Materials Sciences and Applications
Vol.07 No.04(2016), Article ID:65999,8 pages
10.4236/msa.2016.74020
The Thermal Stability of (CaTiO3)1−x (Cr3/4Fe5/4O3)x Ceramic Composites in the Microwave Region
Francisco Nivaldo Aguiar Freire1, Manoel Roberval Pimentel Santos2, Hélio Henrique Barbosa Rocha1, Ana Fabiola Leite Almeida1, Selma Elaine Mazzetto3, Antonio Jefferson Mangueira Sales4, Antonio Sergio Bezerra Sombra4
1Department of Mechanical Engineering, Federal University of Ceará, Campus do Pici, Fortaleza, Brazil
2Western Pará Federal University (UFOPA), Santarem, Brazil
3Department of Organic and Inorganic Chemistry, Federal University of Ceara, Campus do Pici, Fortaleza, Brazil
4Department of Physics, Federal University of Ceará, Campus do Pici, Fortaleza, Brazil

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 21 September 2015; accepted 21 April 2016; published 27 April 2016
ABSTRACT
The thermal stability, structural and dielectric properties of the (CaTiO3)1−x(Cr3/4Fe5/4O3)x ceramic composites with x = 0, 0.1, 0.5, 0.9 and 1 have been examined in microwave region. The samples were produced via the solid-state reaction. The orthorhombic structural phase of CaTiO3 and trigonal structural phase of Cr3/4Fe5/4O3 were confirmed by the X-Ray Powder Diffraction (XRPD). The XRPD patterns for composites reveal the quantities of each original phase present. The infrared spectra of the samples reinforce this structural verification indicating no or minimal occurrence of unwanted reactions. The first sample (x = 0) and last sample (x = 1) in this series exhibit the maximum and the minimum of the relative dielectric permittivity and values range from 140.1 to 8.3 respectively. The measured temperature coefficient of the matrix CaTiO3 was +921 ppm∙˚C−1 and for the matrix Cr3/4Fe5/4O3 was −56 ppm∙˚C−1. With the study of series of composites, it was possible to make a mathematical prediction for a composition reach the temperature coefficient near zero. The proposed ceramic has potential use as thermostable material in the microwave region and can be applied to resonators, low-noise amplifiers, filters, and so on.
Keywords:
Ceramics, Composites, Dielectric Properties

1. Introduction
The development of dielectric elements suitable for telecommunication systems performing at microwave band demands for very stringent criteria. In order to be applied in devices such as amplifiers, filters and resonators the ceramic materials must display a combination of planned properties. A high dielectric permittivity promotes the intended device miniaturization since the wavelength in dielectrics is inversely proportional to the squared root of their relative dielectric permittivities. This produces impacts positively on the signal propagation velocity and cross-coupling effect minimization. Additionally, a low dielectric loss or equivalently high quality factor lead to prominent frequency selectivity and operational stability. Often pointed out as determinant practical requisite, the thermostability at different working temperatures is ensured by a small temperature of the resonant frequency. There is a significant amount of effort devoted to this research field continuously. In part, this is due to the difficulty to attain simultaneously the three requested features highlighted [1] - [3] .
CaTiO3-based ceramics, are attractive candidates for use as dielectric resonators in wireless telecommunication systems, can exhibit dielectric properties of εr~162, Q × f~12,000 GHz and τf ~850 ppm∙˚C−1 [4] . Its temperature coefficient can effectively restrict its application in a microwave device. In general, an effective way to achieve near-zero or zero coefficient and boost the material quality factor is by compensating its large temperature coefficient values using compounds having temperature coefficient opposite signal. Cr3/4Fe5/4O3 is a polycrystalline solid solution and member of the hematite (α-Fe2O3) and eskolaite (Cr2O3) system (CrYFe2-YO3, Y = 3/4) [5] - [7] , in additional exhibited negative τf [8] [9] , which makes candidate to balance the temperature coefficient as composite together with CaTiO3.
This work deals with the investigation of the structural and microwave dielectric properties of a two-phase system derived from CaTiO3 and Cr3/4Fe5/4O3 oxide ceramics. Their synthesis involved mechanical activation followed by thermal treatments of the corresponding raw materials, and their composites were produced via thermal processing by considering determined composition of each original synthesized phase. Along the compositional process of the proposed system, a better combination of microwave dielectric properties was achieved.
2. Experimental Procedure
CaTiO3 and Cr3/4Fe5/4O3 compounds (hereafter referred as CTO and CRFO, in this order) were individually synthesized by the solid-state reaction using high-purity oxide powders: CaCO3 (Aldrich, 99%) and TiO2 (Aldrich, 99.9%) for CTO, Cr2O3 (Reagen, 99.8%) and Fe2O3 (Aldrich, 99%) for CRFO production, weighed according to their specific stoichiometry. The starting materials were hand-grounded in an agate mortar. Prior to the first heat treatment, high-energy grinding of the homogeneous powder mixture was carried out in a planetary mill (Pulverisette 5, Fritsch GmbH, Germany) using stainless steel vials (volume approx. 110 mL) with 20 stainless steel spheres (4 g and 10 mm diameter) experiencing 370 rpm at room temperature. The time of the mechanical milling operation was set at 180 min for the CTO and 60 min for the CRFO. Subsequently, the reagents were calcined in conventional controlled furnaces (EDG1800, EDG Equipamentos, Brazil) at 1100˚C for 3 h to synthesize CTO, and at 1300˚C for 5 h to synthesize CRFO, both in atmospheric air. The calcined powders were mixed according to the molar fraction (x = 0.1, 0.5, 0.9) to produce the (1 − x)CTO:(x)CRFO composite materials. For dielectric microwave properties characterization, the polycrystalline powders were uniaxially pressed (100 MPa) into pellets in a steel die. It was added about 3 wt.% of an organic binder (epoxy resin). The resin (contributing to the pellets preparation) was eliminated along the subsequent thermal stage. In conclusion, all palletized compounds (whose typical dimensions were in diameter and in thickness) were sintered at 1200˚C for 3 h in atmospheric air.
The X-ray powder diffraction experiments were performed in a powder diffractometer (DMAXB, Rigaku, Japan) using the Brag-Brentano geometry in a continuous mode with a scan speed of 0.5˚ min−1. A CuKα radiation tube with the line focus was operated at 40 kV and 25 mA at room temperature (294 K). The X-ray powder diffractions (XRPD) were taken in the range of 20˚ - 60˚ (2θ) in step sizes of 0.02˚. The diffracted X-ray beam coming from the sample is focused into the detector slit with a curved graphite monochromator. Rietveld refinement procedures [10] were applied to diffraction patterns using the interface DBWS9807-Tools [11] , as described by Young and coworkers [12] . The peak shape was assumed to be pseudo-Voigt (pV) function with asymmetry. The background of each pattern was fitted by a polynomial function of degree 5. The fitting of the model to the data was carried out by observing parameters such as the R indexes (RP, RWP), goodness-of-fit (SGoF) and Durbin-Watson d-statistic (dDW). The theoretical densities of the polycrystalline samples calculated (from crystal cell and contents, ρth) in the structural refinement were compared to the bulk (experimental, ρexp) densities of the sintered specimen determined by Archimedes’s method. In a complementary manner, the Fourier-infrared (FT-IR) spectra were obtained using a FT-IR infrared spectrometer (IR-prestige, Shimadzu, Japan), applying standard KBr pellet as dispersant agent (1:100 wt./wt.) in the mid range from 400 up to 1000 cm−1.
Microwave relative dielectric permittivity (εr) and unloaded quality factor (Qu) values at microwave frequencies were measured using the TE01δ resonance mode obtained by the Hakki-Coleman [13] method as modified and improved by Courtney [14] and Kobayashi and Tanaka [15] . The cylindrical pellets were inserted in a cavity connected to a network analyzer (HP 8719ET, Agilent, USA) ranging from 1 to 12 GHz in the transmission setup. The electromagnetic field pattern obtained in this method is widely used in characterization of properties of materials due to the nonexistence of the current crossing both the dielectric and the conducting plates. Hence, possible air gaps between the dielectric and the conducting plates have no effects on resonance properties of the TE01δ mode [16] [17] . By measuring the resonant frequency in the temperature ranging from 25˚C (Ti) to 100˚C (Tf) at a step of 10˚C via temperature controlled thermocouple, the temperature coefficient of the resonant frequency (Tf) was evaluated according to the Equation (1):
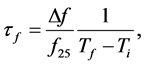 (1)
(1)
where f25 is the resonant frequency at arbitrary standard temperature (25˚C) and 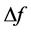 is the difference between the final and initial measured frequencies. Usually,
is the difference between the final and initial measured frequencies. Usually, 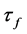 is scaled up by 106 and reported in parts per million per Celsius degree (ppm∙˚C−1).
is scaled up by 106 and reported in parts per million per Celsius degree (ppm∙˚C−1).
3. Results and Discussion
The XRPD observed (yo) and calculated (yc) profiles of CTO (x = 0.0) and CRFO (x = 1.0) are shown in Figure 1(a). The XRPD polycrystalline patterns for the composite members (x = 0.1, 0.5, 0.9 mol%) of the series are shown in Figure 1(b). The red lines (positioned bellow diffractograms) represent the relative difference between the experimentally observed and the computed intensities by the refinement method. According to the XRD results, there are well defined two-phased ceramic composites combining CaTiO3 (perovskite structure) and a Cr3/ 4Fe5/4O3 (hematite structure), both indexed in the Inorganic Crystal Structure Database (ICSD) [18] under the codes 163,528 and 163,943, respectively. The Rietveld refinement of CTO was carried out based on a reference model [19] . The CTO present, at room temperature, orthorhombic structure belonging to the spatial group ( 62), with four molecules per unity cell (Z = 4). In this structure, the calcium is localized in the Wyckoff position 4c, where the oxygen ions occupies the sites 4c and 8d and titanium occupies the site 4b. A crystallographic model based on iron oxide and on chromium oxide was proposed [7] to study the CRFO phase. It was suggested that CRFO presents (at room temperature) a trigonal structure belonging to 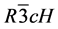 ( 167) spatial group, with six molecules per unity cell (Z = 6). In this proposition the iron and chromium are localized both in the 12c Wyckoff Position, and the oxygen ion occupies the 18e site.
( 167) spatial group, with six molecules per unity cell (Z = 6). In this proposition the iron and chromium are localized both in the 12c Wyckoff Position, and the oxygen ion occupies the 18e site.
The numerical criteria of fit considered here are the weighted profile R-factor (RWP), the value of the Durbin- Watson d-statistic (dDW) and the goodness of fit (SGoF). From a purely mathematical point of view, RWP is the most meaningful of the R’s because the numerator is the residual being minimized, scaled by the weighted intensities [12] . Based on this criterion, the refinement associated to the phase CRFO presented the best result. The value of the Durbin-Watson d-statistic shows the serial correlation of the refinement, where an ideal value should be around 2, indicative of insignificant serial correlation in the refinement process [20] . According to the results, it is suggestive a significant serial correlation, in principle associated with the presence of a slight amount of extra phases present and/or overlapping. Graphical representations of the Rietveld model fit to the measured XRD diffraction patterns for CTO and CRFO are shown in Figure 1(a) & Figure 1(b). The residual exhibits satisfactory matching (minimization of the difference) between observed peaks of the crystalline phases and the employed model. Also, the obtained values for SGoF around 1.0 indicate the adequacy of the proposed models, which justify the effectiveness of structural elucidation for the materials analyzed and validates a good purity grade achieved by them. The crystallographic data, refinement indices and density of the materials under investigation are summarized in the Table 1. The results for the composites are reported in Table 2.
Figure 2 shows the room temperature infrared transmission spectra observed for CTO, CRFO and derived

Figure 1. XRPD refined patterns for (a) CTO (upper frame) and CRFO (lower frame) and for the members of the (1−x)CaTiO3:(x)Cr3/4Fe5/4O3 series (b) x = 0.1 (top), x = 0.5 (middle), 0.9 (bottom).
Figure 2. Room temperature infrared spectra of (1−x)CTO:(x)CRFO microwave dielectric ceramics (x = 0.0, 1.0) and their derived composites (x = 0.1, 0.5, 0.9).
composites. The characteristic vibrational modes, ranging from 400 cm−1 to 600 cm−1, are summarized in the Table 3. The values for CTO are slightly different, but close to previously published results [21] [22] . Evidencing the solid-state reaction, the CTO and CRFO present displacements in relation to that characteristic observed for their reagent oxides: the Fe2O3 transmission spectrum occurs in 575 cm−1 and 480 cm−1, in 580 cm−1 and 445 cm−1 for eskolaite (Cr2O3) and in 423 cm−1 for rutile (TiO2). As suggested by the XRPD patterns, the composites seem to reflect the quantities of each phase therein.
Table 4 presents the microwave dielectric properties of the samples investigated considering the measurement
Table 1. Crystallographic data, numerical criteria of fit and density (theoretical, physical and relative) for CaTiO3 (CTO) and Cr3/4Fe5/4O3 (CRFO) phases.
Table 2. Density (theoretical, physical and relative) and Rietveld indices (numerical criteria of fit) for composites of the (1−x)CTO:(x)CRFO system.
Table 3. Wave numbers (in cm−1) for (1−x)CTO:(x)CRFO compounds.
Table 4. Microwave dielectric properties of the (1 − x)CTO:(x)CRFO series.
methodology described in the literature [13] - [15] . All materials resonated in the range from 2 GHz to 7.94 GHz. The CTO (x = 0.0) and CRFO (x = 1.0) base compounds limited the εr of the series: 140.1 and 8.3, respectively. The decrease in relative dielectric permittivity values accompanies the resonant frequency raising. The dielectric loss (tan(δ)) for the materials is around 1 × 10−3. The behavior of the quality factor is not explained considering only the proportion of CTO/CRFO present in each composite, as seen in the case for (x = 0.1), with the lower result. It seems that additions of CRFO improved this parameter along the system: the Qu × fr increases from 1499 GHz (x = 0.0) to 6853 GHz (x = 1.0). Alteration in the production route, and hence in the resulting microstructure, can be an indicative of the differences observed for literature reports of the same material, presently exemplified by CaTiO3. The temperature coefficient of resonant frequency values verified ranges from high positive (+921 ppm∙˚C−1 for x = 0.0) to moderately negative (−56 ppm∙˚C−1 for x = 1.0). In detriment of other characteristics, relatively better combination of microwave dielectric properties was reached for (x = 0.9) member: Quxfr = 6291 GH, a relatively good factor [23] , and τf = −47 ppm∙˚C−1. The potentiality of these composites would be significantly increased by further improving the quality factor values.
The results for microwave dielectric properties measurements are graphically illustrated in Figure 3. There was employed an exponential PDF based with offset (fit) function to predict the response of τf (Figure 3(a)) between x = 0.0 and x = 1.0 extreme compositions as modeled by the Equations (2)-(3):



Figure 3. Measured microwave dielectric properties of the (1−x)CTO:(x)CRFO (x = 0.0, 0.1, 0.5, 0.9, 1.0) series: (a) temperature coefficient of the resonant frequency, (b) quality factor and (c) relative dielectric permittivity. A fitting for τf is also presented. According to the model (Equations (2)-(3)), τf = 0 ppm∙˚C−1 for x = 0.72.
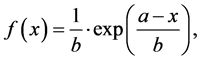 (2)
(2)

This mathematical model probably is restricted to the results observed, not general and neither linked to physical parameters. According to it, approximately for a composition of x = 0.72 it is possible to get a thermostable response (τf = 0 ppm∙˚C−1).
4. Conclusion
The structural and microwave dielectric properties of the (1−x)CaTiO3:(x)Cr3/4Fe5/4O3 (CTO:CRFO) ceramic composites were investigated in this paper. The XRPD patterns and infrared spectra indicated the formation of the extreme phases as well as their preservation in composites, suggesting that, in the thermal processes, it was suppressed the occurrence of unwanted solid-state reactions and/or persistence of reagents. Reportedly, the microwave dielectric properties are related to the composition of the material. The proposed composite (x = 0.9) exhibits a satisfactory combination of er = 8.5, Quxfr = 6291 GHz, and tf = −47 ppm∙˚C −1. With compositional adjustments, the operational and thermal stability of this system can be optimized, fully enabling its application in devices operating at microwave bands. Theoretically, the fitting model (Equations (2)-(3)) provides that the composition with x = 0.72 would have the temperature coefficient near zero.
Acknowledgements
This work was jointly supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) under project No. 407725/2013-0 (Edital MCTI/CNPq/CT-Energ No. 49/2013) grant No. 385487/2013-4, Coordenação de Aperfeioamento de Pessoal de Nível Superior (CAPES), and Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico (FUNCAP), Brazilianfunding agencies. The XRD measurements were performed in the Laboratório multiusuário de caracterização de nanopartículas por difração de raios-X atthe Universidade Federal do Ceará undergrant CNPq 402561/2007-4 (Edital MCT/CNPq No. 10/2007).
Cite this paper
Francisco Nivaldo Aguiar Freire,Manoel Roberval Pimentel Santos,Hélio Henrique Barbosa Rocha,Ana Fabiola Leite Almeida,Selma Elaine Mazzetto,Antonio Jefferson Mangueira Sales,Antonio Sergio Bezerra Sombra, (2016) The Thermal Stability of (CaTiO3)1-x (Cr3/4Fe5/4O3)x Ceramic Composites in the Microwave Region. Materials Sciences and Applications,07,202-209. doi: 10.4236/msa.2016.74020
References
- 1. Plourde, J.K. and Ren, C.-L. (1981) Application of Dielectric Resonators in Microwave Components. IEEE Transactions on Microwave Theory and Techniques, 29, 754-770. http://dx.doi.org/10.1109/tmtt.1981.1130444
- 2. Wersing, W. (1996) Microwave Ceramics for Resonators and Filters. Current Opinion in Solid State and Materials Science, 1, 715-731. http://dx.doi.org/10.1016/s1359-0286(96)80056-8
- 3. Reaney, I.M. and Iddles, D. (2006) Microwave Dielectric Ceramics for Resonators and Filters in Mobile Phone Networks. Journal of the American Ceramic Society, 89, 2063-2072. http://dx.doi.org/10.1111/j.1551-2916.2006.01025.x
- 4. Zhao, F., Yue, Z.X., Zhang, Y.C., Gui, Z.L. and Li, L.T. (2005) Microstructure and Microwave Dielectric Properties of Ca[Ti1-x(Mg1/3Nb2/3)x]O3 Ceramics. Journal of the European Ceramic Society, 25, 3347-3352. http://dx.doi.org/10.1016/j.jeurceramsoc.2004.07.036
- 5. Rocha, H.H.B., Freire, F.N.A., Costa, R.C.S., Sohn, R.S.T.M., Orjubin, G., Junqueira, C.C.M., Cordaro, T. and Sombra, A.S.B. (2007) Dielectric Resonator Antenna: Operation of the Magnetodielectric Composites Cr0.75Fe1.25O3 (CRFO)/Fe0.5Cu0.75Ti0.75O3 (FCTO). Microwave and Optical Technology Letters, 49, 409-413. http://dx.doi.org/10.1002/mop.22160
- 6. Rocha, H.H.B., Freire, F.N.A., Santos, M.R.P., Sasaki, J.M., Cordaro, T. and Sombra, A.S.B. (2008) Radio-Frequency (RF) Studies of the Magneto-Dielectric Composites:Cr0.75Fe1.25O3 (CRFO)-Fe0.5Cu0.75Ti0.75O3 (FCTO). Physica B: Condensed Matter, 403, 2902-2909. http://dx.doi.org/10.1016/j.physb.2008.02.033
- 7. Rocha, H.H.B., Freire, F.N.A., Silva, R.R., Gouveia, D.X., Sasaki, J.M., Santos, M.R.P., Goes, J.C. and Sombra, A.S.B. (2009) Structural Properties Study of the Magneto-Dielectric Composite: Cr0.75Fe1.25O3 (CRFO):Fe0.5Cu0.75Ti0.75O3 (FCTO). Journal of Alloys and Compounds, 481, 438-445. http://dx.doi.org/10.1016/j.jallcom.2009.03.002
- 8. Rocha, H.H.B., Freire, F.N.A., Sohn, R.S.T.M., Silva, M.G., Santos, M.R.P., Junqueira, C.C.M., Cordaro, T. and Sombra, A.S.B. (2008) Bandwidth Enhancement of Stacked Dielectric Resonator Antennas Excited by a Coaxial Probe: An Experimental and Numerical Investigation. IET Microwaves, Antennas & Propagation, 2, 580-587. http://dx.doi.org/10.1049/iet-map:20070292
- 9. Costa, R.C.S., Costa, A.B., Freire, F.N.A., Santos, M.R.P., Almeida, J.S., Sohn, R.S.T.M., Sasaki, J.M. and Sombra, A.S.B. (2009) Structural Properties of CaTi1-x(Nb2/3Li2/3)xO3-δ(CNLTO) and CaTi1-x(Nb1/2Ln1/2)xO3 (Ln=Fe (CNFTO), Bi (CNBTO)), Modified Dielectric Ceramics for Microwave Applications. Physica B: Condensed Matter, 404, 1409- 1414. http://dx.doi.org/10.1016/j.physb.2008.12.037
- 10. Rietveld, H.M. (1967) Line Profiles of Neutron Powder-Diffraction Peaks for Structure Refinement. Acta Crystallographica, 22, 151-152. http://dx.doi.org/10.1107/s0365110x67000234
- 11. Bleicher, L., Sasaki, J.M. and Santos, C.O.P. (2000) Development of a Graphical Interface for the Rietveld Refinement Program DBWS. Journal of Applied Crystallography, 33, 1189. http://dx.doi.org/10.1107/S0021889800005410
- 12. Young, R.A., Sakthivel, A., Moss, T.S. and Paiva-Santos, C.O. (1995) DBWS-9411—An Upgrade of the DBWS*.* Programs for Rietveld Refinement with PC and Mainframe Computers. Journal of Applied Crystallography, 28, 366- 367. http://dx.doi.org/10.1107/S0021889895002160
- 13. Hakki, B.W. and Coleman, P.D. (1960) A Dielectric Resonator Method of Measuring Inductive Capacities in the Millimeter Range. IRE Transactions on Microwave Theory and Techniques, 8, 402-410. http://dx.doi.org/10.1109/TMTT.1960.1124749
- 14. Courtney, W.E. (1970) Analysis and Evaluation of a Method of Measuring the Complex Permittivity and Permeability Microwave Insulators. IEEE Transactions on Microwave Theory and Techniques, 18, 476-485. http://dx.doi.org/10.1109/TMTT.1970.1127271
- 15. Kobayashi, Y. and Tanaka, S. (1980) Resonant Modes of a Dielectric Rod Resonator Short-Circuited at Both Ends by Parallel Conducting Plates. IEEE Transactions on Microwave Theory and Techniques, 28, 1077-1085. http://dx.doi.org/10.1109/TMTT.1980.1130228
- 16. Chen, L., Ong, C., Neo, C., Varadan, V. and Varadan, V. (2004) Microwave Electronics: Measurement and Materials Characterization. Wiley, New York. http://dx.doi.org/10.1002/0470020466
- 17. Castro, P.J. and Nono, M.C.A. (1999) Microwave Properties of Barium Nanotitanate Dielectric Resonators. Journal of Microwaves and Optoelectronics, 1, 12-19.
- 18. Belsky, A., Hellenbrandt, M., Karen, V.L. and Luksch, P. (2002) New Developments in the Inorganic Crystal Structure Database (ICSD): Accessibility in Support of Materials Research and Design. Acta Crystallographica, Section B: Structural Science, Crystal Engineering and Materials, 58, 364-369. http://dx.doi.org/10.1107/S0108768102006948
- 19. Chakhmouradian, A.R. and Mitchell, R.H. (1998) A Structural Study of the Perovskite Series CaTi1−2xFexNbxO3. Journal of Solid State Chemistry, 138, 272-277. http://dx.doi.org/10.1006/jssc.1998.7803
- 20. Durbin, J. and Watson, G.S. (1971) Testing for Serial Correlation in Least Squares Regression. III. Biometrika, 58, 1-19. http://dx.doi.org/10.2307/2334313
- 21. Kim, K.H., Uehara, M., Hess, C., Sharma, P.A. and Cheong, S.-W. (2000) Thermal and Electronic Transport Properties and Two-Phase Mixtures in La5/8−xPrxCa3/8MnO3. Physical Review Letters, 84, 2961-2964. http://dx.doi.org/10.1103/PhysRevLett.84.2961
- 22. Nakamoto, K. (1963) Infrared Spectra of Inorganic and Coordination Compounds. 4th Edition, Wiley, New York.
- 23. Jacob, K.S., Satheesh, R. and Ratheesh, R. (2009) Preparation and Microwave Characterization of BaNd2−xSmxTi4O12 (0≤x≤2) Ceramics and Their Effect on the Temperature Coefficient of Dielectric Constant in Polytetrafluoroethylene Composites. Materials Research Bulletin, 44, 2022-2026. http://dx.doi.org/10.1016/j.materresbull.2009.06.001




