concentrations were determined using the standard solutions prepared in the same acid matrix. All reagents used during analysis were of analytical grade and deionized water was used throughout the study. All the used plastics and glassware were soaked in nitric acid for 15 min and rinsed with deionized water prior to use.
3. RESULTS
3.1. Comparison between SPECIES
The mean metal concentration for Ni and Mg in the three fish species D. macarellus, D macrosoma and D. russelli showed significant differences (p = 0.0002 and p = 0.0129, respectively). The highest Ni concentration was found in D. macarellus (4.16 μg/g), followed by D. macrosoma (3.17 μg/g) and D. russelli (0.60 μg/g), whereas the highest Mg concentration was found in D. russelli (1034 μg/g), followed by D. macarellus (872 μg/g) and D. macrosoma (671 μg/g). On the other hand Cu, Pb, Zn, Cd and Fe did not show any significant differences (Table 1).
3.2. Comparison between Organs
Mean metal concentrations for Cu, Ni, Mg, Pb, Zn, Cd and Fe in D. macarellus in different fish organs such as gills, gonads, kidney, liver and muscle are given in Table 2. The results included significant differences between different organs of D. macrelleus for Cu (p = 0.03), Ni (p = 0.0001), Mg (p = 0.0001), Cd (p = 0.0001) and Fe (p = 0.0001). Whereas, Pb (p = 0.5) and Zn (p = 0.41) did not show any significant differences.
The results in Table 3 showed significant differences between different organs of D. macrosoma for Ni (p = 0.0001), Mg (p = 0.0001), Zn (p = 0.0001), Cd (p = 0.03) and Fe (p = 0.0001). In contrast, Cu (p = 0.56) and Pb (p = 0.67) did not show any significant differences (Table 3).
The results in Table 4 showed significant differences between different organs of D. russelli for Cu (p = 0.02), Mg (p = 0.0001), Zn (p = 0.0001), and Fe (p = 0.0001). There were no significant differences for Ni (p = 0.94), Pb (p = 0.7) and Cd (p = 0.17). In general the lowest metal concentrations were found in muscle tissue, whereas the highest concentrations of Cu, Pb, Cd and Fe
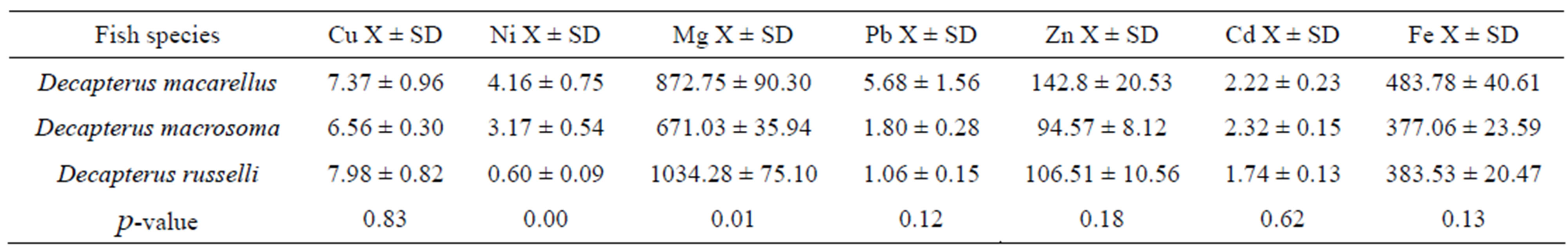
Table 1. Mean metal concentration in μg/g of the fish samples collected from the Jordanian coast/Aqaba, Red Sea and the p-value.
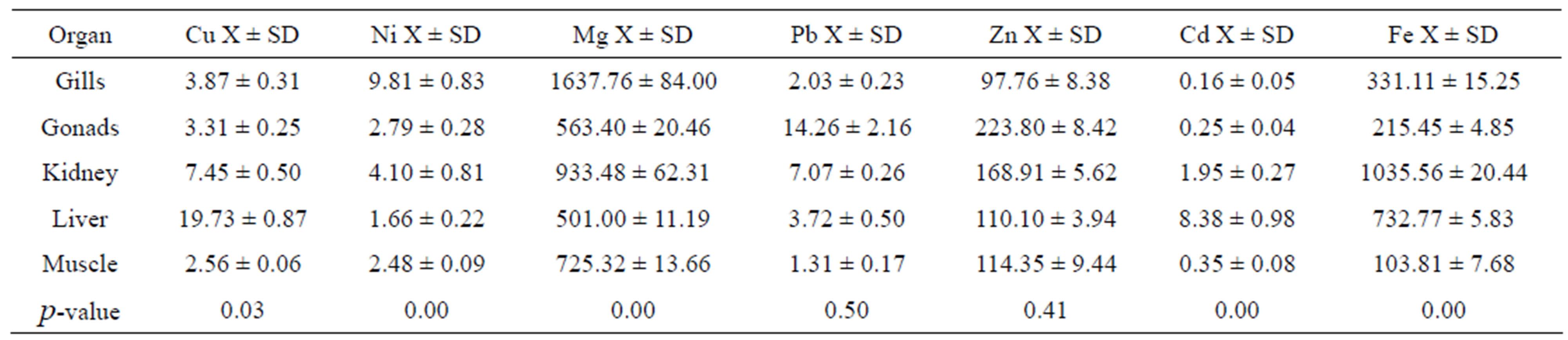
Table 2. Mean metal concentration in μg/g of selected fish organs: Gill, gonads, kidney, liver and muscle of Decapterus macarellus collected from Jordanian coast/Aqaba, Red Sea.

Table 3. Mean metal concentration in μg/g of selected fish organs gill, gonads, kidney, liver and muscle of Decapterus macarosoma collected from Jordanian coast, Aqaba, Red Sea.
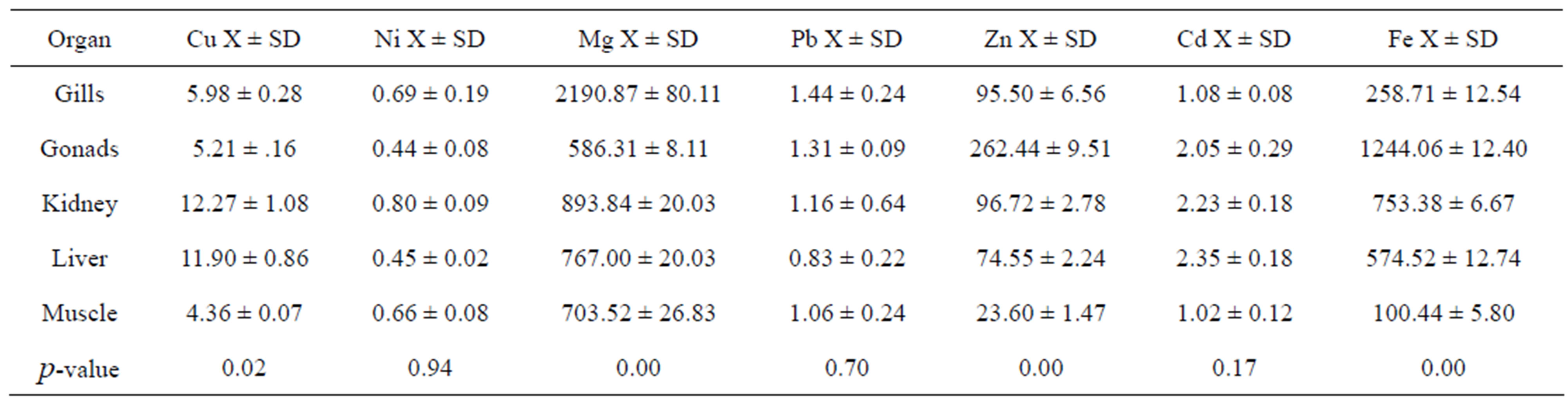
Table 4. Mean metal concentration in μg/g of selected fish organs gill, gonads, kidney, liver and muscle of Decapterus russelli collected from Jordanian coast/Aqaba, Red Sea.
were mainly found in liver and kidney. Mg metal concentrations were highest in gills of all species, and that of Zn were highest in gonads of all species.
The present study indicated significant differences for most of the heavy metal elements among different organs of the same species, particularly the presence of low concentrations of Cu, Pb, Zn, Cd and Fe in the muscle of D. macarellus and low concentrations of Ni, Pb, Zn and Fe in the muscle of D. macrosoma. Similarly, in D. russelli the lowest concentrations of Cu, Pb, Zn, Cd and Fe were found in muscle. In contrast, the highest concentrations of Cu, Pb, Cd and Fe were mainly concentrated in liver and kidney. Mg metal concentration was highest in gills and Zn was highest in gonads.
4. DISCUSSION
The present investigation revealed significant differences in metal concentration between D. macarelus, D macrosoma and D. russelli. Two of the species, Decapterus macarellus and D. macrosoma, occupy the same niche, feeding on similar food (zooplankton, zoobenthos and other planktonic invertebrates). In contrast, the third species, D. russelli, inhabits deep water from middle to benthic zones and feeds on zooplankton, zoobenthos, benthic crustaceans and nektons [17,18]. Our results are in accordance with the findings of Wahbeh [10], who found significant differences in heavy metal concentrations in six coral reef species from the same area. As in our study, they included species with different feeding habits, from piscivorous species such as the lizardfish (Synodus variegates) to those feeding on invertebrates, such as the goatfish (Parupeneus barberinus), or on algae, such as the Sergeant Major fish (Abufefduf saxatilis).
The general pattern was of significant differences for heavy metal concentrations between different organs for the three species D. macarellus, D. macrosoma, and D. russelli. This study revealed low heavy metal concentrations in muscle for most elements. Similar conclusions were reported by Abu Hilal [15], who found lower concentrations of metals in muscle versus liver, gonads, gills or stomach for three coral reef fish species collected from the Jordanian coast. Similarly Wahbeh [10] found that muscle contained lower metal concentrations than either liver or gonads.
In general, gills, liver and kidney contained relatively high metal concentrations for the three Decapterus species studied during this investigation, indicating that these organs might be the major organs for metal uptake (gills) or final metal deposition (liver and kidney). These results are in agreement with those of previous studies [20-23]. In addition, our results indicated high metal concentrations of Cu in liver and kidney. High concentrations of Cu in fish liver has been reported to be associated with high copper-related enzymes [24]. Our results revealed copper levels ranging from 2.6 μ/g in muscle to 19.73 μg/g in liver for D. macarellus, from 3.01 μ/g in gonads to 11.06 μg/g in liver for D. macrosoma, and from 4.36 μg/g in muscle to 12.27 μg/g in kidney for D. russelli. These levels were much lower than the copper level found by [25]. Because the lowest metal concentrations were found in muscle tissue, humans, such as Jordanians, who eat primarily fish muscle as opposed to liver and other organs should be at lower risk of heavy metal contamination. Copper and zinc are essential elements in fish and are thought to be strictly regulated in muscle tissue [26]. They are carefully regulated by physiological mechanisms in most organisms [27]. They are, however, considered to be potential hazards to both animal and human health. Knowledge of their concentrations in fish is important in respect to both nature management and human consumption of fish [28]. Our study showed that Mg, which is considered to be an important essential element, was higher than other essential elements such as Fe, and Zn in almost all organs in the three studied species. Magnesium metal has been found to play an important role in muscle contraction and in the activation of some enzymes [29].
5. ACKNOWLEDGEMENTS
The authors are grateful to the Deanship for Scientific Research, University of Jordan, for funding the whole research, the staff of Marine Science Station particularly Mr. Omar Al-Momaney for their support.
REFERENCES
- Ikem, A. and Egiebor, N.O. (2005) Assessment of trace elements in canned fishes (mackerel, tuna, salmon, sardines and herrings) marketed in Georgia and Alabam (United States of America). Journal of Food Composition and Analysis, 18, 771-787. doi:10.1016/j.jfca.2004.11.002
- El-Zibdeh, M., Khalaf, M. and Odat, N. (2006) The fishery status in Jordan’s Gulf of Aqaba, Red Sea. Dirasat: Pure Science, 33, 127-142.
- Reinfelder, J.R., Fishher, N.S., Luoma, S.N., Nichots, J.W. and Wang, W.X. (1998) Trace element trophic transfer in aquatic organisms: A critique of the kinetic model approach. Science of the Total Environment, 219, 117-135. doi:10.1016/S0048-9697(98)00225-3
- Astorga-Espana, M.S., Pena-Mendez, E.M. and GorciaMontelango, F.J. (1999) Application of principal component analysis to the study of major cations and trace metal in fish from Tenerife (Canary Island). Chemometrics and Intelligent Laboratory Systems, 49, 73-178. doi:10.1016/S0169-7439(99)00038-6
- Cid, B.P., Boia, C., Pombo, L. and Rebelo, E. (2001) Determination of trace metals in fish species of the Ria de Averio (Portugal) by electrothermal atomic absorption spectrometry. Food Chemistry, 75, 93-100. doi:10.1016/S0308-8146(01)00184-4
- Mansour, S.A. and Sidky, M.N. (2002) Ecotoxicological studies. 3. Heavy metals contaminating water and fish from Fayoum Governorate, Egypt. Food Chemistry, 78, 15-22. doi:10.1016/S0308-8146(01)00197-2
- Burger, J., Gaines, K.F., Shane Boring, C., Stephens, W.L., Snodgrass, J. and Dixon, C. (2000) Metal levels in fish from the Savannah River: Potential hazards to fish and other receptors. Environmental Research, 89, 85-97. doi:10.1006/enrs.2002.4330
- Hadson, P.V. (1988) The effect of metabolism on uptake, diposition and toxicity in fish. Aquatic Toxicology, 11, 3- 18. doi:10.1016/0166-445X(88)90003-3
- Wahbeh, M.I. (1985) Levels of zinc, iron, magnesium and cadmium in the tissue of fish from Aqaba, Jordan. Dirasat, 12, 35-42.
- Wahbeh, M.I. and Mahasneh, D. (1987) Concentrations of metals in the tissues of six species of fish from Aqaba, Jordan. Dirasat, 14, 316-326.
- Al-Sayed, M. (2008) Levels of trace metals in some Carnivorous fish of Gulf of Aqaba, Red Sea. Master’s Thesis, Hashemite University, Zarka.
- Al-Zgool, A. (2008) Levels of trace metals in food chain of some carnivores fishes (Family: Carangidae) collected from the Gulf of Aqaba, Red Sea. Master’s Thesis, Hashemite University, Zarka.
- Batayneh, M. (2010) Levels of trace metals in some Herbivorous fish of Gulf of Aqaba, Red Sea. Master’s Thesis, Hashemite University, Zarka.
- Ismail, N.S. and Abu-Hilal, A.H. (2008). Heavy metals in three commonly available coral reef fish species from the Jordan Gulf of Aqaba, Red Sea. Jordan Journal of Biological Sciences, 2, 61-66.
- Abu-Hilal, A.H. and Ismael, N.S. (2008) Heavy metals in eleven common species of fish from the Gulf of Aqaba, Red Sea. Jordan Journal of Biological Sciences, 1, 13-18.
- Hulings, N.C. (1989) A review of the marine science research in the Gulf of Aqaba. Publication of the Marine Science Station, Aqaba.
- FishBase. (1999) A global information system on fishes. ICLARM, Manila. http://www.fishbase.org
- Khalaf, M.A. and Disi, A.M. (1997) Fishes of the Gulf of Aqaba. Publication of the Marine Science Station, Aqaba.
- Stat Soft (1997) Statistica for Windows 5.1. Stat Soft, Tulsa.
- Dallinger, R. and Kautzy, H. (1985) The importance of contaminated food for the uptake of heavy metals by rainbow trout (Salmo gairdneri): Afield study. Oecologia, 67, 82-89. doi:10.1007/BF00378455
- Dallinger, R., Prosi, F., Segner, H. and Back, H. (1987) Contaminated food and uptake of heavy metals by fish: A review and proposal for further research. Oecologia, 73, 91-98. doi:10.1007/BF00376982
- Thomann, R.V., Shkreli, F. and Harrison, S. (2000) A pharmacokinetic model of cadmium in rainbow trout. Environmental Toxicology and Chemistry, 16, 1367-1373.
- Wong, C.K., Wong, P.P. and Chu, L.M. (2001) Heavy metal concentrations in marine fish collected from fish culture sites in Hong Kong. Archives of Environmental Contamination and Toxocology, 40, 60-69. doi:10.1007/s002440010148
- Syed, M.A. and Coombs, T.L. (1982). Copper metabolism in the plaice, Pleuonecetes platessa (L.). Journal of Experimental Marine Biology and Ecology, 63, 293. doi:10.1016/0022-0981(82)90184-8
- Bu-Olayan, A.H. and Subrahmanyam, M.N.V. (1996). Trace metals in fish from the Kuwait coast using the microwave acid digestion technique. Environmental International, 22, 753-758. doi:10.1016/S0160-4120(96)00067-0
- Phillips, D.J.H. (1980) Quantitative aquatic biological indicators. Applied Science Publishers, London.
- Eisler, R. (1988) Zink Hazards to fish, wildlife and invertebrates: A synoptic review. US Fish and Wildlife Service Biological Report, 85, 99.
- Amundsen, P.A., Staldvik, F.J., Lukin, A., Kashulin, N., Popova, O. and Reshetnikov, Y. (1997) Heavy metals contamination in freshwater fish from the border region between Norway and Russia. Science of the Total Environment, 201, 211-224. doi:10.1016/S0048-9697(97)84058-2
- Bowen, H.I.M. (1966) Trace elements in biochemistry. Academic Press, London, 241.

