Materials Sciences and Applications
Vol.4 No.12A(2013), Article ID:41286,8 pages DOI:10.4236/msa.2013.412A003
Effect of Corrosion Inhibitors in Limestone Cement
![]()
Department of Materials Science and Engineering, School of Chemical Engineering, National Technical University of Athens, Athens, Greece.
Email: ezaxaropoulou3@yahoo.gr, aggeliki.zaxaropoulou@gmail.com, atteyeh@gmail.com, gbatis@central.ntua.gr, stsiv@central.ntua.gr,
Copyright © 2013 Evgenia Zacharopoulou et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received October 14th, 2013; revised November 17th, 2013; accepted December 9th, 2013
Keywords: Concrete; Corrosion of Rebar; Limestone Cement
ABSTRACT
In this paper examines the improving durability of different limestone cement and effects of the use of corrosion inhibitor. The target is to experimentally investigate the effect of different types of cement in corrosion of reinforcement in presents of corrosion inhibitors and without it. Three types of cement have been used: CEM II, LC1 and LC2. For this purpose constructed mortar specimens, containing 4 reinforcements, with or without corrosion inhibitors for each group, these exhibited to partial immersion in sodium chloride in 3.5% w.t NaCl solution. The methods, with which the corrosion of reinforcement in concrete was tested, were measurements of corrosion potential, corrosion current and mass loss of reinforcement. The mortars with CEM II cement have better durability than that with limestone cement. The use of VpCI, Cyclohexylammonium benzoate, improves the corrosion protection of mortars with CEM II cement upper 50%. On the other hand, the addition of VpCI, Cyclohexylammonium benzoate, improves the corrosion protection of mortars with limestone cement 30% or lower.
1. Introduction
The cement industry continues to introduce more sustainable practices and products for constructing and maintaining our concrete infrastructure and buildings. That sustainable development focus, proposed implementation of more restrictive environmental regulations on cement manufacturing, and a legislation of potential global climate change has prompted the US cement industry to propose provisions for Portland-limestone cements within specifications ASTM C595 [1] and AASHTO M240 [2]. Portland-limestone cements are in common use around the world.
Limestone that is provided from the European standard EN 197-1 (CEN 2000) allows cements to contain limestone in three different dosage levels. CEM I, “Portland cement”, may contain up to 5% minor additional constituents, of which limestone is one possible material. CEM II/A-L and CEM II/B-L, both called “Portland limestone cement”, contain 6% to 20% and 21% to 35% ground limestone, respectively. Roughly 19% of all cement sold in Europe contains between 6% and 35% limestone [3]. The requirements were specified for limestone use pertaining to effects on performance only.
In most early research it was believed that limestone acted as inert filler; however more recent researches have shown that limestone participates to some extent in hydration reactions. In addition, fine limestone particles may promote silicate hydration by providing nucleation sites for C-S-H precipitation.
Calcium carbonate has been reported to react with the tricalcium aluminate to form high and low forms of carboaluminates [4].
Tsivilis [5] found that the addition of limestone as an intergrinding material increased the reactivity of the clinker. Campiteli and Florindo [6] found that the addition of limestone decreased the optimum SO3 content. Production of CH appears to increase at early ages, which was attributed in part to the dissolution of limestone and in part to the role of the limestone in acting as a nucleation site [7].
Permeability is the key to the durability of a porous material in all but the most protected environments. With the exceptions of abrasion and erosion, deterioration mechanisms involve the ingress of water and/or other harmful species (oxygen, carbon dioxide, chlorine ions, sulfate ions, acids, etc.). Corrosion requires water and oxygen, and is catalyzed by chlorine ions.
In a related study, Tsivilis [8] produced concretes with five cements with limestone contents ranging from 0% to 35%, and conducted the “Rapid Chloride Permeability Test” (RCPT) (ASTM C1202) after 28 days of moist curing. Table 1 shows details of the cements and concrete together with the results of the RCPT. Addition of lime over 10% increases the value of RCPT. The results show little impact due to the increase of limestone content up to 15 % to 20%. The mix with 35% limestone had a higher RCPT value despite being cast with a lower w/cm, indicating that permeability increased at this level of limestone [5].
This paper examines the improving durability of two different limestone cements compared with CEM II cement and the protective effects of the use of corrosion inhibitor. The methods, with which was tested the corrosion of reinforcement in concrete, were: measurements of corrosion potential, corrosion current and electrochemical calculated mass loss of reinforcement.
2. Analytical Investigation
2.1. Materials
2.1.1. Cements
In the present experiment 3 type of cement were used CEMII, LC1 and LC2. Portland limestone cements, contain 15%, 35% w/w limestone, were produced by intergrinding of clinker, limestone and gypsum in a pilot plant ball mill of 5 kg capacity. Preliminary tests with varying grinding time have been done in order to produce cements of appropriate compressive strength. The cements, LC1 and LC2, contain 15%, 35% limestone, respectively. The composition of the cement, as well as their 28 days compressive strength and specific surface are shown in Table 1 [9].
The chemical and mineralogical composition of clinker is shown in Table 2.
The chemical composition of the CEMII cement is shown in Table 3 [10].
Portland cement clinker of industrial origin and limestone of high calcite content (CaCO3: 97.5%) were used. The chemical composition of the above materials is pre-

Table 1. Characteristic of the LC1 and LC2 cements.

Table 2. Chemical and mineralogical composition of clinker.
sent in Tables 3 and 4 respectively. The used clinker has a moderate C3A.
2.1.2. Aggregates
The use of aggregates should be according to EN12620 for normal and heavyweight aggregates and according to EN13055-1 for lightweight aggregates. In this sand have been used aggregate, which confirm all required terms and conditions.
2.1.3. Reinforcement
Concrete reinforcement steel should be protected against corrosion, both before it is incorporated into concrete, and after. During its placement into the final position, steel should be relieved of all visible scaling alterations, or unwanted deformations and damage, which, decides other things, speed up the effects of corrosion.
In this experiment we use steels type of B500C ELOT. The marking for identifying the quality by a grad of concrete reinforcement steel is done with a different configuration of the transverse ribs on the surface of the bar.
Steels with ribs are characterized by their surface geometry, which dictates their adhesion to the concrete. Concrete reinforcement steels with ribs have at least two rows of parallel transverse ribs uniformly distributed on each side of the steels surface and at equal distance throughout both rows. Longitudinal ribs may be added, but are not mandatory.
The tensile strength limits that the mechanical properties of concrete reinforcement steels must meet are given in Table 5.
The values of yield strength fy and fi are calculated according to the nominal cross-section.
The chemical composition and production methods are display in Table 6.

Table 3. Chemical composition of CEMII cement.

Table 4. Chemical analysis of limestone (% w/w).
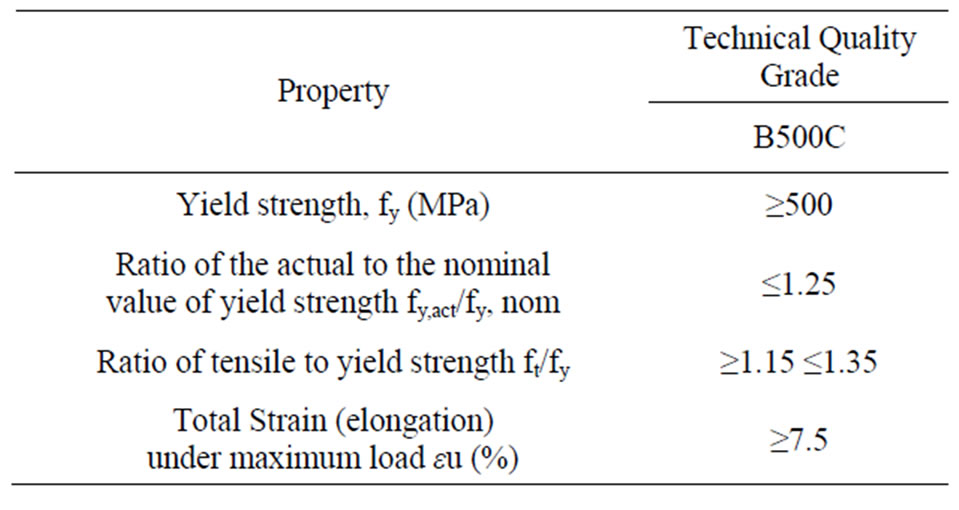
Table 5. The tensile strength limits of the mechanical properties of steel according to ELOT 1421-2 and ELOT 1421-3 (typical values).
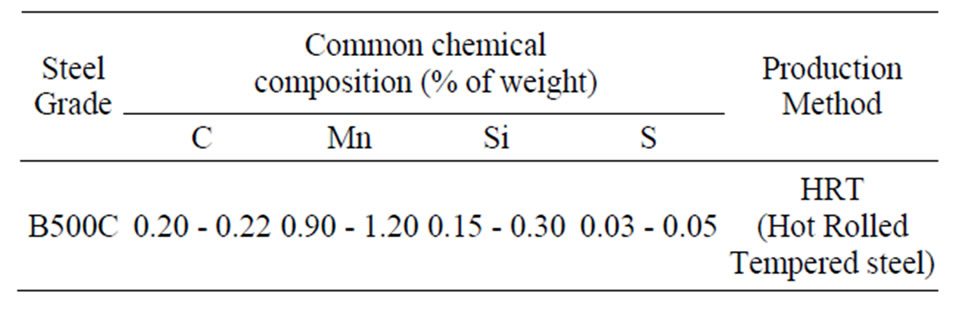
Table 6. Chemical composition, production methods.
2.1.4. Corrosion Inhibitor
The corrosion inhibitor used is Cyclohexylammonium benzoate, as admixtures. Cyclohexylammonium benzoate is a VpCI (Vapor Corrosion inhibitor) volatile low pressure corrosion inhibitor and multifunctional corrosion inhibitor. Protects rebars electrochemically through anodic or cathodic reaction as contact inhibition and diffusion of molecules (potential migration molecules). The nature of the product is to exploit whatever substrate absorption, creating diffusion through the porous concrete, approaching the reinforcing steel (structural steel elements) in order to protect them from further corrosion, extending both the length and the operating limits of construction. The addition of VpCI is 0.4/100g cement. The structural formula Cyclohexylammonium benzoate is the following [11]:

Further technical characteristics and physical properties are presented in Table 7.
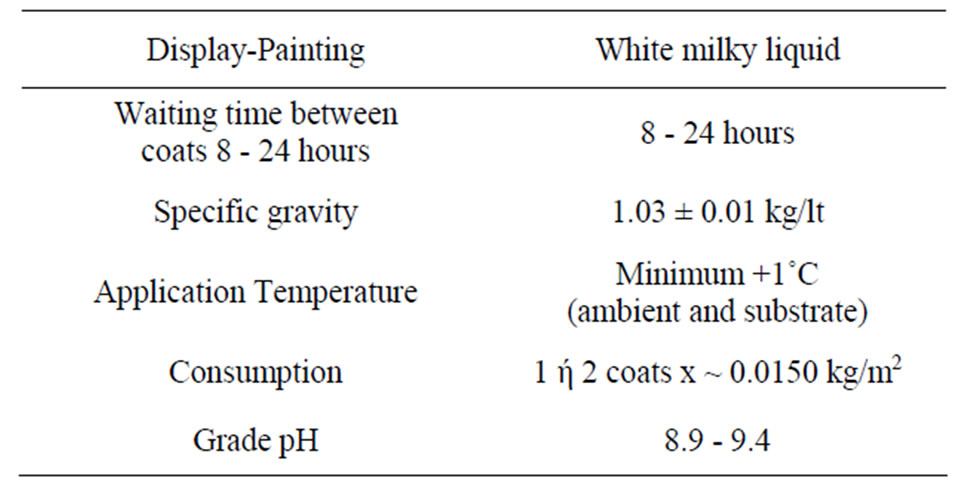
Table 7. Technical characteristics and physical properties of the corrosion inhibitor.
2.2. Methods
As part of the experimental work, electrochemical studies as monitoring the corrosion potentials, linear polarization and mass loss of rebars were carried out as described below.
2.2.1. Monitoring the Corrosion Potentials
The corrosion trend of the samples was estimated by monitoring the corrosion potential versus exposure time. The corrosion potential of steels was measured according to ASTM C876-87, using a Ag/AgCl/KCl (3M) electrode as a reference electrode which was placed in 3.5% NaCl solution. A voltmeter is also needed.
As it’s referred in ASTM C 876-87:
1) Potentials less negative than −0.2 volts generally indicate 90% higher probability of no corrosion taking place at the time of measurement.
2) Potentials in the middle of −0.2 to 0.35 volts are inconclusive.
3) Potentials greater than −0.35 volts generally indicated, 90% or higher probability of active corrosion in the area at the time of testing.
4) Positive potentials, if obtained, generally indicate insufficient moisture in the concrete and should not be considered valid. However, stray DC currents may also cause potential measurements and therefore careful review analysis of the obtained data is required.
2.2.2. Linear Polarization
The linear polarization technique requires us to polarize the steel with an electric current and monitor its effect on the half cell potential. It is carried out with a sophisticated development of the half cell incorporating an auxiliary electrode and a variable low voltage DC power supply. The half cell potential is measured and then a small current is passed from the auxiliary electrode to the reinforcement. The change in the half cell potential is simply related to the corrosion current by the equation:

where B is a constant (in concrete 26 to 52 mV) and it depend on type of cement, water cement ratio and the passivity or active condition of the steel. For steel in passivity condition B = 52 mV and for steel in active condition B = 26 mV. In this present thesis, assuming that steel was in active condition, B = 26 mV. Rp is the polarization resistance (in ohms):

Rp gives the technique its alternative name of polarization resistance. The change in potential must be kept to less than 20 mV or so for the equation to be valid and remain linear.
The change in potential must be kept to less than 20 mV or so for the equation to be valid and remain linear. The “iR drop” must also be removed. This is the voltage that exists because a current is flowing through concrete that has an electrical resistance. This is also referred to as the solution resistance. This means that the current is usually switched off during the measurement process so that the potential without the iR drop is measured [12]. Corrosion is an electrochemical process whereby the amount of corrosion is related to the electrical energy consumed, which is a function of voltage, amperage, and time interval. The amount of corrosion can be estimated using an equation based on Faraday’s low:
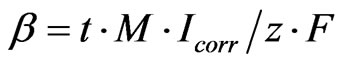
where: t is time (sec), Icorr is corrosion current (Amperes), M is atomic weight of iron (55.847 g/mol), z is in charge (assumed 2 for Fe→Fe2++2e−) and F is Faraday’s constant (96.487 Amp.sec).
3. Experimental Procedure
During this project all Reinforcements were cleaned by immersing in acetone in order to removes grease and oils, immersing in solution HCl and corrosion inhibitors to remove rust from steel. Steel bars were sunk in deionized water, in Acetone and alcohol. Few seconds later they were weighted.
The specimens were prepared using three different cement types CEMII, LC1, LC2. In all specimens the aggregate used was the same. Reinforcing steel bars of steel type B500C and tap water were used. Mix proportions aggregate/water/cement was kept constant and equal to 3/1/0.5. Each specimen was cast into a prismatic mould (80 × 80 × 100 mm), where four identical steel bars (100 × 12 mm) was embedded in position shown in Figure 1.
Specimens were stored at ambient condition for 48 h, then cure in tap water for 7 days and then the part shown in Figure 1 was insulated with epoxy glue. Finally, all specimens were partially immersed up to 2/3 of their height in 3.5% NaCl.
4. Measurements
4.1. Measurement of Corrosion Potential
Corrosion potentials were measured for a period of 305 days for specimens. Measurement of corrosion potentials for specimens started after passing 12 days from immersing in 3.5% NaCl. The measurements of corrosion potential for CEM II, LC1 and LC2 without corrosion inhibitor are shown in Figure 2.
Figure 2 shows the comparison of corrosion potential for CEM II, LC1 and LC2 without corrosion inhibitor. It is noticed that CEM II without corrosion inhibitor has better behavior than LC2 without corrosion inhibitor and LC2 without corrosion inhibitor has better behavior than LC1 without corrosion inhibitor.
The measurements of corrosion potential for CEM II, LC1 and LC2 with corrosion inhibitor are shown in Figure 3.
Figure 3 shows the comparison of corrosion potential for CEM II, LC 1 and LC 2 with corrosion inhibitor. It is noticed that CEM II with corrosion inhibitor has better behavior than LC2 with corrosion inhibitor and LC2 with corrosion inhibitor has better behavior than LC1 with corrosion inhibitor.
4.2. Electrochemical Calculated Mass Loss
The corrosion rate of the bars was determined by meas-
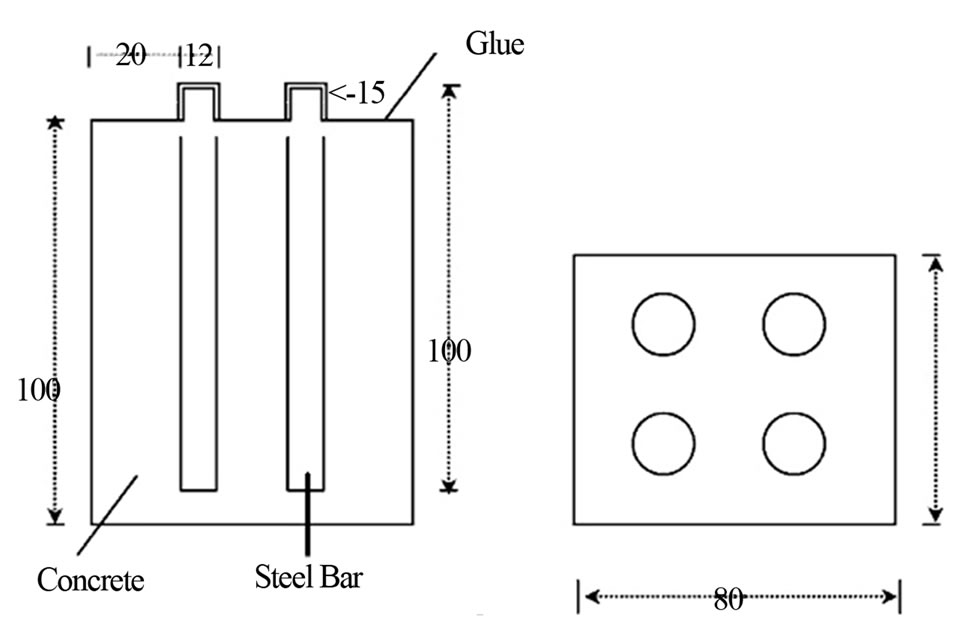
Figure 1. Schematic representation and dimensions (mm) of specimens.

Figure 2. Figure of corrosion potentials for CEM II, LC1 and LC2 without corrosion inhibitor.
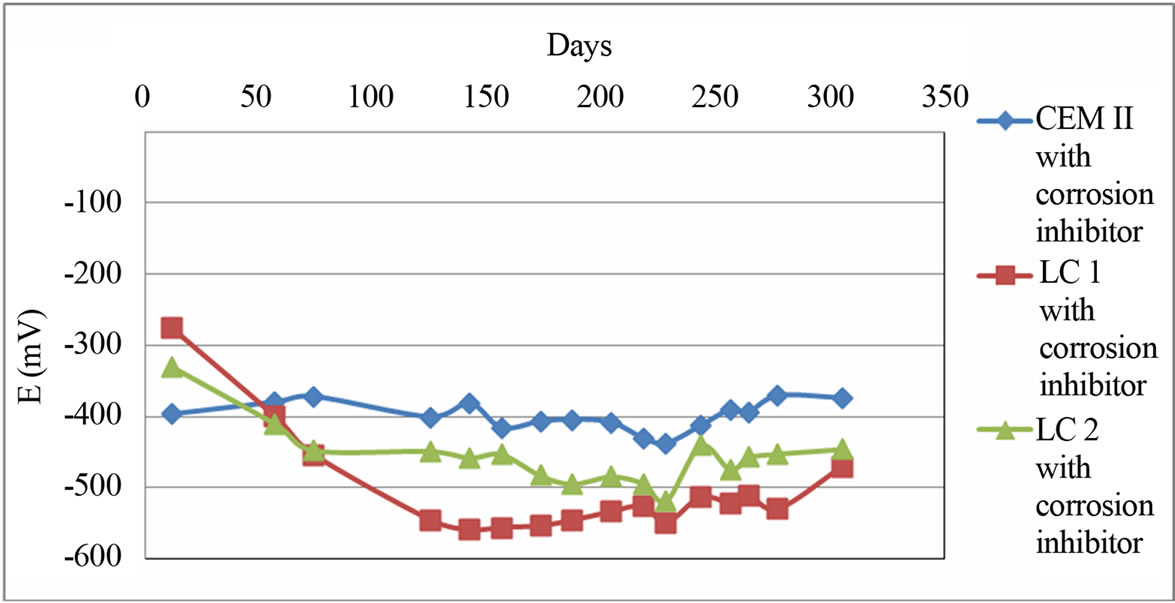
Figure 3. Figure of corrosion potentials for CEM II, LC1 and LC2 with corrosion inhibitor.
uring their mass losses at predetermined exposure time intervals in the corrosive environments. In order to find the mass loss the polarization resistance (Rp) was measured and corrosion current (Icorr) and mass loss were calculated.
The measurements of mass loss for CEM II, LC1, LC2 without corrosion inhibitor are shown in Figure 4.
From Figure 4 is obvious that the durability of mortars with CEM II cement is better than LC1 and LC2.
The measurements of mass loss for CEM II with and without corrosion inhibitor are shown in Figure 5.
Figure 5 shows the comparison of mass loss of CEM II with and without corrosion inhibitor. It is noticed that the CEM II without corrosion inhibitor appears greater mass loss than the CEM II with corrosion inhibitor. It is obvious that the corrosion inhibitor protects the rebars from the corrosion.
The measurements of mass loss for LC1 with and without corrosion inhibitor are shown in Figure 6. It is known that the concrete or mortars with limestone cement have lower durability as concrete or mortars with CEM I and CEM II cement [13].
Figure 6 shows the comparison of mass loss of LC1 with and without corrosion inhibitor. It is important to mention that the mass loss of LC1 with and without corrosion inhibitor is approximately the same. From the measurements of mass loss of LC1 with and without corrosion inhibitor, there is no significant difference.
The measurements of mass loss for LC2 with and without corrosion inhibitor are shown in Figure 7.
Figure 7 shows the comparison of mass loss of LC2 with and without corrosion inhibitor. It is noticed that the LC2 without corrosion inhibitor appears greater mass loss than the CEM II with corrosion inhibitor. It is obvious that the corrosion inhibitor protects the rebars from the corrosion.
The measurements of mass loss for CEM II, LC1, LC2 with corrosion inhibitor are shown in Figure 8.
Figure 8 show that the protective action of corrosion inhibitor is greater in mortars with CEM II cement.
5. Discussion
It is known that the concrete or mortars with limestone cement have lower durability as concrete or mortars with CEM I and CEM II cement [13]. It is also known that the addition of corrosion inhibitors such as calcium nitrite, N-N’-dimethylaminoethanol increases the protection of rebars of mortars or concrete from corrosion in chloride environment [6,10]. In this study is made an effort to promote the protection of rebars, of mortars with lime-

Figure 4. Figure of (electrochemical calculated) mass loss for CEM II, LC1, LC2 without corrosion inhibitor.
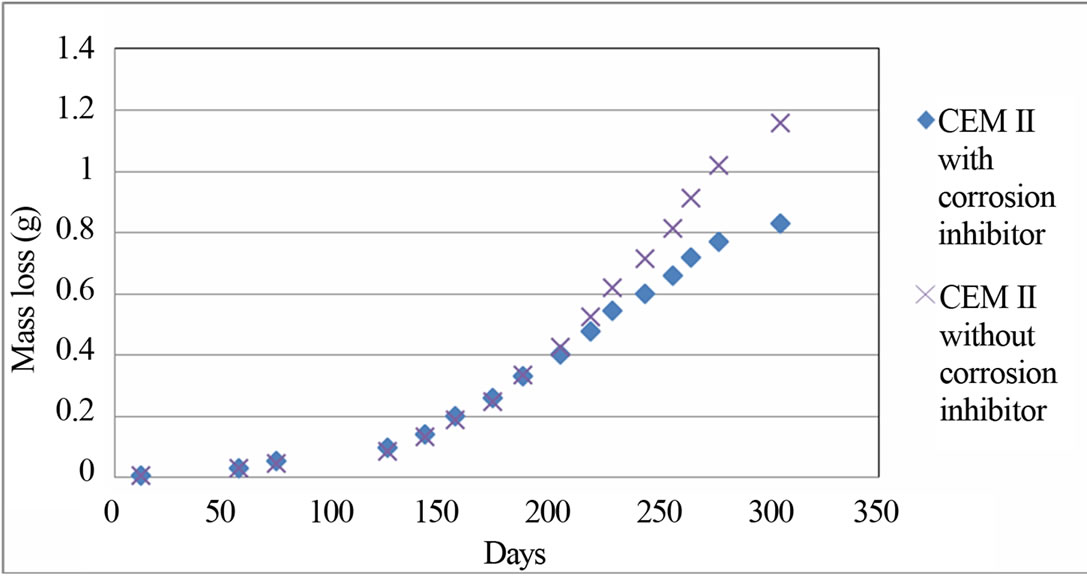
Figure 5. Figure of (electrochemical calculated) mass loss for CEM II with and without corrosion inhibitor.
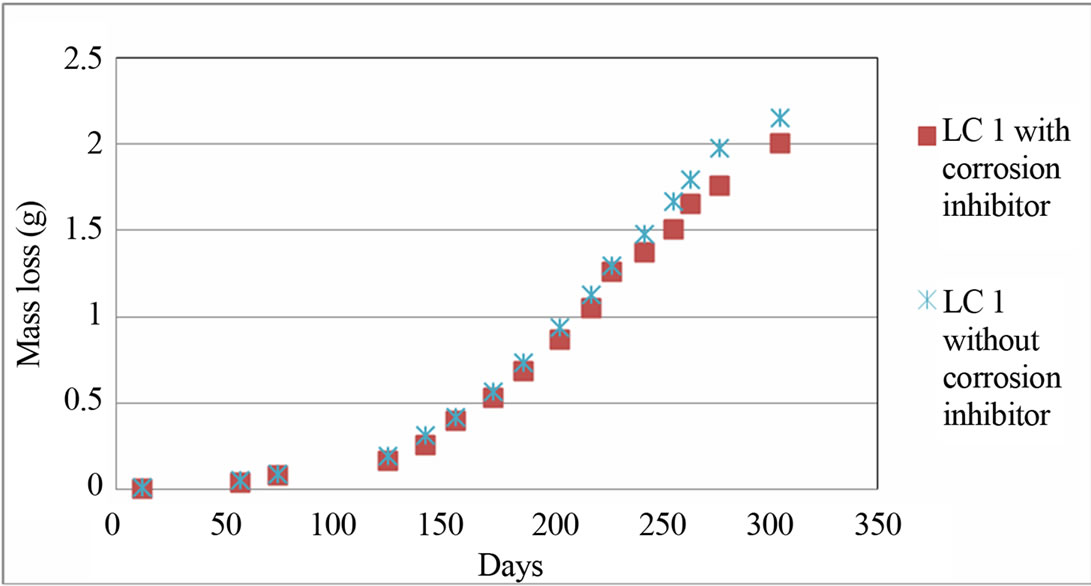
Figure 6. Diagram of (electrochemical calculated) mass loss for LC1 with and without corrosion inhibitor.

Figure 7. Diagram of (electrochemical calculated) mass loss for LC2 with and without corrosion inhibitor.

Figure 8. Figure of (electrochemical calculated) mass loss for CEM II, LC1, LC2 with corrosion inhibitor.
stone cement, with VpCI Cyclohexylammonium benzoate in chloride contaminated environment.
From the half-cell potential of rebars versus time (see Figures 2 and 3), it is no possible to establish a protective action of corrosion inhibitor. From the electrochemical calculated mass loss (see Figures 4-6) the VpCI Cyclohexylammonium benzoate has a protective action to corrosion of rebars after 200 days.
The addition of VpCI in mortar with CM II cement has corrosion protective effect about 52% (calculated for 300 days). On the same time, the LC2 mortar with limestone cement has corrosion protective effect of 30%. For the LC1 the protective effect is lower.
The corrosion protective effect of mortar with CEM II cement is compared with the general opinion that the corrosion inhibitors duplicate the technical service life of concrete [4,9,14]. On the other hand, the corrosion protective effect of mortars with limestone cement has lower protective effect. It is possible that the VpCI Cyclohexylammonium benzoate cannot be used in mortar and concrete with limestone cement.
The degreasing of corrosion protective effect, it is possible to explain through the observation, that the use of concrete with limestone cement is entrapped in large air voids that concrete with CEM I and II cement [12,15]. In this case the simultaneously use of VpCI and air-entraining can be improved the percentage of VpCI corrosion protection.
6. Results
Based on the results of this experimental investigation under corrosive environment, the following conclusions are drawn:
• The mortars with CEM II cement have better durability than the limestone cements.
• The use of VpCI Cyclohexylammonium benzoate improves the corrosion protection of mortars with CEM II cement 52% (calculated for 300 days).
• The use of VpCI Cyclohexylammonium benzoate improves the corrosion protection of mortars with limestone cement LC2 (35% limestone) 30%.
• The use of VpCI Cyclohexylammonium benzoate improves the corrosion protection of mortars with limestone cement LC1 (15% limestone) only 10%.
REFERENCES
- ASTM C595, “Standard Specification for Blended Cements.”
- AASHTO M240, “Standard Specification for Blended Cement.”
- V. C. Campiteli and M. C. Florindo, “The Influence of Limestone Additions on Optimum Sulfur Trioxide Content in Portland Cements,” In: P. Klieger and R. D Hooton, Eds. Carbonate Additions to Cement, ASTM STP 1064, American Society for Testing and Materials, Philadelphia, 1990, pp. 30-40.
- D. K. Yfantis, “Materials—Corrosion and Protection,” Edition NTUA, Athens, 2000.
- K. Sotiriadis, E. Nikolipoulou and S. Tsivilis, “Sulfate Resistance of Limestone Cement Concrete Exposed to Combined Chloride and Sulfate Environment at Low Temperature,” Cement and Concrete Composites, Vol. 34, No. 8, 2012, pp. 903-910.
- G. Batis, K. K. Sideris and P. Pantazopoulou, “Influence of Calcium Nitrite Inhibitor on the Durability of Mortars under Contaminated Chloride and Sulphate Environments,” Anti-Corrosion Methods and Materials, Vol. 51 No. 2, 2004, pp. 112-120. http://dx.doi.org/10.1108/00035590410523201
- S. Tsivilis, E. Chaniotakis, G. Kakali and G. Batis, “An Analysis of the Properties of Portland Limestone Cements and Concrete,” Cement and Concrete Composites, Vol. 24, No. 3, 2002, pp. 371-378.
- S. Tsivilis, G. Batis, E. Chaniotakis, G. Grigoriadis and D. Theodossis, “Properties and Behavior of Limestone Cement Concrete and Mortar,” Cement and Concrete Research, Vol. 30, No. 10, 2000, pp. 1679-1683. http://dx.doi.org/10.1016/S0008-8846(00)00372-0
- M. C. Brown, “Assessment of Commercial Corrosion Inhibiting Admixtures for Reinforced Concrete,” Virginia Polytechnic Institute, Blacksburg, 1999.
- G. Batis, N. Kouloumbi and P. Pantazopoulou, “Protection of Reinforced Concrete by Coatings and Corrosion Inhibitors,” Pigment and Resin Technology, Vol. 29, No. 3, 2000, pp. 159-163. http://dx.doi.org/10.1108/03699420010334312
- “Carbonation of Concrete,” Concrete Experts International, Denmark, 2002.
- P. Turker and K. Erdoğdu, “Effects of Limestone Addition on Microstructure and Hydration of Cements,” Proceedings of the Twenty Second International Conference on Cement Microscopy, Montreal, 29 April-4 May 2000.
- R. D. Hooton, M. Nokken and M. D. A. T. Thomas, “Portland-Limestone Cement: State-of-the-Art Report and Gap Analysis for CSA A 3000,” Report SN3053, Cement Association of Canada, Toronto, 2007, 60 p. http://www.cement.org/bookstore/results_quicksearch.asp?store=main&id=&cat2ID=3& searchterm=SN3053
- B. P. John, “Corrosion of Steel in Concrete,” E&FN Spon, London, 1997. http://dx.doi.org/10.4324/9780203414606
- S. Tsivilis, E. Chaniotakis, G. Batis, C. Meletiou, V. Kasselouri, G. Kakali, A. Sakellariou, G. Pavlakis and C. Psimadas, “The Effect of Clinker and Lime Stone Quality on the Gas Permeability, Water Absorption and Pore Structure of Limestone Cement Concrete,” Cement and Concrete Composites, Vol. 21, No. 2, 1999, pp. 139-146.

