Materials Sciences and Applications
Vol.4 No.1(2013), Article ID:27062,8 pages DOI:10.4236/msa.2013.41003
Physicochemical Characteristics of Some Cameroonian Pozzolans for Use in Sustainable Cement Like Materials
![]()
1Materials Analysis Laboratory, Yaoundé, Cameroon; 2Physico-Chemistry of Mineral Materials Laboratory, University of Yaoundé I, Yaoundé, Cameroon; 3Groupe d’Etude des Matériaux Hétérogènes, Ecole Nationale Supérieure de Céramique Industrielle, Limoges, France.
Email: *nbillong@yahoo.fr
Received September 3rd, 2012; revised October 2nd, 2012; accepted October 31st, 2012
Keywords: Pozzolan; Characteristic; Geopolymer; Supplementary Cementing Material; Cameroon
ABSTRACT
In the present study, physico-chemical investigations have been carried out on the possibility of using Cameroonian volcanic or clay pozzolans as raw material for geopolymer or pozzolanic binder. The research had made some suggestive results and conclusions. Powders of less than 100 µm of five sampled pozzolans from volcanic or clay origins have been subjected to chemical and mineralogical analysis, BET specific surface, absolute density, granulometry and pozzolanic activity in solution tests. The results obtained showed that, geopolymers or pozzolanic binders can be produced from samples studied. The samples contain significant amounts of glassy or amorphous phase ready to dissolve in an alkaline solution. The high alkali content of volcanic pozzolans makes them more appropriate for geopolymer application. Clay pozzolans are the easier to grind in order to obtain the appropriate fineness and can be used for both geopolymers and pozzolanic binders.
1. Introduction
Environmental considerations and energy requirements have motivated researchers in recent decades to search for sustainability in the Portland cement industry [1-4]. During the production of Portland cement clinker, a significant amount of CO2 is released into the atmosphere. It is estimated that the production of every one metric ton of Portland clinker results in the emission of about one metric ton of CO2, a major greenhouse gas implicated in global warming. The World’s Portland cement clinker production is responsible for about 7% of total CO2 emissions [4]. To reduce the use of Portland cement clinker without decreasing the needed amount of cement for the construction industry, pozzolanic admixtures are partially added to Portland clinker during the grinding process [5-8]. ASTM C618 defines a pozzolan as a siliceous or siliceous-aluminous material which, in itself, possesses little or no cement binding value but which will, in finely divided form in the presence of moisture, react chemically with calcium hydroxide at ordinary temperature to form compounds possessing binding properties. The amorphous silica, alumina or aluminosilicates present in pozzolans have also permitted the design of inorganic polymers (geopolymers) with promising mechanical behavior in the presence of an alkali solution [9-12]. Essentially, geopolymers are a group of cement-like materials that are formed by reacting silicarich or alumina-rich solids with a solution of alkali-salts resulting in a mixture of gels and crystalline coumpounds that eventually harden into new strong compounds. They can gain reasonable strength in a short time. 70% of the final compressive strength can be obtained in the first 4 hours of setting [11]. Pozzolanic materials can be natural or artificial. Natural pozzolans are derived from volcanic projections (ash and scoria) or from natural amorphous rocks like opale [13]. Artificial pozzolans are made from activated alumino-silicates (clay, bauxite, laterite, etc.) or from industrial by-products and wastes like silica fume, fly ash, blast furnace slag and rice hush ash [13]. Theoretically, any pozzolanic mineral or source of silica and alumina that is readily soluble in alkali may serve as a geopolymer precursor [10]. Geopolymers from volcanic pozzolans have shown promising performance [11,14], but little attention is still given to this type of geopolymer compared to fly-ash or metakaolin geopolymers. Volcanic pozzolans are abundant in countries with past or present volcanism. These minerals, which are deposited at the surface during volcanic activity are readily accessible and have the advantage that they can be economically mined, with enormous benefits of low cost and limited negative environmental impact compared to traditional open pit quarry-type clay mining [15]. Cameroon has a large reserve of raw materials for use as pozzolans. Several deposits of volcanic ash and scoria exist along the “Cameroon’s line”. Particularly on the slopes of Mount Cameroon, the Kumba plain, the slopes of Mount Manengouba, the Tombel plain, the Noun plain, the Lake Nyos and the Adamawa plateau [16]. Several clay deposits of a greater or lesser importance are scattered throughout the territory [16]. Some of them such as the deposits of Mayoum (Western Region) and Etoa (Central Region) have been studied for use in ceramics [17,18]. However, these materials can have physico-chemical characteristics favorable or not for their use in the formulation of pozzolanic binders or geopolymers according to international standards and specifications.
In the present study, samples of five Cameroonian pozzolans materials from volcanic and clay origins have been studied for use in the elaboration of pozzolanic binders or geopolymers. The major chemical elements, the mineralogy, the grindability, the densities and the pozzolanic activity in solution of samples were investigated.
2. Materials and Experimental Procedure
The pozzolanic materials studied were: three volcanic scoria samples from Gouogouo (FVS1) and Fessang (FVS2) in the locality of Foumbot in the West Region and Djoungo (DVS) in the Littoral Region of Cameroon. A small part of volcanic scoria from Djoungo is being used as supplementary cementing material by a local cement factory [19]. A calcined common alluvial clay (TAC) from Etoa in the South-West of Yaoundé (Center Region) and metakaolin (MK) from the kaolin of Mayoum near Foumban in the West Region have also been studied. Previous studies on samples of the Etoa clay and the kaolin of Mayoum for use in ceramics have showed that they contain respectively 41% and 79% kaolinite respectively [20,21]. Samples of volcanic scoria were dried, pulverized at 500 µm, milled for 5 h using an AMACO 132 ball mill and sieved totally at 100 µm. The common clay and kaolin samples were also pulverized at 500 µm and fired in an electric furnace at 750˚C for 1 hour at a heating rate of 3˚C/minute before milling and sieving using the same process as volcanic scoria. The fired products (TAC and MK) were left to cool freely in the furnace. The chemical and mineralogical analyses of samples were carried out using the Atomic Emission Spectrometer by Inductively Coupled Plasma (ICP-AES) and X-Ray Diffraction (XRD) respectively. MK and TAC also underwent Differential Thermal (DTA) and Gravimetric (TG) Analyses to evaluate the state of amorphisation of the materials. BET specific surface test, absolute densities test using an Accupyc1330 helium pycnometer, granulometry analysis by laser and pozzolanic activity in solution were also performed on samples. The pozzolanic activity test in solution was used to determine the lowering of the concentration of hydroxyl ions per gram of pozzolan in a lime saturated solution for a given time compared to the same lime solution containing no pozzolan. That method combined the requirements of the Chapelle test [22] and that of EN 196-5: 1994 standard. 20.00 g of pozzolan were introduced carefully in 100 ml of lime saturated solution. The mixture was maintained at 40˚C ± 1˚C statically for 8 days in hermetically sealed plastic boxes. 100 ml of lime saturated solution containing no pozzolan (control solution) was also maintained under the same conditions. The saturated solution was prepared by dissolving hydrated lime in excess in distilled water and filtered by the mean of vacuum filtration with an ash-less filter paper No. 111. At the end of the reaction period, the suspension was cooled and filtered. The filtrate was kept hermetically closed and the determination of the hydroxyl ion concentration of every filtrate was performed by titration with a hydrochloric acid (HCl) solution of concentration 0.1 mol/l. Compared to the result provided by the control solution, the lowering of the hydroxyl ions in solution was deduced. The end result is the average of three test results.
An industrial slaked lime (SL) of EN 459-1 CL 90-S type produced by the SB-Mercier company in France was used for the pozzolanic activity in solution test.
3. Results and Discussion
3.1. Chemical Analysis of Materials Used in the Study
Table 1 gives the chemical elements of materials used in the study. Sample SL is essentially made of CaO. The pozzolans samples are made of SiO2, Al2O3 and Fe2O3 as major chemical elements (weight% more than 10%) and CaO, MgO, Na2O, K2O and TiO2 as minor elements (% less than 10). In general, the chemical composition of pozzolans fluctuate between the following limits: 45% - 65% SiO2, 15% - 30% Al2O3 + Fe2O3 and about 15% CaO + MgO + alkali [23] with the exception of silica fume which contains more than 85% amorphous silica [24] and blast furnace slag with 29% - 36% SiO2, 13% - 19% Al2O3, 40% - 43% CaO and other oxides. ASTM C-618 [25] recommends the following compositions for thermally activated or natural pozzolans used as additives in cement (class N): SiO2 + Al2O3 + Fe2O3: 70.0% minimum; SO3: 4.0% maximum; MgO: 5.0% maximum; Na2O: 1.5% maximum; Loss on ignition: 10.0% maximum. The Indian Standard IS 1344 - 1981 [26] for calcined clays as pozzolans recommends the chemical composition limits as follows: SiO2 + Al2O3 + Fe2O3: 70.0% minimum; SiO2: 40% minimum; CaO: 10% maximum; MgO: 3% maximum; SO3: 3% maximum; Na2O + K2O: 3% maximum; Loss on ignition: 10% maximum. The results showed that, pozzolans from clay origin (TAC and MK) compliy with the specifications of ASTM C 618 and IS 1344 - 1981 but pozzolans from volcanic origin (FVS1, FVS2, DVS) have MgO, Na2O and Na2O + K2O greater than the recommended compositions.
From the chemical point of view, all the pozzolans are suitable for use as geopolymer raw materials. Although standards are not yet available for geopolymers, this partial conclusion was made by comparing the chemical composition of pozzolans studied to that of some alumino-silicates used to elaborate geopolymers with satisfactory results by several authors: Elimbi et al. [12] used kaolinite as raw material (sample I and II in Table 2), calcined between 450˚C and 800˚C and showed that the compressive strength of geopolymers cement paste made with this material increases with the increasing calcina-
Table 1. Chemical analysis of pozzolans and hydrated lime samples.

Table 2. Chemical analysis of some geopolymer’s raw materials [11,12,14].

tion temperature of kaolinite, with the maximum being at 700˚C; Kamseu et al. [14] and Lemougna et al. [11] used volcanic ash (sample III and IV in Table 2) and (sample V in Table 2) respectively and concluded that, glassy and deshydroxylated aluminosilicates which characterize volcanic ash can be dissolved in alkaline solution into silicate and alumino-silicate monomers prior to polycondensation into geopolymers structural materials. Dissolution and hardening time can be longer compared to metakaolin geopolymers but thermal curing between 200˚C and 400˚C can be applied as an activation factor. The good densification behavior, good mechanical properties and lower porosity of volcanic based geopolymers indicated that these materials can be suitable for building application. When sodium hydroxide as the sole alkaline solution is used as the activator, the result showed that this low-energy geopolymerization can synthesize these natural pozzolanic raw materials into viable products with properties suitable for building and low-grade refractory applications. The chemical composition of raw materials used by these various authors is shown in Table 2.
3.2. Mineralogical Analysis
The XRD patterns of the volcanic scoria are shown in Figure 1. The result indicated the presence of anorthite (CaAl2Si2O8), diopside (CaMg(SiO3)2), plagioclase which is a mixture of anorthite and albite (NaAlSi3O8), enstatite (MgxFe2−x Si2O6) and quartz (SiO2). Most scoria have no quartz, but according to Dron et al. [23], the absence of quartz in some natural pozzolans is due to the fact that the total silica of sialic phases is in deficit compared to the composition of feldspars (except in special case where it would be in the exact ratio). The absence of quartz indicates the presence of feldspathoids which are silicates close to feldspars but containing less silica. Therefore, the coexistence of these minerals with free silica (quartz) is impossible. The detection of quartz in the scoria studied excluded the presence of feldspathoids like leucite, pollicite, analcime, sodalite, etc. and confirmed the presence of the mineral phases found in samples. The profile of the base of the XRD patterns of volcanic scoria indicated the presence of a considerable amount of glassy phase in samples. From the work of Millet et al. [27] concerning natural pozzolans, it has been noted that the surface of the diffraction band due to the presence of glass in scoria is directly proportional to the quantity of glassy phase in samples. The rapid cooling of magma in the atmosphere during volcanic eruption is at the origin of the glassy phase in scoria. Millet et al. [27] have also demonstrated that, the glassy phase content of natural pozzolans is related to the content of SiO2 and CaO in samples. When the difference between SiO2 and CaO content is below 34%, pozzolans would not include the glassy phase. After calculating this difference for the three samples of volcanic scoria using the data in Table 1, we obtained 39.13%, 35.46% and 36.6% for FVS1, FVS2 and DVS respectively. This is a confirmation of the presence of the glassy phase in volcanic pozzolans studied. The presence of that glassy phase would play a major role in the reactivity of the scoria sample in the presence of Ca(OH)2 and water to form pozzolanic binders or in the presence of NaOH or KaOH and water to form geopolymers. Figures 2 and 3 indicated the presence of kaolinite, quartz and hematite as major minerals in the raw material of TAC; kaolinite, illite, quartz and anatase are the major minerals present in the raw material of MK respectively. When the clay samples were activated at 750˚C, the kaolinite crystal structure was transformed into an amorphous state not detectable by X-rays. It is for this reason that the peak of kaolinite is absent on XRD patterns of TAC and MK in Figures 2 and 3. More noticeable, the kaolinite peak around two theta = 12.29˚ no longer exist. The total transformation of the kaolinite of TAC and MK in an amorphous state was confirmed by DTA-TG analysis of samples in Figure 4 in which no thermal reaction was observed in samples up to 900˚C, except the transformation of quartz α to quartz β shown by TAC at about 600˚C. The quartz present in theses samples is not reactive but, it will play a filler role in the cement matrix obtained from the pozzolanic or the polymerization reactions by reinforcing the strength, the durability and the resistance to chemical attacks of the cement matrix [14,28]. Figure 5 indicated that, sample SL is consisted essentially with Ca(OH)2 and quartz is present as impurity.
3.3. Specific Surface, Absolute Densities and Granulometry
Table 3 indicates the specific surfaces and absolute densities of samples. Compared to clay pozzolans, volcanic pozzolans are samples with lower specific surfaces, less than the general specific BET surface of Portland cement

Figure 1. XRD patterns of volcanic pozzolans: a—anorthite, d—diopside, e—enstatite, p—plagioclase, q—quartz, 1—FVS1, 2—FVS2, 3—DVS.

Figure 2. XRD patterns of TAC and its raw clay.
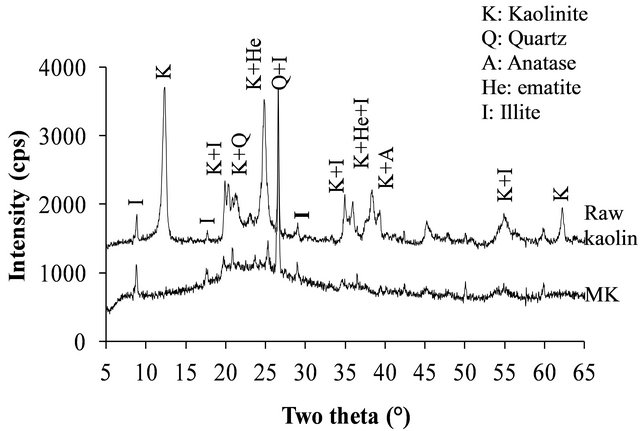
Figure 3. XRD patterns of MK and its raw kaolin.
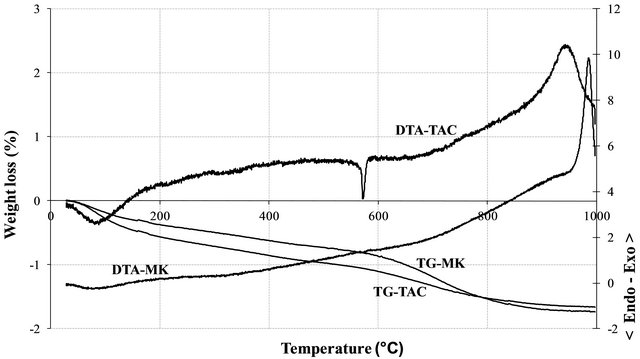
Figure 4. DTA-TG thermograms of TAC and MK.
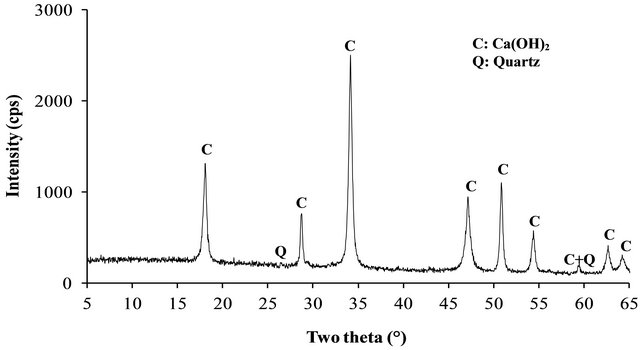
Figure 5. XRD patterns of SL.
Table 3. BET specific surfaces and absolute densities of pozzolans samples.
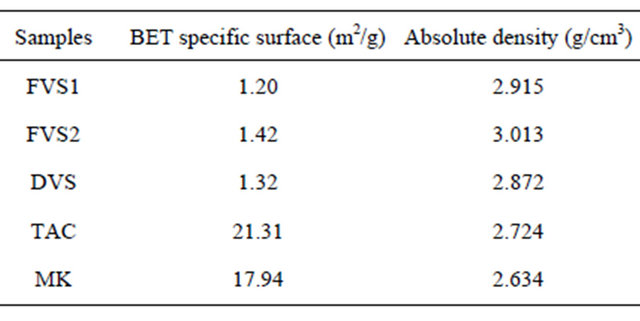
which is 1.5 m2/g. In solution, when the pH is favorable, the reactivity of pozzolans depends on the kinetic of the solubility of reactive compounds which itself depends on the specific surface of particles [29]. If some pozzolans like silica fume are naturally in a very fine form, others acquire their fineness from grinding. Volcanic pozzolans will need more grinding energy to achieve the proper fineness than clay pozzolans. The high fineness of clay pozzolans can be explained by the fine nature of raw clay compared to volcanic scorias which are also heavier in term of absolute density. Those high absolute densities can be explained by the high Fe2O3 and CaO content of volcanic pozzolans compared to clay pozzolans. The granulometry analysis of the samples is shown in Figure 6. Samples TAC and MK are those having high proportions of fine particles with 36% and 35% particles less than 10 µm respectively. FVS1, FSV2 and DSV have respectively about 27%, 17% and 20% of their particles less than 10 µm. For particles less than 45 µm, the proportions are 80% for the clay pozzolans and 53%, 67% and 70% respectively for FSV1, FSV2 and DSV. Those proportions are in accordance with ASTM C 618 standard which recommend a minimum of 34% particles less than 45 µm for supplementary cementing materials. The high fineness of clay pozzolans is at the origin of their high BET specific surfaces.
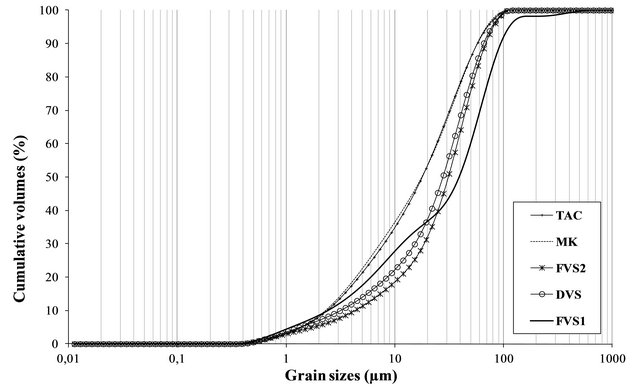
Figure 6. Grain sizes distribution of pozzolans powders (<100 µm).

Figure 7. Reactivity of pozzolans with Ca(OH)2 in the presence of water.
3.4. Pozzolanic Activity in Solution
The study of the pozzolanic activity of samples in solution gave the result in Figure 7. The concentration of the sodium hydroxide solution used for titration was 0.0912 mol/l. It was calculated by assuming that all OH− ions in the lime saturated solution are from the Ca(OH)2 which was the main component of SL. From this result, the pozzolans from clay origin (TAC and MK) were samples that absorbed more Ca(OH)2 in solution compared to volcanic pozzolans. This high reactivity of clay pozzolan can be explained by their high fineness. Globally, the result of the pozzolanic activity of samples in solution was in concordance with the result of BET specific surface test. When samples entered in contact with the Ca(OH)2 solution, their acidic and amphoteric compounds reacted through an acido-basic reaction. The mechanism of that reaction differs from clay pozzolans to volcanic ones. In the case of volcanic pozzolans a mechanism was proposed by Dron [30], by using orthoclase (KAlSi3O8) which is a feldspath with a structure made by three tetrahedral units of SiO2 and one 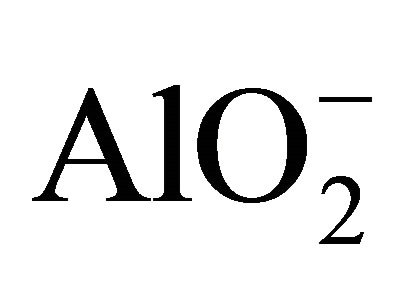 unit. The negative charge is compensated by K+. Inside SiO2 units, each O2− ion at the summits of the tetrahedral is linked to one central Si4+ ion and also linked to an O2− ion of another unit. In solution, external O2− ions face more the presence of water molecules and reaction (1) can happen. The two negative charges produced in this reaction can find compensation out of the surface and inside the tetrahedral. Consequently the tetrahedral units can become less and less linked, move from their initial positions and enter in solution in the form of H3SiO4− ions. It is those last ions that react with Ca2+ ions to form hydrated calcium silicates. The glassy structure having less strong bonds is more predisposed to the replacement of O2− ions by OH− ions and thus the dissolution of tetrahedral units.
unit. The negative charge is compensated by K+. Inside SiO2 units, each O2− ion at the summits of the tetrahedral is linked to one central Si4+ ion and also linked to an O2− ion of another unit. In solution, external O2− ions face more the presence of water molecules and reaction (1) can happen. The two negative charges produced in this reaction can find compensation out of the surface and inside the tetrahedral. Consequently the tetrahedral units can become less and less linked, move from their initial positions and enter in solution in the form of H3SiO4− ions. It is those last ions that react with Ca2+ ions to form hydrated calcium silicates. The glassy structure having less strong bonds is more predisposed to the replacement of O2− ions by OH− ions and thus the dissolution of tetrahedral units.
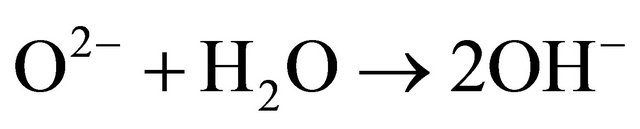 (1)
(1)
In the case of clay pozzolans, as the reactive mineral is kaolinite (Al2Si2O5(OH)4 or Al2O3·2SiO2·2H2O), the thermal activation of the samples at 750˚C causes the departure of the structural water. The metakaolinite
(Al2O3·2SiO2) formed in reaction (2) is amorphous to X-rays. In the presence of Ca(OH)2 and water, the metakaolinite reacted to form hydrated calcium silicates and alumino-silicates like CSH, C4AH13, C3AH6 and C2ASH8 as indicated in reactions (3) to (5).
 (2)
(2)
 (3)
(3)
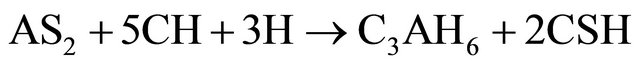 (4)
(4)
 (5)
(5)
According to the chemistry of cement conventions,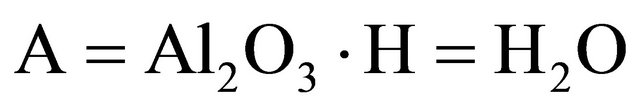
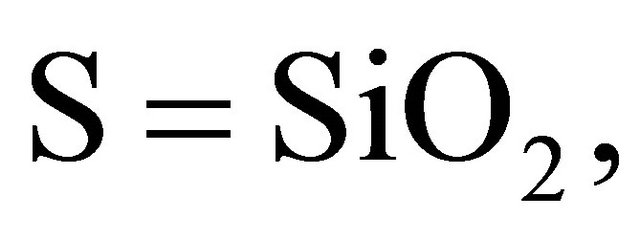
 .
.
4. Conclusions
From the results of the various tests performed, the following conclusions can be drawn:
• Volcanic pozzolans are more suitable for geopolymer than for pozzolanic binder applications because of their high alkali content.
• Clay pozzolans are suitable for both geopolymer and pozzolanic binder applications.
• A significant amount of glassy or amorphous phase is present in volcanic and clay pozzolan that will make those samples ready to dissolve in alkaline solution to form geopolymers or pozzolanic binder.
• Finer particles with appropriate specific surface can easily be obtained with clay pozzolans than with volcanic pozzolans.
• Both volcanic and clay pozzolans studied are reactive in solution with Ca(OH)2 and water. The reaction highly depends on the fineness of samples.
REFERENCES
- M. J. Shannag and A. Yeginobali, “Properties of Pastes, Mortars and Concretes Containing Natural Pozzolan,” Cement and Concrete Research, Vol. 25, No. 3, 1995, pp. 647-657. doi:10.1016/0008-8846(95)00053-F
- G. Barger, E. R. Hansen, M. R. Wood, T. Neary, D. Beach and D. Jaquier, “Production and Use of Calcined Natural Pozzolans in Concrete,” Cement, Concrete and Aggregates, Vol. 23, No. 2, 2001, pp. 73-80. doi:10.1520/CCA10478J
- D. D. Vu, P. Stroeven and V. B. Bui, “Strength and Durability Aspect of Calcined Kaolin-Blended Portland Cement Mortar and Concrete,” Cement and Concrete Composites, Vol. 23, No. 6, 2001, pp. 471-478. doi:10.1016/S0958-9465(00)00091-3
- M. Ghrici, E. Kenai and E. Meziane, “Mechanical and Durability Properties of Cement Mortar with Algerian Natural Pozzolan,” Journal of Material Science, Vol. 41, No. 21, 2006, pp. 6956-6972. doi:10.1007/s10853-006-0227-0
- N. Kaid, M. Cyr, S. Julien and H. Khelafi, “Durability of Concrete Containing a Natural Pozzolan as Defined by a Performance-Based Approach,” Construction and Building Materials, Vol. 23, No. 12, 2009, pp. 3457-3467. doi:10.1016/j.conbuildmat.2009.08.002
- M. J. Shannag, “High Strength Concrete Containing Natural Pozzolan and Silica Fume,” Cement and Concrete Composites, Vol. 22, No. 6, 2000, pp. 399-406. doi:10.1016/S0958-9465(00)00037-8
- R. E. Rodriguez-Camacho and R. Uribe-Afif, “Importance of Using the Natural Pozzolans on Concrete Durability,” Cement and Concrete Research, Vol. 32, No. 12, 2002, pp. 1851-1858. doi:10.1016/S0008-8846(01)00714-1
- B. B. Sabir, S. Wild and J. Bai, “Metakaolin and Calcined Clays as Pozzolans for Concrete: A Review,” Cement and Concrete Composites, Vol. 23, No. 6, 2001, pp. 441-454. doi:10.1016/S0958-9465(00)00092-5
- J. Tailby and K. J. D. MacKenzie, “Structure and Mechanical Properties of Aluminosilicate Geopolymer Composites with Portland Cement and Its Constituent Minerals,” Cement and Concrete Research, Vol. 40, No. 5, 2010, pp. 787-794. doi:10.1016/j.cemconres.2009.12.003
- J. Davidovits, “Geopolymer Chemistry and Applications,” Second Edition, Institut Geopolymère, Paris, 2008.
- P. N. Lemougna, K. J. D. MacKenzie and U. F. C. Melo, “Synthesis and Thermal Properties of Inorganic Polymers (Geopolymers) for Structural and Refractory Applications from Volcanic Ash,” Ceramics International, Vol. 37, No. 8, 2011, pp. 3011-3018. doi:10.1016/j.ceramint.2011.05.002
- A. Elimbi, H. K. Tchakoute and D. Njopwouo, “Effects of Calcination Temperature of Kaolinite Clays on the Properties of Geopolymer Cements,” Construction and Building Materials, Vol. 25, No. 6, 2011, pp. 2805-2812. doi:10.1016/j.conbuildmat.2010.12.055
- H. Neville, H. Stafford and D. Mather, “Lime and Other Alternative Cements,” Intermediate Technology Publications, London, 1992.
- E. Kamseu, C. Leonelli, D. S. Perera, U. C. Melo and P. N. Lemougna, “Investigation of Volcanic Ash-Based Geopolymers as Potential Building Materials,” Interceram, Vol. 58, No. 2, 2009, pp. 136-140.
- C. Leonelli, E. Kamseu, D. N. Boccaccini, U. C. Melo, A. Rizzuti, N. Billong and P. Misselli, “Volcanic Ash as alternative Raw Materials for Traditional Vitrified Ceramic Products,” Advances in Applied Ceramics, Vol. 106, No. 3, 2007, pp. 135-141. doi:10.1179/174367607X159329
- O. R. Amougou, “Données Sur les Argiles, Calcaires, Pouzzolanes et Sables au Cameroun,” Bureau de Recherches Géologiques et Minières, Yaoundé, 1993.
- E. Kamseu, C. Leonelli, D. N. Boccaccini, P. Veronesi, P. Miselli, G. Pellacani and U. C. Melo, “Characterisation of Porcelain Compositions Using Two China Clays from Cameroon,” Ceramics International, Vol. 33, No. 5, 2007, pp. 851-857. doi:10.1016/j.ceramint.2006.01.025
- C. N. Djangang, E. Kamseu, M. K. Ndikontar, G. L. L. Nana, J. Soro, U. C. Melo, A. Elimbi, P. Blanchart and D. Njopwouo, “Sintering Behavior of Porous Ceramic Kaolin-Corundum Composites: Phase Evolution and Densification,” Materials Science and Engineering, Vol. 528, No. 29-30, 2011, pp. 8311-8318. doi:10.1016/j.msea.2011.07.006
- C. Bidjocka, J. Tusset, A. Messi and J. Perra, “Etude et Evaluation de l’Activité Pouzzolanique des Pouzzolanes de Djoungo (Cameroun),” Annales de la Faculté des Sciences de l’Université de Yaoundé, 1993, pp. 133-145.
- Ndigui Billong, U.C. Melo, F. Louvet and D. Njopwouo, “Properties of Compressed Lateritic Soil Stabilized with a Burnt Clay-Lime Binder: Effect of Mixture Components,” Construction and Building Materials, Vol. 23, No. 6, 2009, pp. 2457-2460. doi:10.1016/j.conbuildmat.2008.09.017
- Ndigui Billong, U. C. Melo, D. Njopwouo, F. Louvet and J. P. Bonnet, “Effect of Mixture Constituents on Properties of Slaked Lime-Metakaolin-Sand Mortars Containing Sodium Hydroxide,” Cement and Concrete Composites, Vol. 31, No. 9, 2009, pp. 658-662.
- O. Benoit, “Détermination de l’Activité Pouzzolanique d’Une Pouzzolane Par Voie Chimique,” Bulletin de Liaison des Laboratoires Routiers, Ponts et Chaussées, Vol. 1, No. 26, 1967, pp. D1-D2.
- R. Dron and F. Brivot, “Bases Minéralogiques de Sélection des Pouzzolanes,” Bulletin de Liaison des Laboratoires de Ponts et Chaussées, Vol. 93, 1977, pp. 61-65.
- P. C. Aitcin, “High Performance Concrete,” FN Spon, New York, 1998.
- W. B. Butler, “A Critical Look at ASTM C 618 and C 311,” Cement, Concrete and Aggregates, Vol. 4, No. 2, 1982, pp. 68-72. doi:10.1520/CCA10230J
- Indian Bureau of Standards, “IS 1344, Specification for Burnt Clay Pozzolans,” India, 1968.
- J. Millet, R. Hommey and F. Brivot, “Dosage de la Phase Vitreuse dans les Matériaux Pouzzolaniques,” Bulletin de Liaison des Laboratoires de Ponts et Chaussées, Vol. 92, 1977, pp. 101-104.
- J. C. Benezet and A. Benhassaine, “The Influence of Particle Size on the Pozzolanic Reactivity of Quartz Powder,” Powder Technology, Vol. 103, No. 1, 1999, pp. 26- 29. doi:10.1016/S0032-5910(99)00010-8
- S. Caijun, “An Overview on the Activation of Reactivity of Natural Pozzolans,” Canadian Journal of Civil Engineering, Vol. 28, No. 5, 2001, pp. 778-786. doi:10.1139/l01-041
- R. Dron, “L’Activité Pouzzolanique,” Bulletin de Liaison des Laboratoires de Ponts et Chaussées, Vol. 93, 1978, pp. 101-104.
NOTES
*Corresponding author.

