Materials Sciences and Applications
Vol.4 No.7A2(2013), Article ID:34841,8 pages DOI:10.4236/msa.2013.47A2003
Osmium-Polymer Modified Carbon Nanotube Paste Electrode for Detection of Sucrose and Fructose
![]()
1Department of Chemistry and Drug Technologies, Sapienza University of Rome, Rome, Italy; 2Department of Chemistry, Universidad de Santiago de Chile, Santiago, Chile.
Email: *riccarda.antiochia@uniroma1.it
Copyright © 2013 Riccarda Antiochia et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received May 14th, 2013; revised June 15th, 2013; accepted June 27th, 2013
Keywords: Osmium Redox Polymer; Electrode Surface Modification; Carbon Nanotube Paste Electrode; Sucrose; Fructose
ABSTRACT
The aim of the work was the modification of a carbon nanotube paste electrode with a highly original osmium-polymer hydrogel for the development of a new amperometric biosensor for detection of sucrose and fructose. The biosensor for sucrose is based on the activity of the enzymes invertase and fructose dehydrogenase (FDH) immobilized into a carbon nanotube paste (CNTP) electrode properly modified with the Os-polymer. A second biosensor, for fructose only, is constructed containing inactive invertase and used for detection of fructose and for signal subtraction. The biosensors exhibit a detection limit for sucrose of 2 mM and for fructose of 1 mM, linearity up to 5 mM for both biosensors, high sensitivity (1.98 mA·cm−2·mM for sucrose and 1.95 mA·cm−2·mM for fructose), a good reproducibility (RSD = 2.5% for sucrose and 2.1% for fructose), fast response time (8 s for sucrose and 4 s for fructose) and a stability of about 4 months for both biosensors when stored under wet conditions at 4˚C. Finally, the biosensors were applied for specific determination of sucrose and fructose in several commercial fruit juice samples and validated with a commercial spectrophotometric enzymatic kit.
1. Introduction
Chemically modified electrodes (CMEs) are electrodes prepared by surface modification to produce an electrode suited for a particular function. These electrodes have a molecular monolayer coated on the electrode surface which confers to the electrodes new chemical, physical, electrochemical, optical and electrical properties [1]. In their first stages, CMEs were merely applied for electrochemical investigations but they also provide a route for other purposes such as chemical sensing, energy conversion, storing and protecting corrosion, developing molecular electronics and electrochromic devices [1]. The electron transfer reactions of biological molecules are frequently very slow at ordinary electrodes. To overcome this problem an thus to facilitate the direct coupling of biological redox reactions to electrodes for biosensor applications, different types of modified electrodes have been developed. These include electrodes modified by the covalent attachment of species to the surface, by the reversible adsorption of promotors, or by the deposition of polymeric species and the use of conducting polymers as electrode materials [2].
In recent years, nanotechnology and nanomaterials have been revolutionizing the area of biosensors. In particular carbon nanotubes have begun to attract enormous interest in electrochemistry for biosensor construction because of their small size and their good electrochemical properties [3-5]. Recently, for this reason and for their easy preparation and the possibility of renewal of their surface, carbon nanotube paste (CNTP) electrodes have started to become popular for electrode modification [6-8].
The objective of the present work is to describe the modification of a CNTP electrode with a new osmium redox polymer for the development of an alternative biosensor for detection of sucrose and fructose. The efficient electron shuttling properties of the osmium redox polymer allowed its utilization for electrical wiring of cells and enzymes [9,10] and for biosensor construction [11- 13].
Sucrose and fructose are important sugars contained in many food staffs and sweet drinks. The determination of both sucrose and fructose is therefore extremely important for food and beverage industries. Conventional methods for sugar analysis use techniques such as chromatography, spectrophotometry, electrophoresis and titration [14,15] but these methods do not allow an easy and rapid monitoring because they require expensive instrumentation, well trained operators and often elaborate sample pretreatment with an increasing time of analysis. A great deal of research is therefore needed to develop a simple, fast and sensitive method, which could be effectively used by the food industries. Biosensors offer a promising alternative: besides their good selectivity and low cost, they can be used to develop simple and portable equipment allowing fast in-situ monitoring of raw materials and food processing steps [16-18]. Three enzymes, invertase, mutarotase and glucose oxidase (GOx) are commonly used for amperometric sucrose biosensor development [19,20]. These methods are laborious and time-consuming because of a cascade of three or four enzyme system with consequent problems of reproducibility and stability. We propose a more convenient method which used only two enzymes, invertase and fructose dehydrogenase, immobilized into a carbon nanotube paste (CNTP) electrode modified with a highly original osmium-polymer hydrogel. Fructose dehydrogenase (FDH; E.C. 1.1.99.11), first described by Ameyamaand Adachi [21], catalyses the oxidation of fructose to 5-keto-D-fructose with the concomitant reduction of the bound cofactor flavin adenine dinucleotide (FAD) [22,23], thus representing an ideal enzyme for biosensor construction because no addition of the well-known NAD(P)+/NAD(P)H cofactor is required. Invertase enzyme (E.C. 3.2.1.26) catalyzes the hydrolysis of sucrose to glucose and fructose. The coupling of the two enzymes can be therefore successfully utilized for the construction of the sucrose biosensor.
In our work the osmium redox polymer was used as redox mediator to shuttle the electrons between the immobilized enzyme and the single-walled carbon nanotube paste (SWCNTP) electrode and also as a support for direct wiring of both invertase and FDH into the paste by using poly(ethylene glycol) diglycidyl ether (PEDGE) as a cross-linking agent. As well as optimization studies, application of the proposed biosensor for sucrose and fructose analysis in real samples was carried out and the results obtained were in good agreement with those determined with the standard spectrophotometric method.
2. Experimental
2.1. Chemicals
Invertase (E.C. 3.2.1.26) from baker’s yeast, fructose dehydrogenase (FDH) (E.C. 1.1.1.47) from Gluconobacter sp., sucrose and D-fructose were purchased from Sigma (St. Louis, MO, USA). Poly(ethylene glycol) (400) diglycidyl ether (PEDGE) was obtained from Polyscience (Warrington, PA, USA). Poly(1-vinylimidazole)12- [osmium (4,4’-dimethyl-2,2’-dipyridyl)2Cl2]2+/+ (osmium redox polymer) was generously provided as a gift from There Sense Inc. (Alameda, CA, USA). Single-walled carbon nanotubes Carbolex (diameter 1 - 2 mm) were obtained from Aldrich (Steinheim, Germany). Mineral oil was obtained from Fluka (Buchs, Switzerland). All other chemicals were from Carlo Erba (Milan, Italy). All solutions were prepared with high purity water produced by a Milli-Q System (Millipore, Bedford; MA, USA).
2.2. Construction of the Os-Polymer Modified CNTP Electrode
The CNTP electrodes were prepared by hand-mixing SWCNTs and graphite powder with mineral oil at a 60%:40% ratio (w/w) [3]. The paste was mixed in a mortar and packed into a cavity (3 mm diameter, 0.5 mm depth) at the end of a Teflon tube and electrical contact was established via a copper wire connected to the paste. The electrode surface was gently smoothed by rubbing it on a piece of filter paper before use.
The Os-polymer CNTP electrode was prepared by depositing on the CNTP electrode surface 10 mL of a solution (10 mg/mL) of the Os-polymer in Milli-Q water and 1 mL of an aqueous solution (2.5 mg/mL) of the cross-linker agent PEDGE [11]. After the deposition, the electrodes were left to dry overnight at room temperature.
2.3. Construction of the Modified CNTP Sucrose and Fructose Biosensors
The sucrose biosensor was assembled according to the following procedure: invertase and FDH were directly wired into the Os-polymer hydrogel by thorough mixing of 10 mL of a solution of the Os-polymer (10 mg/ml) in Milli-Q water, 1 mL of an aqueous solution of PEDGE (2.5 mg/mL), 10 mL of a solution of FDH (5 U) and 10 mL of a solution of invertase (5 U). Successively, a 10 mL aliquot of this solution was deposited on the CNTP electrode surface and left to dry overnight at room temperature [11]. The modified electrode was rinsed carefully with 0.1 mol/L phosphate buffer at pH 7.0 before use. A schematic representation of the wiring of invertase and FDH through the Os-polymer redox hydrogel on the CNTP electrode is shown in Figure 1.
A second biosensor, for fructose only, was constructed following the same procedure containing inactive invertase and used also for signal subtraction.
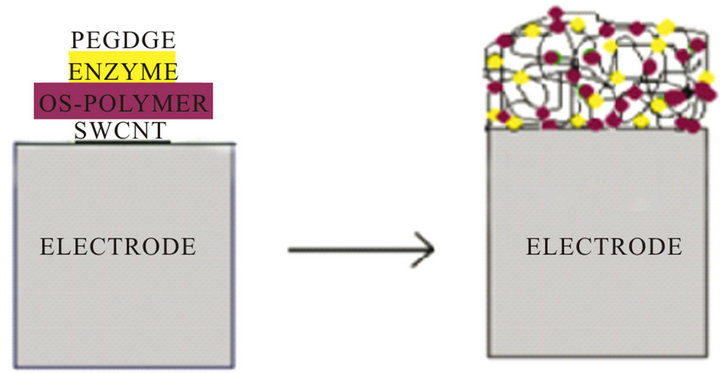
Figure 1. Schematic representation of the inveratse-FDH wiring through redox hydrogel of Os-polymer on CNTP electrode.
2.4. Electrochemical Characterization
Electrochemical measurements were performed using an Autolab electrochemical system equipped with PGSTS-12 with GPES software (Eco Chemie, Utrecht, The Netherlands). All electrochemical experiments were carried out in a conventional three-electrode cell at room temperature with the modified CNTP electrode (3 mm diameter) as working electrode, an Ag/AgCl/KCl (sat) as the reference and a platinum wire as the counter electrode. The electrochemical cell contained 10 mL of 0.1 M phosphate buffer or 0.1 M acetate buffer at various pHs. A fixed potential, +200 mV versus Ag/AgCl, was used to make the amperometric experiments.
2.5. Biosensor Response
For sucrose and fructose determinations aliquots of a stock solution of, respectively, sucrose and fructose in 0.1 M acetate buffer at pH 5.0 were successively added in the electrochemical cell and the steady-state current, proportional to the sucrose concentration, was recorded. The steady-state current was achieved within 20 - 25 s.
2.6. Application in Food Analysis
The sucrose and fructose biosensors were tested for the detection of sucrose and fructose in commercial fruit juices purchased from local supermarkets. Sucrose concentrations in real samples were determined in five replicates on the basis of the calibration curve obtained for the standard sucrose solution. As fructose content present in the samples causes interference with sucrose determination, the fructose content was previously measured with the biosensor with inactive invertase and the current signal was subtracted from the total response of the sucrose biosensor.
The results obtained were compared with those obtained with the enzymatic spectrophotometric assay kit (Mannheim, Germany, Cat. N. 139106). The determination of sucrose is related to the amount of NADPH formed and spectrophotometrically measured at 340 nm.
Fruit juices samples did not require any pretreatment. The beverage samples were analysed by adding 50 mL directly to the electrochemical cell containing 10 mL of buffer.
3. Results and Discussion
The principle of operation of the sucrose biosensor is based on the conversion of sucrose to D-fructose (reaction (1)) and then on the oxidation of D-fructose to 5-keto-D-fructose by FDH with the concomitant reduction of the osmium-polymer mediator (reaction (2)), according to the following reactions:
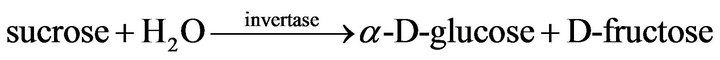 (1)
(1)
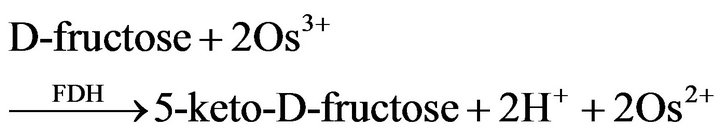 (2)
(2)
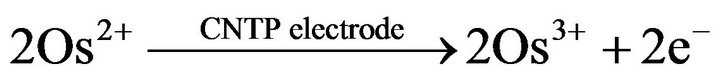 (3)
(3)
reactions (2) and (3) represent the basis of the principle of operation of the fructose biosensor. The reduced mediator is successively reoxidized at the CNTP electrode at a potential of +200 mV (reaction (3)). The current values due to the enzymatic reaction are proportional to the concentration of sucrose in the reaction medium for sucrose concentrations lower than the Michaelis-Menten constant. The simplified reaction sequence of this biosensor given by reactions (2) and (3) is due to the characteristics of FDH enzyme, which contains the FAD cofactor tightly bound to the enzyme and does not require the adding of NAD+ as most other dehydrogenase enzymes thus allowing the construction of oxygen independent reagentless sensors [24]. The structure of the mediator is shown in Figure 2.
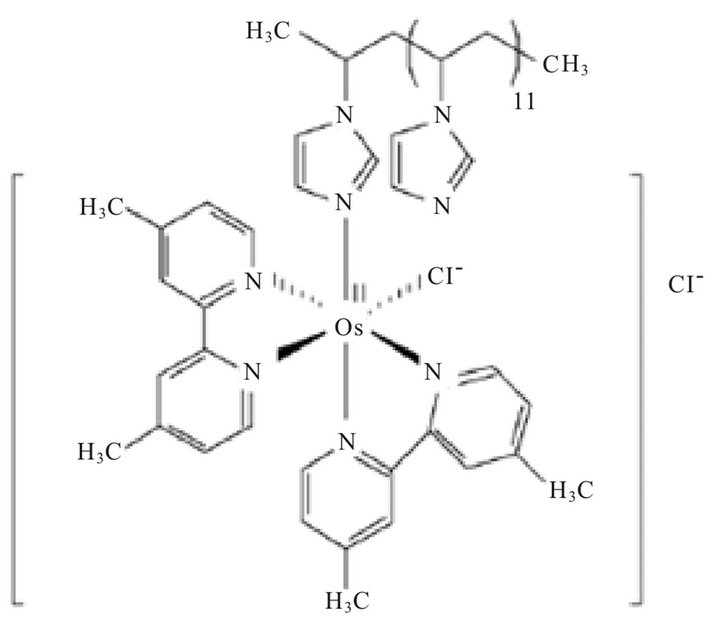
Figure 2. The chemical structure of poly(1-vinylimidazole)12-[osmium(4,4’-dimethyl-2,2’-bipyridyl)2Cl2]2+/+ (osmium redox polymer).
3.1. Electrochemical Characterization of the Invertase/FDH/Os-Polymer/CNTP Electrode
The electrochemical behavior of a similar Os-polymer CNTP electrode was already investigated in our previous work [11]. Figure 3 shows a cyclic voltammogram obtained with the Os-polymer CNTP modified electrode at a scan rate of 10 mV/s in the absence (curve a) and in the presence (curve b) of invertase and FDH enzymes. As expected, a small peak separation typical of surface-bound species is shown by all voltammograms, showing that the presence of invertase and FDH does not influence the electrochemical behaviour of the modified electrode. The formal redox potential of the Os-polymer was found to be of about +180 mV vs. Ag/AgCl, in good agreement with that already reported previously [9-11]. The proper working potential for the biosensor was therefore choosen at +200 mV.
3.2. Sucrose and Fructose Biosensor Response
In order to optimize the sucrose-Os-polymer CNTP biosensor different amounts of the two enzymes were tested. Figure 4 shows the sucrose calibration curves derived from the amperometric response and relative to an Os-polymer CNTP electrode with invertase and FDH directly wired to the Os-polymer hydrogel. The effect of enzyme loading on the current response of the biosensor was investigated in the range from 2 U to 10 U for both enzymes. All curves showed a linear part at the beginning with a deviation from linearity at higher sucrose concentrations. This behaviour is probably due to the
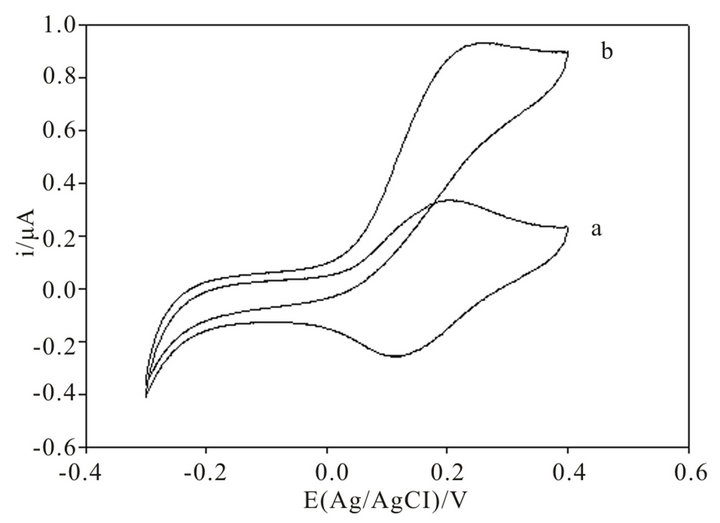
Figure 3. Cyclic voltammograms obtained with an invertase-FDH-Os-polymer modified CNTP electrode in the absence (a) and in the presence of 5 × 10−3 M sucrose concentration with invertase and FDH immobilized directly into the Os-polymer hydrogel (b). Applied potential: 0.2 V (vs. Ag/AgCl). Experimental conditions: Os-polymer: 10 μL of a 10 mg/mL solution; PEDGE: 1 μL of a 2.5 mg/mL solution; invertase: 5U; ν = 10 mV/s; 0.1 M phosphate buffer pH 7.0; electrode diammeter: 3 mm.
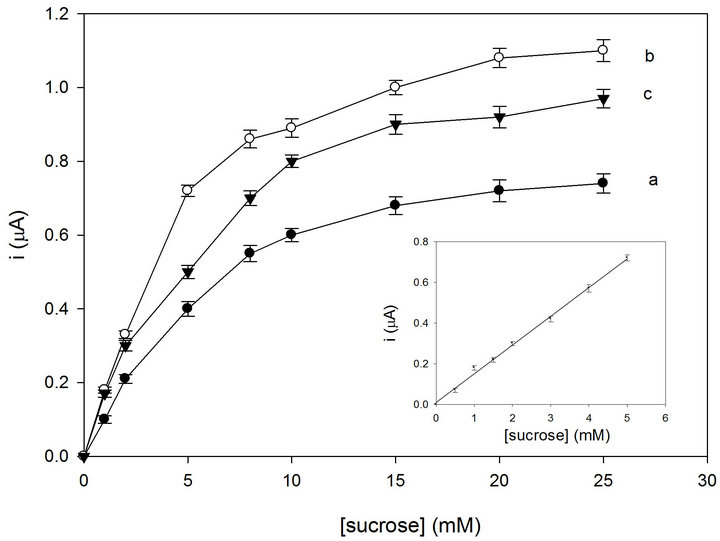
Figure 4. Calibration graphs for the sucrose biosensor with the following invertase-FDH amounts directly immobilized into the Os-polymer hydrogel: 2 U/2 U (a); 5 U/5 U (b); 10 U/10 U (c). The inset shows the linear part of curve b. RSD% values calculated for each point of the calibration curves are between 0.8 and 3% (n = 6). Applied potential: 0.2 V (vs. Ag/AgCl). Experimental conditions: as in Figure 3.
saturation of the active site of the enzyme. The current gradually increased with increasing invrtase and FDH values from 2 to 5 U (curves a and b, Figure 4) but it showeda slight decrease for amounts higher than 5 U (curve c, Figure 4). This fact indicates that the amount of enzyme is rate limiting at quite low enzyme amounts employed but then the rate of the reaction becomes limited by other factors. An amount of 5 U of invertase and 5 U of FDH was found to give the maximum current (curve b, Figure 4) and therefore 5 U of FDH and 5 U of inveratse were used to prepare the biosensor in further experiments.
The low amount of both enzymes employed and the relatively high current response can be explained by the fact that the enzymes are now wired through the redox hydrogel of the Os-polymer onto the CNTP electrode surface. The hydrogel allows the immobilization of the enzyme without suffering major structural changes or loss of activity but at the same time the high surface area of the SWCNTs allows a bigger exposure of the enzyme’s catalytic sites as well as a high loading of invertase and FDH with a possible enhancement of the resulting signal.
The inset of Figure 4 shows the linear part of the for sucrose registered by the optimized biosensor. It shows a good linearity over the range of 0.1 - 5 mM. At higher sucrose concentrations the curve becomes nonlinear approaching a saturation value. Statistical analysis gave the following equation y = 0.14x + 1.03 × 10−2 (r = 0.9969, n = 6), where y indicates the current (mA) and x the sucrose concentration (mM). The sensitivity was found to be 1.98 µA·cm−2·mM and the detection limit was found to be about 2 µM, calculated using the relation 3 S.D. a/b, where S.D. a is the absolute standard deviation of the intercept and b the slope of the calibration curve. The biosensor showed also a fast response time of about 8 s.
As for the fructose biosensor response, Figure 5 shows the fructose calibration curve registered with three different amounts of FDH. Once again an FDH amount of 5 U was found to give the maximum current. The biosensor shows a good linearity over the range 0.1 - 5 mM. At higher fructose concentrations the curve becomes nonlinear approaching a saturation value (KM = 3.66 mM). Statistical analysis gave the following equation y = 0.14x + 5.24 × 10−3 (r = 0.9992, n = 6), where y indicates the current (mA) and x the fructose concentration (mM). The sensitivity was found to be 1.95 µA∙cm−2∙mM and the detection limit was found to be about 1 µM, calculated using the relation 3 S.D. a/b, where S.D. a is the absolute standard deviation of the intercept and b the slope of the calibration curve. The biosensor showed also a fast response time of about 4 s.
3.3. Reproducibility
The reproducibility of the biosensors was found to be very good, obtaining an RSD of 2.5% for a 0.5 mM sucrose solution and an RSD of 2.1% for a 0.5 mM fructose solution, both for n = 6, where n represents the number of sensors used for the test.
3.4. Storage Stability and Lifetime
The stability and lifetime of both biosensors were also tested by consecutive measurements of the current re-
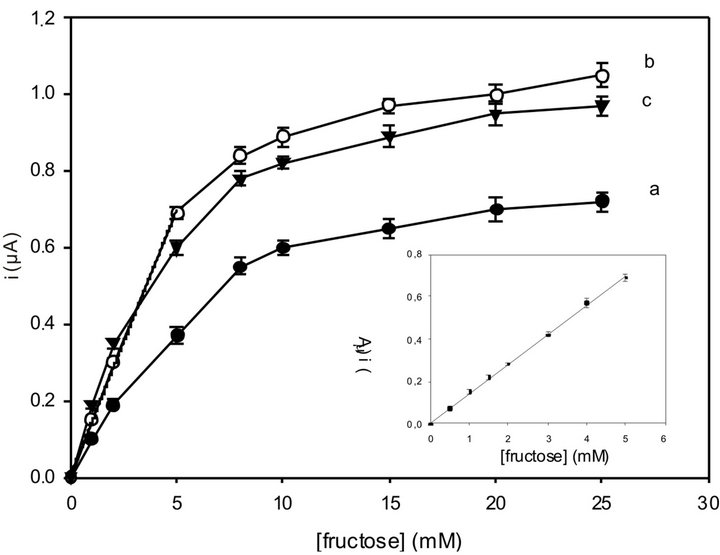
Figure 5. Calibration graphs for the fructose biosensor with the following FDH amounts directly immobilized into the Os-polymer hydrogel: 2 U (a); 5 U (b); 10 U (c). The inset shows the linear part of curve b. RSD% values calculated for each point of the calibration curves are between 0.8 and 3% (n = 6). Applied potential: 0.2 V (vs. Ag/AgCl). Experimental conditions: as in Figure 3.
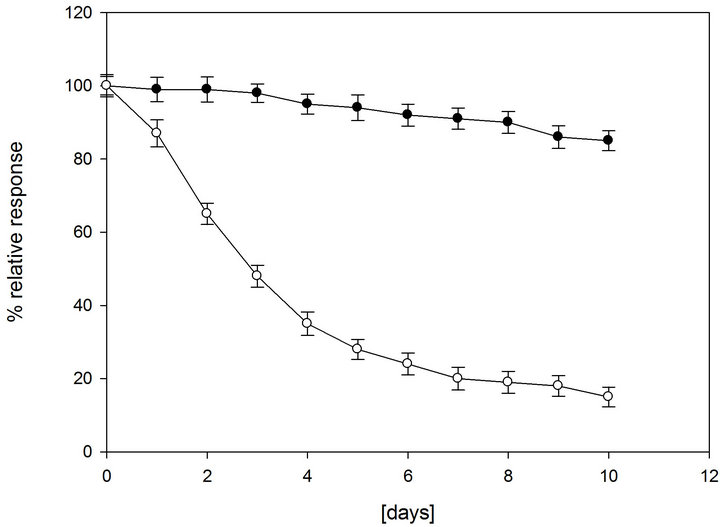
sponse to a 1 mM sucrose and fructose solutions. As most enzymes lose their activity if not stored in the refrigerator (4˚C), both sucrose and fructose biosensors were carefully stored at 4˚C when not in use. Figure 6 shows the biosensors lifetime under dry and wet storage conditions at 4˚C. Both biosensors showed a very similar behavior as they contain the same enzymes (fructose biosensor with inactivated invertase) with the same immobilization procedure. Under dry storage conditions, the biosensors lost about 20% of their initial activity after 1 day of 8 h/day operation and 80% after 7 days (Figures 6(a) and (b), white dots).Under wet conditions with a use of a maximum 8 h/day operation and storage at 4˚C, both biosensors retain more than 90% of their original response after 7 days (Figures 6(a) and (b), black dots) and are able to retain 80% of their original response even up to about 4 months (data not shown). The stability presented for the proposed biosensor is quite superior to
 (a)
(a)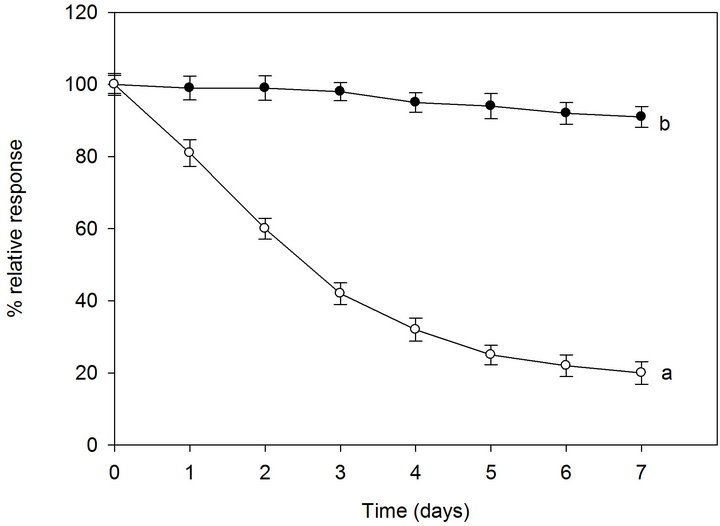 (b)
(b)
Figure 6. Lifetime of the sucrose biosensor (a) and fructose biosensor (b) stored at 4˚C in dry conditions (white dots, a) and wet conditions (black dots, b). RSD% values calculated for each point of the curves are between 2.5% and 3.7% (n = 6). Other conditions as in Figure 4.
most of the amperometric biosensors for sucrose reported in literature [25]. This result is consistent with the fact that under wet conditions the biosensor is kept in a closed bottle with cotton wet with buffer solution and this allows the maintenance of a higher water content in the immobilized hydrogel layer.
3.5. Selectivity of the Biosensors
In order to confirm the selectivity of both biosensors, possible interfering species such as ascorbic acid, ethanol and other mono and disaccharide compounds present in food such as glucose, lactose, galactose and maltose were checked by adding equal quantities of the interferent and of sucrose or fructose. The results are shown in Table 1. No significant influence was observed for all interferences with the only exception of ascorbic acid, with a slight interference of about 7% for sucrose biosensor and 8% for fructose biosensor.
As for fructose, the sucrose biosensor is based on FDH and therefore reacts also towards fructose present in solution (Table 1). For this reason, all measurements of sucrose in real samples must be coupled with a measurement of the fructose concentration in the same sample by using the same sensor with inactive invertase and subtracting this current response from the total response of the sucrose biosensor.
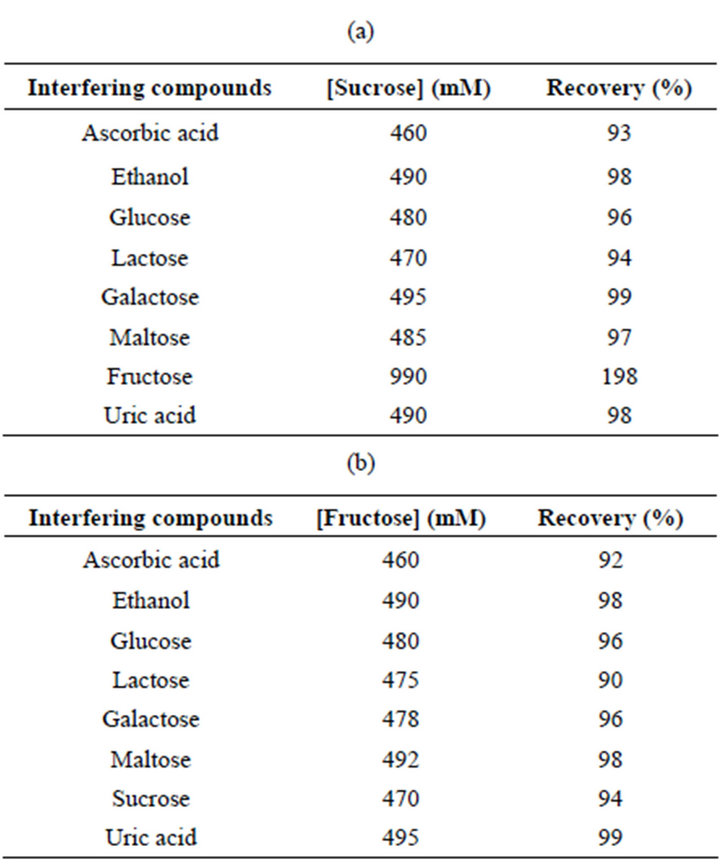
Table 1. Influence of interfering compounds on sucrose and fructose response of the FDH-Os-polymer-CNTP sucrose and fructose biosensors. The amounts of interferents added were 500 mM.
3.6. Analysis of Food Samples
The proposed invertase-FDH sucrose and fructose biosensors were applied to the analysis of foodstuffs like commercial fruit juice samples. The sucrose and fructose content was determined applying the calibration graph method and the samples were analysed in five replicates. The results were compared to those obtained with the enzymatic commercial spectrophotometric assay kit. As can be seen from Table 2, there is a good agreement between the results obtained with the biosensors and the reference spectrophotometric kit. It is interesting to observe that the results obtained with the reference spectrophotometric kit all show slightly higher values, probably because of the multienzyme cascade reactions, which take place in the kit and that can cause interferences from other compounds, especially other sugars.
4. Conclusions
In this work the osmium redox polymer was successfully used for the modification of a carbon nanotube paste electrode. In particular, the osmium-polymer resulted to be able to shuttle the electrons between the immobilized enzyme and the single-walled carbon nanotube paste
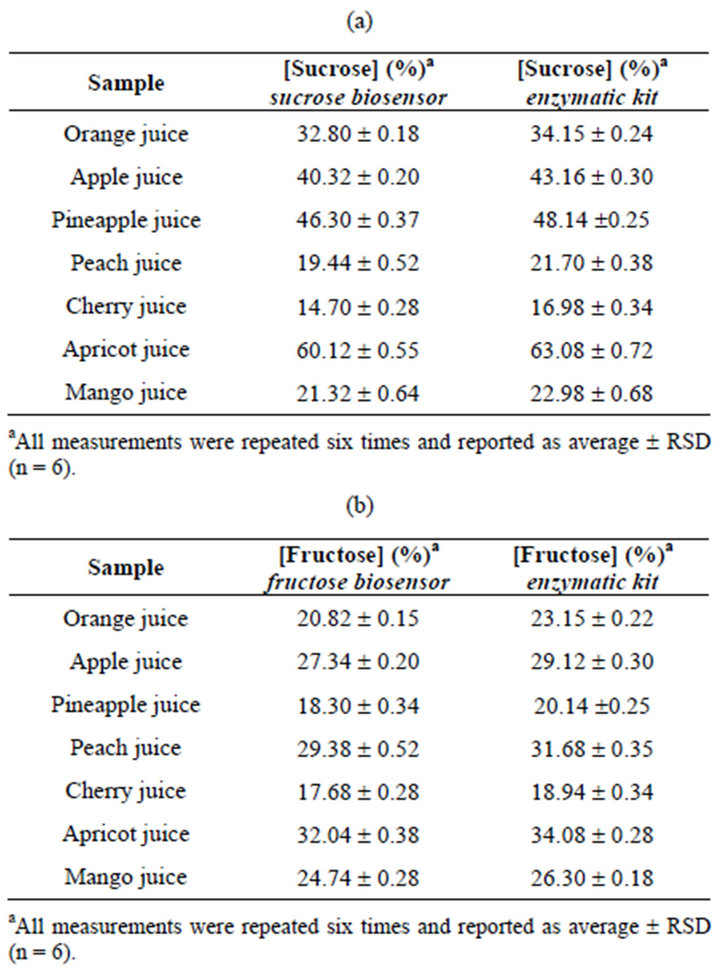
Table 2. Results for sucrose and fructose determination in real samples using the osmium-polymer-FDH biosensor and the reference enzymatic kit.
(SWCNTP) electrode and was used also as a support for direct wiring of both invertase and FDH into the paste by using poly(ethylene glycol) diglycidyl ether (PEDGE) as a cross-linking agent.
The so modified electrode was used to develop a sucrose and a fructose biosensor based on the immobilization of invertase and fructose dehydrogenase on a carbon nanotube electrode. The preparation of the biosensors is very simple, cheap and not time-consuming. The biosensors showed good linear ranges, low detection limits, high reproducibility, good stability and fast response times. They were also not influenced by possible interferents which can be present in food matrices. The sucrose and fructose biosensors were successively tested for detection of sucrose and fructose in fruit juices.
Therefore the new osmium-polymer proved to be an excellent agent for electrode modification ensuring a fast electron transfer to the electrode. The realized sucrose and fructose biosensors based on the osmium-polymer modified electrode will form the basis of a new, rapid and portable method for determination of sucrose and fructose in food analysis.
5. Acknowledgements
The authors thank the Swedish Research Council and the Nanometer Consortium at Lund University for financial support.
REFERENCES
- R. A. Durst, A. J. Baumner, R. W. Murray, R. P. Buck and C. P. Andrieux, “Chemically Modified Electrodes: Recommended Terminology and Definitions,” Pure and Applied Chemistry, Vol. 69, No. 6, 1997, pp. 1317-1323. doi:10.1351/pac199769061317
- P. N. Bartlett, “Modified Electrode Surface in Amperometric Biosensors,” Medical & Biological Engineering & Computing, Vol. 28, No. 3, 1990, pp. 10-17. doi:10.1007/BF02442675
- F. Valentini, A. Amine, S. Orlanducci, M. L. Terranova and G. Palleschi, “Carbon Nanotubes Purification: Preparation and Characterization of Carbon Nanotube Paste Electrodes,” Analytical Chemistry, Vol. 75, No. 20, 2003, pp. 5413-5421. doi:10.1021/ac0300237
- R. Antiochia, I. Lavagnini, F. Magno, F. Valentini and G. Palleschi, “Single-Wall Carbon Nanotube Paste Electrodes: A Comparison with Carbon Paste, Platinum and Glassy Carbon Electrodes via Cyclic Voltammetric Data,” Electroanalysis, Vol. 16, 2004, pp.1451-1458. doi:10.1002/elan.200302971
- R. Antiochia, I. Lavagnini and F. Magno, “Electrocatalytic Oxidation of NADH at Single-Wall Carbon Nanotube-Paste Electrodes: Kinetic Considerations for Use of a Redox Mediator in Solution and Dissolved in the Paste,” Analytical Bioanalytical Chemistry, Vol. 381, No. 7, 2005, pp. 1355-1458. doi:10.1007/s00216-005-3079-6
- J. J. Gooding, “Nanostructuring Electrodes with Carbon Nanotubes: A Review on Electrochemistry Applications for Sensing,” Electrochimica Acta, Vol. 50, No. 15, 2005, 3049-3060. doi:10.1016/j.electacta.2004.08.052
- J. Wang, “Carbon-Nanotube Based Electrochemical Biosensors: A Review,” Electroanalysis, Vol. 17, No. 1, 2005, pp. 7-14. doi:10.1002/elan.200403113
- R. Antiochia, I. Lavagnini and F. Magno, “Amperometrioc Mediated Carbon Nanotube Paste Biosensor for Fructose Determination,” Analytical Letters, Vol. 37, No. 8, 2004, pp. 1657-1669. doi:10.1081/AL-120037594
- S. Timur, B. Haghighi, J. Tkac, N. Pazarlioglu, A. Telefoncu and L. Gorton, “Electrical Wiring of Pseudomonas putida and Pseudomonas Fluorescens with Osmium Redox Polymers,” Bioelectrochemistry, Vol. 71, No. 1, 2006, pp. 38-45. doi:10.1016/j.bioelechem.2006.08.001
- S. Timur, Y. Yigzae and L. Gorton, “Electrical Wiring of Pyranose Oxidase with Osmium Redox Polymers,” Sensor and Actuators B: Chemical, Vol. 113, No. 2, 2006, pp. 684-691. doi:10.1016/j.snb.2005.07.017
- R. Antiochia and L. Gorton, “Development of a Carbon Nanotube Paste Electrode Osmium Polymer-Mediated Biosensor for Determination of Glucose in Alcoholic Beverages,” Biosensor & Bioelectronics, Vol. 22, No. 11, 2007, pp. 2611-2617. doi:10.1016/j.bios.2006.10.023
- A. Heller, “Electrical Connection of Enzyme Redox Centers to Electrodes,” The Journal of Physical Chemistry, Vol. 96, No. 9, 1992, pp. 3579-3587. doi:10.1021/j100188a007
- A. Heller and B. Feldman, “Electrochemical Glucose Sensors and Their Applications in Diabetes Management,” Chemical Reviews, Vol. 108, No. 7, 2008, pp. 2482-2505. doi:10.1021/cr068069y
- AOAC International, “Official Methods of Analysis,” 16th Edition, AOAC International, Arlington, 1995, Vol. 2.
- H. O. Beutler, “D-Fructose, Methods of Enzymatic Analysis,” 3rd Edition, Verlag, London.
- M. C. Tran and T. M. Cahn, “Biosensors,” Springer, London, 1993.
- B. R. Eggins, “Chemical Sensor and Biosensor,” Wiley & Sons, London, 2002.
- G. Wagner and G. Guilbault, “Food Biosensor Analysis,” Marck Dekker, New York, 1994.
- I. H. Boyaci and M. Mutlu, “Measurement of Glucose, Sucrose and Lactose in Food Samples with EnzymeImmobilized Packed-Bed Column Reactors Integrated to an Amperometric Enzyme Electrode,” Nahrung/Food, Vol. 46, No. 3, 2002, 174-178.
- F. Mizutani and S. Yabuki, “Rapid Determination of Glucose and Sucrose by an Amperometric Glucose-Sensing Electrode Combined with an Invertase/Mutarotase-Attached Measuring Cell,” Biosensors & Bioelectronics, Vol. 12, No. 9-10, 1997, pp. 1013-1020. doi:10.1016/S0956-5663(97)00057-2
- M. Ameyama and O. Adachi, “D-Fructose Dehydrogenase from Gluconobacter Industrius, Membrane-Bound,” Methods in Enzymology, Vol. 89, 1982, pp. 154-159. doi:10.1016/S0076-6879(82)89027-7
- M. Ameyama, E. Shinagawa, K. Matsushita and O. Adachi, “D-Fructose Dehydrogenase of Gluconobacter Industrius: Purification, Characterization and Application to Enzymatic Microdetermination of D-Fructose,” Journal of Bacteriology, Vol. 145, No. 2, 1981, pp. 814-823.
- Y. Kamitaka, S. Tsujimura, N. Setoyama, T. Kajno and K. Kano, “Fructose/Dioxygen Biofuel Cell Based on Direct Electron Transfer-Type Bioelectrocatalysis,” Physical Chemistry Chemical Physics, Vol. 9, No. 15, 2007, pp. 1793- 1801. doi:10.1039/b617650j
- V. L. Davidson and L. H. Jones, “Intermolecular Electron Transfer from Quinoproteins and Its Relevance to Biosensor Technology,” Analytical Chimica Acta, Vol. 249, No. 1, 1991, pp. 235-240. doi:10.1016/0003-2670(91)87028-6
- K. Damar and D. O. Demirkol, “Modified Gold Surfaces by Poly(amidoamine)dendrimers and Fructose Dehydrogenase for Mediated Fructose Sensing,” Talanta, Vol. 87, 2011, pp. 67-73. doi:10.1016/j.talanta.2011.09.042
NOTES
*Corresponding author.

