Advances in Bioscience and Biotechnology
Vol.1 No.2(2010), Article ID:2075,12 pages DOI:10.4236/abb.2010.12012
Isolation, purification and characterization of an N-acetyl-D-lactosamine binding mitogenic and anti-proliferative lectin from tubers of a cobra lily Arisaema utile Schott
![]()
1Department of Molecular Biology and Biochemistry, Guru Nanak Dev University, Amritsar, India;
2Department of Pharmacology, Indian Institute of Integrative medicine, Jammu Tawi, India.
Email: vikramdhuna@gmail.com
Received 18 April 2010; revised 7 May 2010; accepted 8 May 2010.
Keywords: N-acetyl-D-lactosamine; Arisaema utile; affinity purification; anti-proliferative; asialofetuin; chemical modification; lectin; mitogenicity
ABSTRACT
Lectins are the carbohydrate-binding proteins of non-immune origin which have been the subject of intense investigation over the last few decades owing to the variety of interesting biological properties. Most of the lectins which have been purified and characterized from plants have been obtained from dicotyledons. In the present study a lectin was purified from tubers of a monocot plant Arisaema utile (AUL) Schott by affinity chromatography on asialofetuin-linked amino activated silica beads. AUL gave a single band in SDS-PAGE at pH 8.3 corresponding to subunit Mr 13.5 kDa. The native molecular mass of AUL was 54 kDa suggesting a homotetrameric structure. AUL gave multiple bands in isoelectric focusing and in native PAGE at pH 8.3. AUL was inhibited by N-acetyl-D-lactosamine (Lac NAc), a disaccharide and asialofetuin, a complex desialylated serum glycoprotein. When treated with denaturing agents, the lectin was stable in the presence of urea (3 M), thiourea (4 M) and guanidine HCl (4 M). AUL was a glycoprotein with a carbohydrate content of 1.2%. Complete loss of activity was observed upon modification of tryptophan residues of the lectin. The activity was reduced to 25% after modification of tyrosine. Chemical modification of arginine, histidine, serine and cysteine residues of AUL did not affect its activity. Using Far UV CD spectra the estimated secondary structure was 37% α-helix, 25% β-sheet and 38% random contributions. The lectin showed potent mitogenic response towards human lymphocytes. In vitro anti-proliferative assay using 11 human cancer cell lines resulted in 50% inhibition of six cell lines viz. SW-620, HCT-15, SK-N-SH, IMR-32, Colo-205 and HT-29 at 38, 42, 43, 49, 50 and 89 µg/ml, respectively.
1. INTRODUCTION
Lectins are proteins or glycoproteins of non-immune origin that bind specifically to carbohydrates [1]. Most lectins are usually multivalent and able to agglutinate erythrocytes and other cells [1,2]. They have been proved excellent and versatile macromolecular tools for the study of normal or transformed cell surfaces, for the isolation of glycoconjugates and for use in other areas of biomedical science [3]. One of the most exiting properties resulting out of the interaction of lectins with lymphocytes is mitogenicity, i.e. the triggering of quiescent, non-dividing lymphocytes into a state of growth and proliferation. The discovery of first mitogenic lectin Nowell [4] led to the detection of many other such lectins, most notably concanavalin A [5], Wheat germ agglutinin [6] and Pokeweed mitogen [7]. However, every new lectin may have slight differences in the ligand binding specificity to serve as a diagnostic tool to find immunocompetence of patients suffering from a variety of diseases with immunological abnormalities [8]. Thus, such mitogenic lectins are invaluable tools to study the proliferation of various immune cells and biochemical changes associated with lymphocyte activation.
Very few lectins have been investigated for their use in cancer research and therapy. Preliminary investigations suggest that some lectins but not all can detect alterations of malignant cells as well as reduce the cancer cell tumorigenicity by immunomodulation and thus may be helpful for prognosis of the immune status of the patients [9]. The lectin from Viscum album (mistletoe), for instance, increases the reactivity of the lymphocytes of tumor-bearing mice in vitro, indicating its immune stimulating effects on the cancer-immunosuppressed lymphocytes. It also inhibits the protein synthesis in various malignant cell lines [10]. Some lectins inhibit cell proliferation while others stimulate it [11,12]. A few plant lectins have been identified which induce apoptosis in tumor cells, as for example, Viscum album L. [13,14]. Lectins interact with specific carbohydrate structures on the tumor cell surface and may be used to differentiate malignant from normal cells [15]. We report herein purification, characterization and amino acid modifications of a new monocot plant lectin from Himalayan cobra lily Arisaema utile Schott belonging to family Araceae which has shown significant proliferation inhibition towards six human cancer cell-lines and potent mitogenic response towards human lymphocytes.
2. MATERIALS AND METHODS
2.1. Materials and Chemicals
Underground tubers of Himalayan cobra lily A. utile were collected from Khajjiar, Dalhousie, India. Fetal calf serum from Sera Lab (GB) and RPMI-1640 from GIBCO-BRL (New York, USA) were procured and stored at 4℃. Carbohydrates, diethylpyrocarbonate (DEP), N-acetylimidazole, (NAI), N-bromosuccinimide (NBS), 2-hydroxy-5-nitrobenzyl bromide (HNB-Br), bis-dithionitrobenzoic acid, (DTNB), bovine serum albumin, sodium azide, Con A, 5-fluorouracil, adriamycin, mitomycin-C and other general chemicals were obtained from Sigma Chemical Co. (St. Louis, USA). Standard molecular weight markers, gel filtration markers and ampholine (pH 3.0-10.0) were procured from Amersham Pharmacia (New Jersey, USA). Amino activated silica beads used were from Clifmar, UK. The Cell-lines viz. MCF-7 (Breast), SK-N-SH (CNS), 502713 (Colon), Colo-205 (Colon), HCT-15 (Colon), HT-29 (Colon), SW-620 (Colon), Hep-2 (Liver), IMR-32 (Neuroblastoma), DU-145 (Prostate) and PC-3 (Prostate) were procured from National Center for Cell Sciences, Pune, India. These cell lines were maintained in RPMI 1640 medium with 10% FCS, 10 U/ml penicillin and 100 μg/ml streptomycin at 37℃, in humidified atmosphere (90% air and 10% CO2) in CO2 incubator (Heracell, Heraeus).
2.2. Isolation and Purification of AUL
Lectin from homogenized A. utile tubers was extracted overnight at 4℃ with 10 mM phosphate-buffered saline (PBS), pH 7.2 (1:5 w/v). After centrifugation at 20,000 xg for 30 min, the supernatant was chromatographed on asialofetuin-linked amino activated silica beads (1000Å; pore size, 100 μ; diameter) as described by Shangary et al. [16]. The bound lectin was eluted with 100 mM glycine-HCl buffer, pH 2.5. The fractions were neutralized immediately with 2 M Tris-HCl buffer, pH 8.3. The protein rich fractions were dialyzed against PBS and stored at 4℃ for further analysis.
2.3. Lectin-Activity Assay and Sugar Inhibition of Lectin
To 30 μl (1 mg/ml) of serial two fold dilution of the lectin in microtitre plate, an equal volume of 2% (v/v) suspension of rabbit erythrocytes (3.5 × 108 cells/ml) was added [17]. Agglutination was assessed after 1h at 37℃ when the RBCs in the control well had fully settled to form buttons. Agglutination activity was expressed as the reciprocal of the highest dilution that gave a positive result and was reckoned as one hemagglutination unit. To find the carbohydrate specificity of AUL, sugar inhibition was performed in a manner analogous to the hemagglutination test [17]. For this purpose, a series of 42 sugars/derivatives were used which included 4 pentoses: D-Arabinose, L-arabinose, D-ribose, D-xylose; 18 hexoses or their derivatives: D-fructose, D-galactose, Dglucose, D-mannose, L-sorbose, L-fucose, L-rhamnose, N-acetyl-D-galactosamine, N-acetyl-D-glucosamine, Nacetyl-β-D-mannosamine, phenyl-D-glucosamine, αmethyl-D-glucopyranoside, α-methyl-D-mannopyranoside, β-methyl-D-glucopyranoside, β-phenyl-D-glucopyranoside, sialic acid, adonitol, myo-inositol; 10 disaccharides: β-gentiobiose, D-lactose, α-maltose, D-melibiose, D-trehalose, α-D-man (1,2)-D-man, α-D-man (1,3)-Dman, α-D-man (1,6)-D-man, N-acetyl-D-lactosamine and N-acetyl neuraminic acid; 3 trisaccharides: melezitose, raffinose and N,N’,N”-triacetylchitotriose; 3 polysaccharides: chitin, inulin and yeast mannan. Four glycoproteins, i.e. asialofetuin, fetuin, mucin and thyroglobulin were also used. Sugars or their derivatives were tested at a concentration of 100 mM while polysaccharides and glycoproteins at a concentration of 4 mg/ml. Lectin was used at twice the lowest concentration causing agglutination of rabbit RBCs as determined through double dilution technique. The minimum concentration of the sugar in the final mixture that completely inhibited the lectin induced hemagglutination was taken as minimal inhibitory sugar concentration (MISC). To ascertain the biological specificity of AUL, the hemagglutination activity was tested against both normal as well as neuraminidase treated erythrocytes from rabbit, goat, sheep, guinea pig, rat, and human (ABO) blood and human lymphocytes [17].
3. BIOCHEMICAL AND BIOPHYSICAL CHARACTERIZATION
3.1. Protein and Neutral Sugar Content Analysis
Protein concentration in the crude and purified lectin was determined by the method of Lowry et al. [18] using bovine serum albumin as standard while neutral sugar content of the purified lectin preparation was estimated by anthrone method [19] using D-glucose as standard.
3.2. Polyacrylamide Gel Electrophoresis of Native and Denatured Lectin
Native polyacrylamide gel electrophoresis (PAGE) was carried out using 7.5% (w/v) gel at pH 4.5 [20] and 10% (w/v) gel at pH 8.3 [21,22]. SDS–PAGE was performed according to Laemmli [23] using 11% (w/v) separating gel. The lectin sample was heated in the presence/ absence of 2-mercaptoethanol for 10min in boiling water bath. The gels were stained with Coomassie Brilliant Blue. The subunit molecular mass of the purified lectin was determined by comparing its electrophoretic mobility with those of molecular weight markers (14.4-94 kDa).
3.3. Isoelectric Focusing
It was carried out in 5% polyacrylamide tube gels containing 5% ampholine of pI range 3.0-9.5 according to Robertson et al. [24]. A set of pI markers was also loaded on a separate tube gel. Before staining, ampholine were eluted from the gel by incubation in 10% trichloroacetic acid (TCA) for 10 min followed by 1% TCA for 30 min at room temperature. The gels were stained with Coomassie brilliant blue. Isoelectric point was calculated by comparing the mobility of lectin with that of pI markers.
3.4. Gel-Exclusion Chromatography
The molecular mass of the native lectin was determined by gel filtration chromatography on Biogel P-200 column (1.6 × 62 cm) calibrated with molecular weight markers in the range of 12.4-66 kDa according to the method of Andrews [25]. The column was equilibrated and eluted with 10mM PBS, pH 7.2.
3.5. Effect of Temperature, PH, Denaturants, and Chelating Agents on Lectin Activity
To determine thermal stability of affinity purified AUL, 100 μl of it was heated for 15min at a defined temperature ranging from 20 to 100℃ and cooled to room temperature. As the bound lectin was desorbed from the affinity matrix by employing glycine-HCl buffer, pH 2.5, the effect of such a low pH on lectin-induced hemagglutination was ascertained before standardizing the purification protocol. The lectin sample was incubated with the above-mentioned buffer for time intervals ranging from 15 min to 6 h, followed by neutralization with Tris-HCl buffer, pH 8.3. Thereafter, titer of each treated sample was compared with that of controls i.e. lectin sample mixed with glycine-HCl followed by immediate neutralization and lectin sample in PBS alone. The effect of three denaturing agents i.e. urea, thiourea, and guanidine-HCl, at a concentration range of 1.0-8.0 M was tested on lectin activity by incubating 100 µl of each denaturant solution with equal volume of AUL at 37℃ for 1h. To examine divalent cation requirement of AUL for hemagglutination, demetallization of purified lectin was carried out by the method of Paulova et al. [26] by using EDTA followed by dialysis of sample with 0.1M CaCl2 and MnCl2. Following these treatments hemagglutination assay was performed with each sample and titer was compared with that of respective untreated samples.
4. SPECTROSCOPIC MEASUREMENTS
4.1. Fluorescence Studies
Fluorescence measurements were performed on a Shimadzu RF-1501 Spectrofluorophotometer. Samples were excited at 295 nm and emission spectra were recorded between 250 nm to 500 nm. Excitation and emission slit widths were 5 nm. Measurements were made using lectin at a concentration of 0.1 mg/ml protein in deionized water. Base-line corrections were carried out with deionized water without protein in all cases. The fluorescence spectra of native and NBS modified lectin samples were recorded.
4.2. Circular Dichroism Studies
Circular dichroism spectra of AUL were recorded using a Jasco J-715 spectropolarimeter over a wavelength of 200-250 nm at a scan speed of 50 nm/min, under constant N2 purging according to the manufacturer’s instructions (Jasco). The lectin was used at a concentration of 0.15 mg/ml in deionized water, in quartz cuvettes of 0.1 mm path length. The accumulated average of five protein spectra was corrected by subtraction of the spectra measured from deionized water blank. Analysis of CD spectra in terms of secondary structure content was performed using K2D programme.
4.3. Chemical Modification Studies of Amino Acids
The modification of tryptophan residues was carried out using NBS [27] and HNB-Br [28] Modification of tyrosine residues was carried out using NAI [29]. Arginine, histidine, serine and cysteine residues of AUL were modified as per defined conditions in Table 1. Both modified and unmodified lectin samples were dialyzed and residual activity was determined after appropriate dilution. Percentage residual activities were calculated using the native lectin as control possessing 100% activity. For ligand protection, AUL was pre-incubated for 30 minutes with N-acetyl-D-lactosamine and then modification was done.
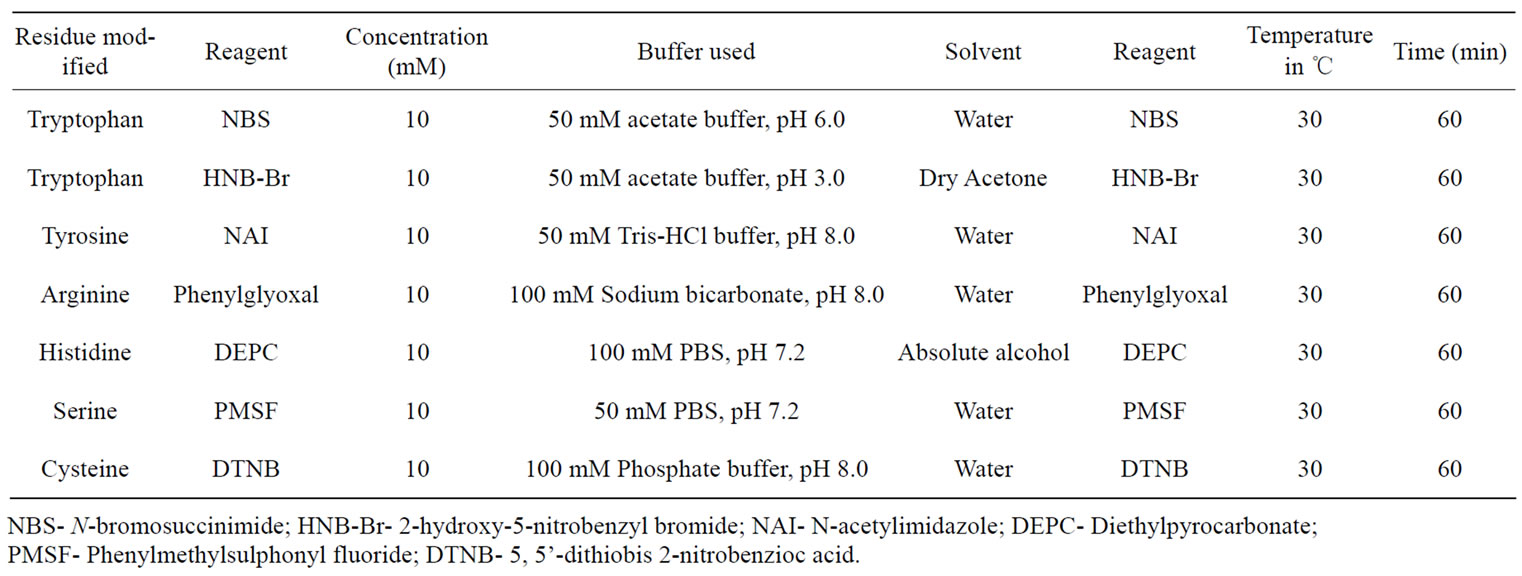
Table 1. Condition for the chemical modification studies of amino acids of AUL.
5. BIOLOGICAL CHARACTERIZATION
5.1. Serological Studies
Antiserum against Purified AUL was raised by immunizing healthy rabbits with one mg/ml of the lectin and 1 mL of Freund’s complete adjuvant (Sigma). Three doses were given at one-week interval each. After a weak of the last dose, the blood was collected by puncturing the ear pinna vein of the animal. Antiserum separated was preserved at –20℃ in aliquots containing 0.01% sodium azide. Double immunodiffusion (Ouchterlony) was performed to study serological cross-reactions. The gels stained for 30 minutes with 0.1% amido black and destained with 7% glacial acetic acid to visualize the stained precipitin lines.
5.2. Assay of Anti-Fungal Activity
Anti-fungal activity of AUL against selected plant pathogenic fungi, i.e., Fusarium oxysporum, Fusarium graminearum, Trichoderma reesei, Colletotrichum lindemuthianum, Alternaria solani, Rhizoctonia solani and Botrytis cinerea was performed as described by Wang et al. [30].
5.3. MTT (3, 4, 5-Dimethylthiazol-2yl-2, 5-Diphenyltetrazolium Bromide) Assay
A non-radioactive method [31] was employed to test the mitogenic potential of lectin on human peripheral blood mononuclear cells (HPBMC) separated by the method of Boyum [32]. The assay was carried out in quadruplicates in microtitre plate having 96 U-shaped wells (Nunc, Denmark). The test lectin and standard mitogen con A were filtered through 0.22 µ membrane filters (13 mm diameter, Schleicher and Schull, Germany) and stored at 4℃ in aliquots of 1 ml each. The protein content was estimated for all the lectin samples after filtration. The lectins were diluted in RPMI-1640 medium supplemented with 10% FCS to obtain the desirable concentration required for the experiment. A volume of 50 ml of each affinity purified lectin at concentrations ranging between 1.25-80.0 mg/ml along with standard Con A (1 mg/ml) was dispensed into 96 well microtitre plate. Now, 50 ml of lymphocyte suspension containing 2 × 106 cells/ml was added to each well of the microtitre plate and incubated for 72 hours. Four hours before the termination of cultures, 25 ml of MTT having a concentration of 2 mg/ml was added to each well. After 4 hours, 100 ml of acidified isopropanol was added. The blue colour of formazan formed was read at 492 nm with Labsystems Multiskan EX ELISA reader against a reagent blank.
5.4. Proliferation Inhibition Assay
To check that the lectin receptors for mitogenicity are glyco-components in nature, the inhibitors revealed by sugar inhibition assay AUL was used to analyze their effect on the proliferation by MTT assay. AUL had been were inhibited by a complex desialylated glycoprotein i.e. asialofetuin. Therefore, asialofetuin (Sigma, USA) was dissolved in RPMI-1640 medium supplemented with 10% FCS at a final concentration of 2 mg/ml. In the wells of a microtitre plate, asialofetuin from its stock solution was serially double diluted in a total volume of 25 µl. To this an equal volume of each lectin at double the optimum concentration was added and the plate was incubated at 37℃for one hour. For the positive controls, 25 µl each of test lectin and ConA at their optimum concentrations with an equal volume of supplemented medium, were set in quarduplicates, thus constituting a total volume of 50 µl/well. After the incubation step, 50 µl of lymphocyte suspension at a concentration of 2 × 106 cells/ml was added to each well.
5.5. In Vitro Anti-Proliferative Potential of AUL on Human Cancer Cell Lines
Inhibitory potential of the lectin was tested against eleven human cancer cell lines viz. MCF-7 (Breast), SK-N-SH (CNS), 502713 (Colon), Colo-205 (Colon), HCT-15 (Colon), HT-29 (Colon), SW-620 (Colon), Hep-2 (Liver), IMR-32 (Neuroblastoma), DU-145 (Prostate) and PC-3 (Prostate) according to the method of Monks et al. [33] known as SRB assay. Cells were seeded at 104 cells/well in 100 μl RPMI medium containing 10% FCS in 96-well tissue culture plate and incubated for 24 h in CO2 incubator. Subsequently, 100 μl of lectin solution (100 μg/ml), prepared in RPMI 1640 medium, was added to cells and the cultures were incubated for 48 h. After incubation period, adherent cell cultures were fixed in situ by adding 50 μl of 50% (v/v) trichloroacetic acid (final concentration 10% TCA) and incubated for 1h at 4℃. The supernatant was discarded and plates were washed five times with deionized water and dried. Hundred microliters of sulforhodamine B (SRB, 0.4% w/v in acetic acid) was added to each well and the cultures were incubated for 30min at room temperature. The unbound SRB was removed by washing with 1% acetic acid and plates were air-dried. The dye bound to basic amino acids of the cell membranes was solubilized with Tris buffer (10 mM, pH 10.5) and the absorption was measured at 540 nm using ELISA reader to determine the relative cell growth viability in the treated and untreated cells. Anti-cancer agents 5-flurouracil at a concentration of 1 × 10-5 M and mytomycin C and adriamycin at a concentration of 1 × 10-6 M were used as positive controls.
6. RESULTS AND DISCUSSION
6.1. Lectin Purification, Activity and Sugar Inhibition
In the present study, a plant lectin having mitogenic and antiproliferative activity has been purified from tubers of Arisaema utile Schott by affinity chromatography using asialofetuin as affinity ligand. The results of isolation of the lectin are summarized in Table 2. The lectin was eluted with 0.1 M glycine-HCl buffer, pH 2.5 (Figure 1). In a separate experiment, AUL was found to be stable at pH 2.0-9 for 1 hour. The efficiency of single step affinity purification protocol is apparent from the fact that there was 76% recovery of the lectin activity. It is also noteworthy that lectin constitutes a sizable (21%) proportion of the total extractable tuber protein and may serve as a major storage protein in the tubers as also reported for Arum maculatum [34].
Out of the 42 sugars/derivatives tested, N-acetyl-Dlactosamine (LacNAc) and asialofetuin inhibited lectininduced hemagglutination. Earlier reports on monocot lectins from the family araceae have shown their inhibition by, N-acetyl-D-lactosamine [16,17] and mannose [35]. It is noteworthy that monocot lectins from families amaryllidaceae and alliaceae bind mannose with high affinity [36] but lectins from family araceae are refractory towards mannose except in Acorus lectins [35]. The specific inhibitor of AUL i.e. LacNAc is one of the important cancer markers [37]. In this context the AUL specific for this disaccharide may serve as a marker for the detection of various types of cancers. Minimal inhibitory sugar concentration with LacNAc was 25.0 mM, while for asialofetuin was 250 µg/ml. Inhibition of hemagglutination with asialofetuin and not with fetuin may suggest that sialic acid hinders the binding of the lectin to the recognition sites on fetuin. The structure of asialofetuin reveals that it consists of 80% Asn-linked oligosaccharides terminating in LacNAc (Gal-β-1, 4 GlcNAc) and 20% Ser/Thr-linked oligosaccharides having T-Disa-ccharide (Gal-β-1, 3-GalNAc) [38]. In the sugar inhibition assay lectin showed binding with LacNAc but not to T-Disaccharide and similar may be the case of asialofetuin which has both disaccharides in its structure.
AUL was reactive towards erythrocytes from rabbit, rat, goat, sheep, and guinea pig and human lymphocytes but non-reactive towards human ABO blood group erythrocytes even after neuraminidase treatment (Table 3). In this regard AUL has broad biological specificity as
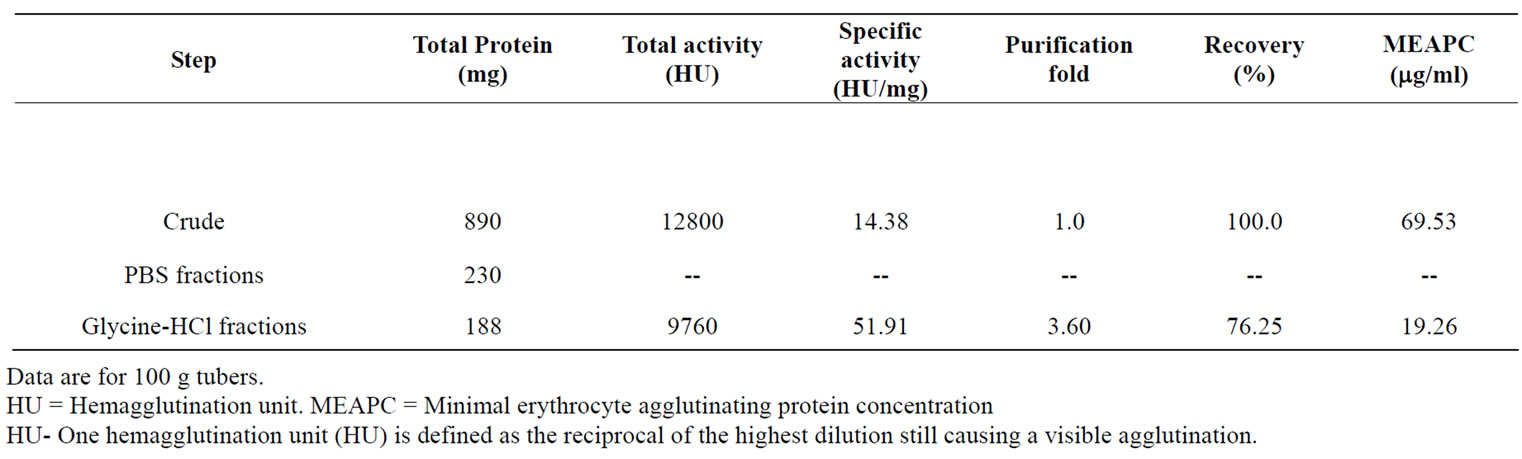
Table 2. Affinity purification of Arisaema utile lectin on asialofetuin-linked amino activated silica beads.
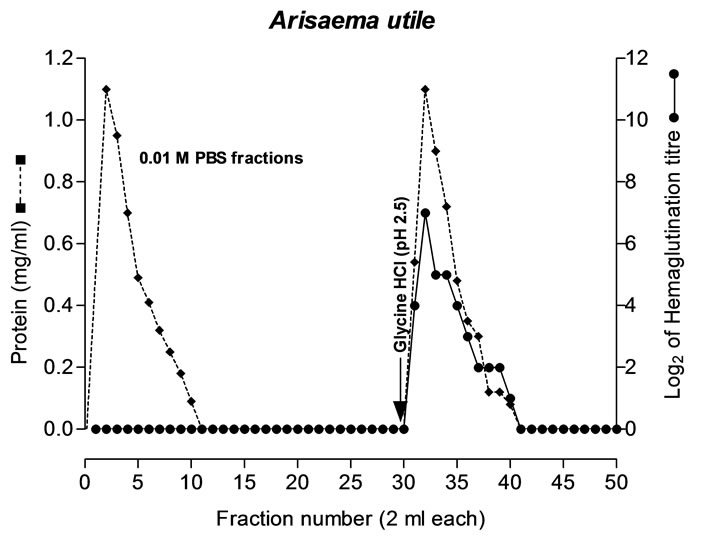
Figure 1. Affinity purification of AUL from tuber extract on asialofetuin-linked amino activated silica beads. Crude dialyzed tuber extract of A. utile was applied to the column (0.8 × 6.0cm), pre-equilibrated with 10mM PBS, pH 7.2. Bound lectin was eluted with 100mM glycine-HCl, pH 2.5 at flow rate 30 ml/h. [● ●] Absorbance of the complex formed, in protein estimation by Lowry et al. (1951), was taken at 540 nm. [♦----♦] Log2 hemagglutination titre was determined using 2% suspension of rabbit erythrocytes.
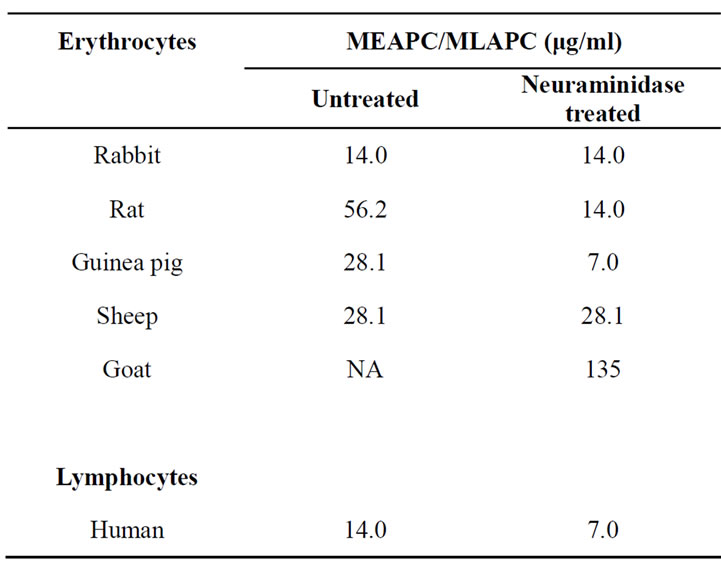
Table 3. Reactivity of AUL towards different types of erythrocytes and human lymphocytes.
reported earlier for other araceous lectins [11,12,16]. The preferential agglutination of rabbit RBCs over other animal blood cells may suggest easy availability of AUL receptors on these cells, their scanty presence on RBCs of rat, sheep and guinea pig and complete absence on human red blood cells. Further, increase in reactivity of rat and guinea pig erythrocytes and human lymphocytes following neuraminidase treatment supports the fact that sialic acid hinders access of lectin to their receptors.
6.2. Biochemical and Biophysical Characterization
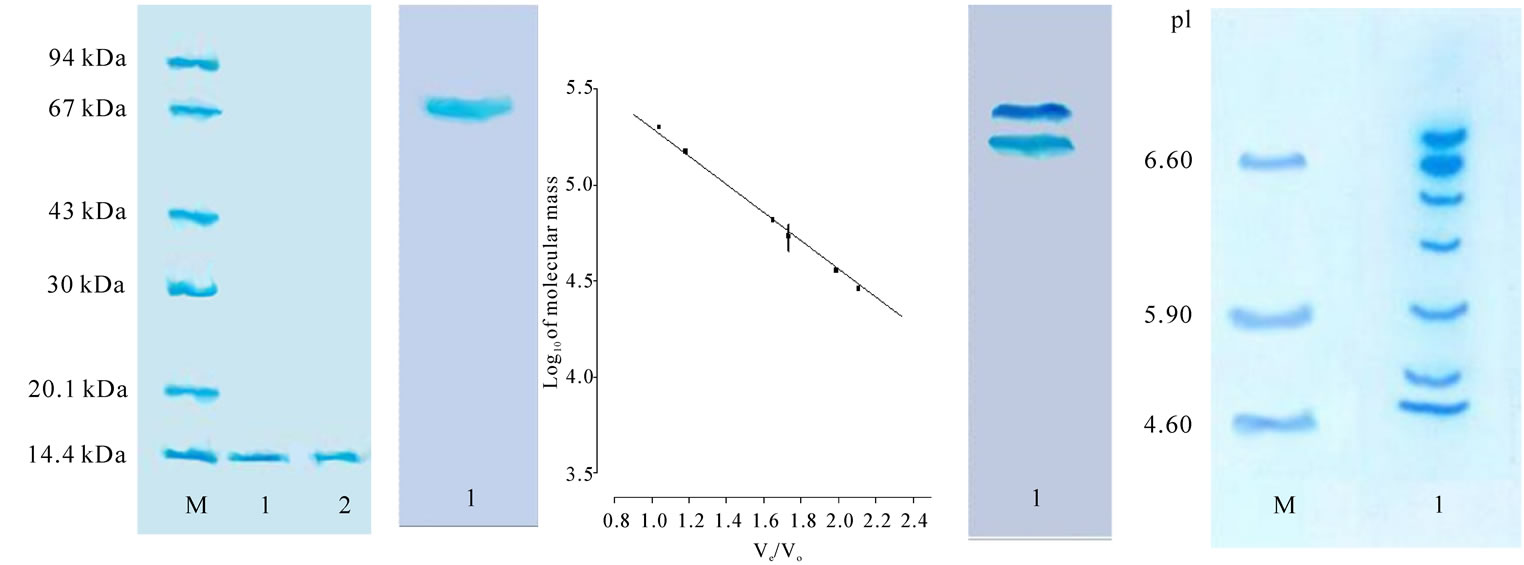
Purified AUL was subjected to various tests to check the purity of the preparation. In SDS-PAGE at pH 8.3, under both reducing and non-reducing conditions, AUL migrated as a single band of 13.5 kDa (Figure 2(a)). Similarly, in native PAGE at pH 4.5 it moved as single band (Figure 2(b)). The native molecular mass of AUL was 54 kDa, as determined by gel filtration chromatography on calibrated Biogel P-200 column (Figure 2(c)). The results of SDS-PAGE under reducing and non-reducing conditions and gel filtration chromatography revealed that the lectin exists as a homotetramer of four identical subunits which are not held together by disulphide linkages [12,35]. However, AUL gave two bands when subjected to native PAGE at pH 8.3 (Figure 2(d)). These results indicate the presence of isolectins in the affinity purified AUL. Similarly, when subjected to isoelectric focusing, multiple bands were seen representing a mixture of isolectins, mostly in acidic range (Figure 2(e)) as reported in many lectins. These findings for AUL corroborate with earlier observations on various monocot as well as dicot lectins [39-41]. The lectin isomers may be due to variations in oligosaccharide chains [42] or it may stem from few altered amino acids in the lectins [43]. The carbohydrate content of the lectin was 1.2% indicating the lectin to be a glycoprotein. Lectin remained stable up to 55℃ for 15 minutes beyond which activity was gradually decreased and completely lost at 80℃ (data not shown) indicating high thermal stability of AUL as reported earlier for other araceous lectins [16]. AUL was stable in 3M Urea, 4M thiourea and 4M Guanidine-HCl. The activity declined at higher concentrations of denaturants but was not completely lost even at 8 M concentration of urea, thiourea and guanidine hydrochloride (data not shown). The denaturation by these agents indicates the globular nature of lectins, stabilized mainly by hydrophobic interactions (Nelson and Cox, 2001). EDTA treatment cations showed no effect on lectin activity suggesting that either the lectin activity was not dependent on metal cations or these metal ions are too strongly held in lectin structure and cannot be removed by dialysis (data not shown).
6.3. Spectroscopic Measurements and Amino Acid Modifications
Native AUL upon excitation at 295 nm exhibited a fluorescence emission maximum (λmax) at 340 nm. When compared with emission maximum of free tryptophan (348 nm), the emission peak of the tryptophan residue in AUL blue-shifted by about 8 nm to 340 nm, which showed that tryptophan residue of AUL is located in hydrophobic areas [44]. As shown above, the modification of tryptophan residues in AUL were essential groups and the hydrophobic areas where they were located help in lectin-sugar binding. The decline in fluorescence spectrum on modification of tryptophan residues of AUL was caused by modification of Trp (Figure 3).
 (a) (b) (c) (d) (e)
(a) (b) (c) (d) (e)
Figure 2. (a) SDS–PAGE, pH 8.3, patterns of purified AUL (lane 1) using 12% gel with (lanes 1) and without (lanes 2) 2% 2-mercaptoethanol (running time 3 h at a constant 100 V). The amount of purified lectin loaded is 20 μg. 40 µg of marker mixture loaded in Lane M, (from top to bottom): phosphorylase b (94 kDa); albumin bovine (67 kDa); ovalbumin (43 kDa); and carbonic anhydrase (30 kDa); trypsin inhibitor (20.1 kDa); and a-lactalbumin (14.4 kDa). The gel was stained with Coomassie brilliant blue. (b) Discontinuous-PAGE, pH 4.5, using 7.5% gel (running time 6 h at a constant 100 V); protein load, 30 μg; lane 1, AUL. (c) Native molecular mass estimation by standard plot of AUL on Biogel P-200 superfine gel filtration chromatography column. Standards used for gel filtration analysis were 1) b-amylase 2) Alcohol dehydrogenase 3) Albumin bovine serum 4) Carbonic anhydrase 5) Cytochrome C (d) Discontinuous-PAGE, pH 8.3, using 10% gel (running time 6 h at a constant 100 V); amount of protein loaded, 30 μg; lane 1, AUL. (e) Isoelectric focussing of non-denatured AUL lectin on 7.5% polyacrylamide gel using carrier ampholine of pH range 3.5-10.0 (running time 12 h at a constant 200 V); protein load, 30 μg; lane M, position of pI marker proteins; 1) carbonic anhydrase I, human erythrocytes (pI 6.6); 2) carbonic anhydrase II, bovine erythrocytes (pI 5.9); and 3) trypsin inhibitor, soybean (pI 4.6); lane (1) AUL.
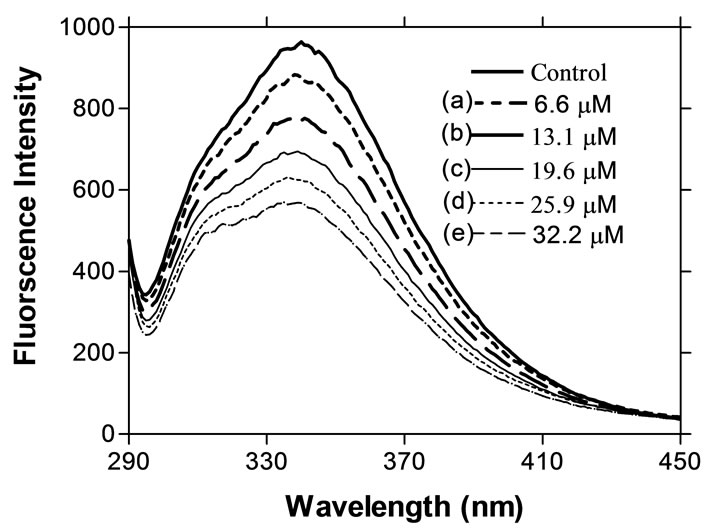
Figure 3. The decline of fluorescence intensity of AUL during tryptophan modification (with NBS) of AUL (100 µg/ml) was excited at 295 nm, and spectra were recorded between 250 and 500 nm. Control is without NBS and rest values (a, b, c, d and e) indicate the concentration of NBS in protein solution.
Far UV CD spectra of AUL were recorded (Figure 4). The spectrum is characterized by two negative minima at around 224 and 228 nm and a positive to negative crossover at around 205 nm. Using K2D software the estimated secondary structures are 37% α-helix, 25% β-sheet and 38% random contributions. The secondary
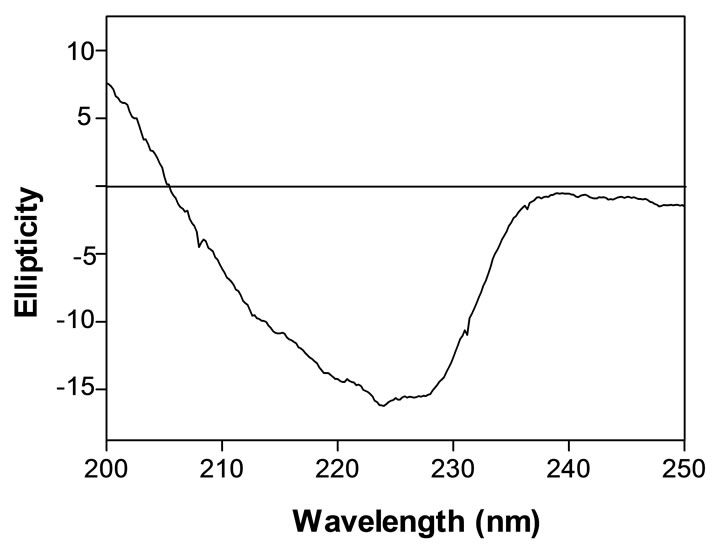
Figure 4. Far-UV CD spectrum of AUL in water.
structure of AUL is comparable with Con A [45] and Arisaema helliborifolium [46].
Upon chemical modification of tryptophan with Nbromosuccinimide (NBS) as well as 2-hydroxy-5-nitrobenzyl bromide (HNB-Br), AUL completely lost its activity. Ligand protection with N-acetyl-D-lactosamine did not protect the lectin from losing activity suggesting that tryptophan may not be present within the sugar binding site but is essential in maintaining the functional three dimensional structure of the lectin. Tyrosine modification with N-acetylimidazole (NAI) led to 50% inactivation of AUL lectin-sugar interaction. Ligand protection assay before tyrosine modification completely protected its activity which further supported the presence of tyrosine in the sugar binding site of the lectin. On the contrary, modification of arginine, histidine, serine and cysteine residues with pyridoxal, diethylpyrocarbonate (DEPC), phenylmethylsulphonyl fluoride (PMSF) and bis-dithionitrobenzoic acid (DTNB) respectively did not affect hemagglutinating activity of AUL suggesting that these amino acids may not play any important role in lectin-sugar interactions. Earlier, tryptophan modification studies on Glycine max [47], Erythrina indica [48] and Erythrina speciosa [49] have been shown to have deleterious effect on the lectin activity. In Trichosanthes dioica lectin [50], tyrosine residues are essential in carbohydrate binding and hemagglutination activities. Both tryptophan and tyrosine seem to play an important role in AUL activity in the present study.
6.4. Biological Characterization
In Oucterlony’s double immunodiffusion, AUL antisera gave lines of identity with lectins from other araceous species (Figure 5), thus indicating evolutionary conservation of antigenic determinants on lectins from these species. In case of Amaryllidaceae and Alliaceae, serological relationship has been reported, as lectins from them resemble each other in their molecular structure and carbohydrate-binding properties. When the purified lectins from Allium moly, A. sativum, A. vineale and A. ursinum were loaded against antiserum raised against Narcissus cv Carlton lectins, single precipitin lines of identity were observed between the Alliaceae and Amaryllidaceae lectins thus indicating the evolutionary conservation of these monocot mannose-binding lectins [36]. When tested for anti-fungal activity against plant pathogenic fungi, AUL was found non-inhibitory.
In MTT assay, AUL gave potent mitogenic response towards human peripheral blood mononuclear cells. The relative mitogenic stimulation of AUL towards human lymphocytes was almost equal to that of Con A, a well-known standard plant mitogen (Figure 6). The optimum proliferation dose of AUL was 20 μg/ml. The mitogenic response of AUL was inhibited in a concentration dependent manner in the presence of asialofetuin that has been found inhibitory towards A. utile (Figure 7). The inhibition of hemagglutination and mitogenicity induced by AUL in the presence of asialofetuin confirms that lectin is producing mitogenic effect.
The in vitro anti-proliferative activity of AUL was carried out on eleven human cancer cell lines viz. MCF-7 (Breast), SK-N-SH (CNS), 502713 (Colon), Colo-205 (Colon), HCT-15 (Colon), HT-29 (Colon), SW-620 (Colon), Hep-2 (Liver), IMR-32 (Neuroblastoma), DU-145 (Prostate) and PC-3 (Prostate) (Table 4). AUL produced
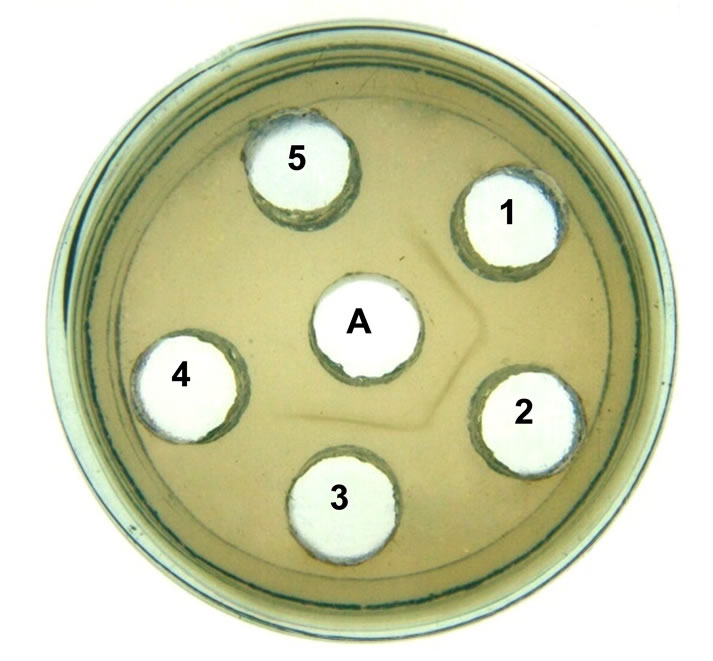
Figure 5. Double immunodiffusion. AUL antiserum was loaded in the central well (A), 15 mg of the purified AUL was loaded in the peripheral (well 1) and the same amount of purified lectin from Sauromatum guttatum (well 2), Gonatanthus pumilus (well 3). The wells 4 and 5 were without lectin.
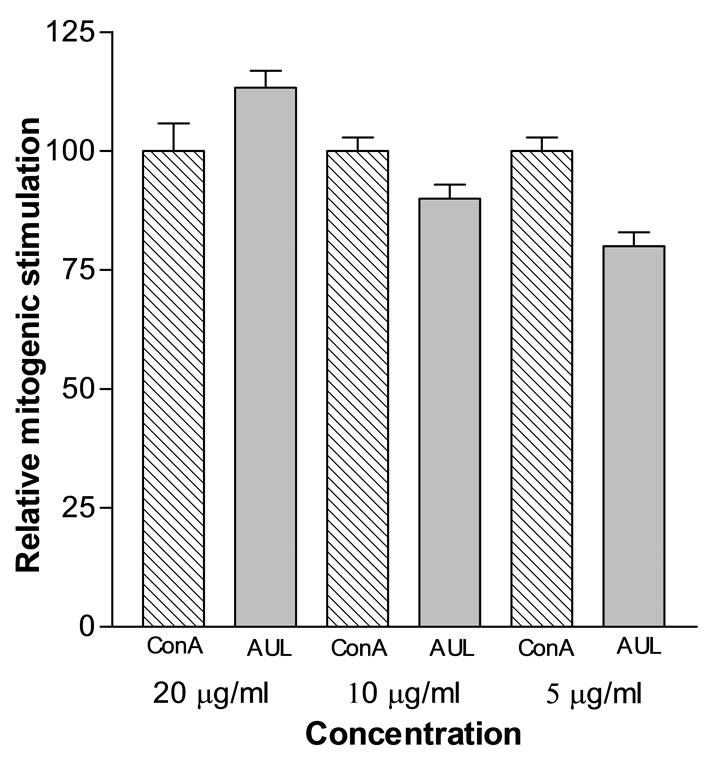
Figure 6. Relative mitogenic index of AUL. Human peripheral blood lymphocytes (1 × 105 cells/well) were cultured with AUL at different concentrations. A positive control is also shown using Con A at different concentrations. In the control wells, cells were cultured with medium alone (no lectin). The proliferation of lymphocytes was measured by MTT assay and the mitogenic index was calculated by dividing absorbance of AUL-stimulated lymphocytes with absorbance of lymphocytes without lectin. The relative mitogenic index of AUL is its mitogenic index relative to mitogenic index of Con A (taken as 100). (Data represent means ± SD, n = 4.)
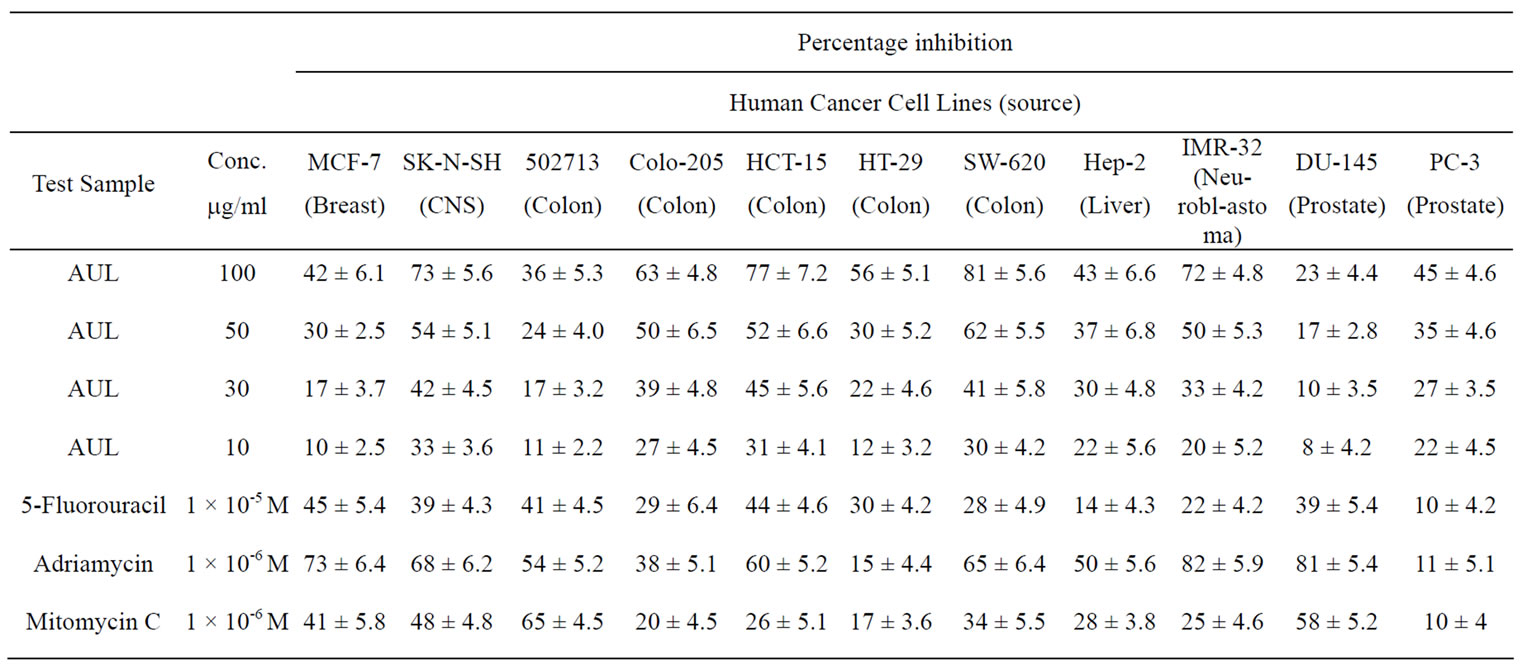
Table 4. In vitro antiproliferative activity of AUL against human cancer cell lines.
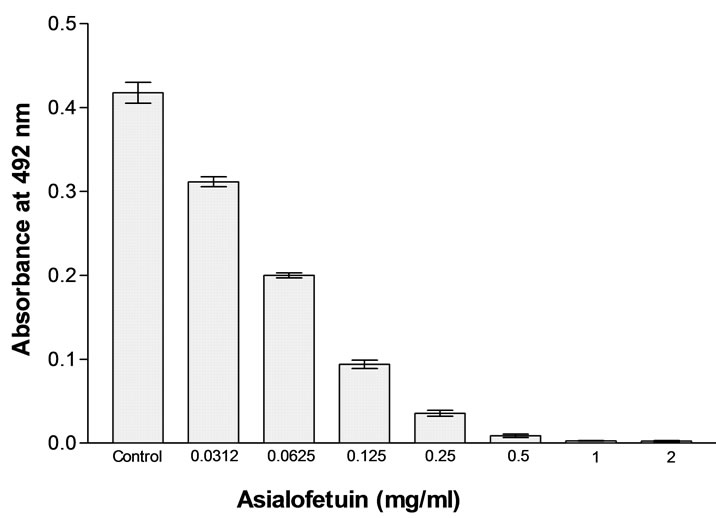
Figure 7. Inhibition of lectin-induced mitogenic stimulation of Human peripheral blood lymphocytes with asialofetuin at a concentration ranging between 2 and 0.0312 mg/ml. Bars represents the percentage inhibition of proliferation. (Data represent means ± SD, n = 4).
50% inhibition (IC50) of cancer cell lines viz. SW-620, HCT-15, SK-N-SH, IMR-32, Colo-205 and HT-29 at 38, 42, 43, 49, 50 and 89 µg/ml respectively as calculated from graph plotted between percentage inhibition and concentration of AUL (Figure 8). However, IC50 was not achieved in MCF-7, 502713, Hep-2, DU-145 and PC-3 cell lines even at 100 μg/ml. The difference in proliferation inhibition of various cell-lines may be due to slight differences in the glycoconjugates expressed on the surface of cancer cells. In the present study, AUL was found specific for LacNAc which has been reported as one of the important cancer markers [37]. It is possible that LacNAc may be one of the components expressed on the tumor cells under investigation and responsible for in teraction with AUL. As every lectin has unique fine sugar specificity, there is a need to investigate a range of
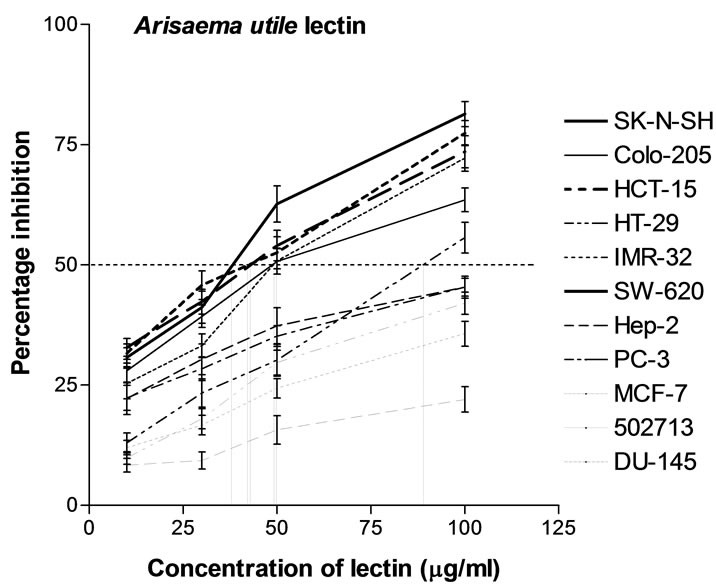
Figure 8. In vitro anti-proliferative effect of Arisaema utile lectin. Inhibitory effect of AUL was tested on human cancer cell lines MCF-7 (Breast), SK-N-SH (CNS), 502713 (Colon), Colo-205 (Colon), HCT-15 (Colon), HT-29 (Colon), SW-620 (Colon), Hep-2 (Liver), IMR-32 (Neuroblastoma), DU-145 (Prostate) and PC-3 (Prostate). Anticancer drugs 5-Fluorouracil, Adriamycin, Mitomycin C were used as positive control. Four different concentrations of lectin i.e. 10, 30, 50 and 100 μg/ml lectin were used for each cancer cell line. Plot between percentage inhibition of cancer cell lines and concentration of AUL was used to calculate the lectin concentration required for 50% inhibition. In the control wells cells were cultured with medium alone (no lectin added). Growth inhibition of cancer cell lines was measured by sulphorhodamine B dye staining assay (Data represent means ± SD, n = 4).
lectins against a number of cancer cell-lines to generate a battery of anti-cancer reagents. There are a few reports of mitogenic lectins which also possess anti-proliferative activity. In this aspect AUL is similar to the lectins isolated from mushrooms having potent mitogenic activity as well as anti-proliferative activity [51-53]. Although several hypotheses have been put forward which suggests that this effect is associated with the ability of lectins to modulate the growth, differentiation, proliferation and apoptosis of premature cells in vivo and in vitro yet the exact molecular mechanism(s) of the antiproliferative effect of plant lectins is not clear at present.
7. CONCLUSIONS
A lectin with mitogenic as well as anti-proliferative activities have been purified and characterized from Arisaema utile Schott in the present study. The lectin was inhibited by N-acetyl-D-lactosamine and asialofetuin. Pure lectin is a homotetrameric molecule of 54 kDa with subunit molecular mass of 13.5 kDa. The lectin is as mitogenic as con A so it could also be used for mitogenic studies to explore the mechanism of lymphocyte activation as lectin bind to sugars of their specificity. Antiproliferative property of AUL suggests the binding of lectin to certain receptor on the cell surface which are responsible for cancerous growth. Therefore this lectin may also be detected as histochemical marker in these type of cancers. The information from clinical studies using pure lectins is promising therefore additional research, including clinical trials, mechanisms of action at the molecular level, and structure-function relationships, should help researchers continue to examine and elucidate the therapeutic effects of lectins Although there is still more to know about the effects of lectins on cancer detection and treatment, this area of research holds great potential.
REFERENCES
- Goldstein, I.J., Hughes, R.C., Monsigny, M., Osawa, T. and Sharon, N. (1980) What should be called a lectin? Nature, 285(5760), 66.
- Sharon, N. and Lis, H. (1989) Lectins as cell recognition molecules. Science, 246(4927), 227-234.
- Sharon, N. and Lis, H. (2003) Lectins. 2nd Edition, Kluwer Academic Publishers, Dordrecht.
- Nowell, P.C. (1960) Phytohemagglutinin: An initiator of mitosis in cultures of normal human leucocytes. Cancer research, 20, 462-464.
- Harris, H. and Robson, E.B. (1963) Precipitin reactions between extracts of seeds of Canavalia ensiformis (jack bean) and normal and pathological serum proteins. Vox Sanguinis, 8(3), 348-355.
- Aub, J.C., Sanford, B.H. and Wang, L.H. (1965) Reactions of normal and leukemic cell surfaces to a wheat germ agglutinin. Proceedings of the National Academy of Sciences, USA, 54, 400-402.
- Brittinger, G. and Konig, E. (1969) Lymphocyte stimulation by pokeweed mitogen (pwm). Klin. Wochenschr, 47, 1307-1313.
- Krickeberg, H., Mauff, G., Mertens, T., Plum, G. and Heitmann, K. (1990) Lymphocyte proliferation in aidsrelated complex/walter-reed 5 patients: response to herpes simplex virus and tuberculin antigen and mitogen during intravenous immunoglobulin treatment,” Vox Sanguinis, 59, 38-43.
- Gabius, H.J. (1987) Endogenous lectins in tumors and the immune system. Cancer investigations, 5, 39-46.
- Zarkovic, N., Vukovic, T., Loncaric, I., Miletic, M., Zarkovic, K. and Borovic, S. (2001) An overview on anticancer activities of the Viscum album extract isorel. Cancer Biotherapy and Radiopharmaceuticals, 16, 55- 62.
- Singh, J., Singh, J. and Kamboj, S.S. (2004) A novel mitogenic and antiproliferative lectin from a wild cobra lily. Arisaema flavum. Biochemical and Biophysical Research Communications, 318, 1057-1065.
- Kaur, A., Kamboj, S.S., Singh, J., Saxena, A.K. and Dhuna, V. (2005) Isolation of a novel N-acetyl-D-lactosamine specific lectin from Alocasia cucullata. Biotechnology Letters, 27, 1815-1820.
- Bantel, H., Engels, I.H., Voelter, W., Schulze-osthoff, K. and Wesselborg, S. (1999) Mistletoe lectin activates caspase-8/flice independently of death receptor signaling and enhances anticancer drug-induced apoptosis. Cancer Research, 59, 2083-2090.
- Park, R., Kim, M.S., So, H.S., Jung, B.H., Moon, S.R. and Chung, S.Y. (2000) Activation of cJun N-terminal kinase 1 (JNK1) in mistletoe lectin II-induced apoptosis ofhuman myeloleukemic U937 cells. Biochemical Pharmacology, 60, 1685-1691.
- Ohba, H., Bakalova, R., Moriwaki, S. and Nakamura, O. (2002) Fractionation of normal and leukemic t-cells by lectin affinity column chromatography. Cancer letters, 184, 207-214.
- Shangary, S., Singh, J., Kamboj, S.S., Kamboj, K.K. and R. S. Sandhu (1995) Purification and properties of four monocot lectins from the family araceae. Phytochemistry, 40, 449-455.
- Dhuna, V., Singh, J., Kamboj, S.S., Singh, J., Shanmugavel and Saxena, A.K. (2005) Purification and characterization of a lectin from Arisaema tortuosum schott having in-vitro anticancer activity against human cancer cell lines. Journal of Biochemistry and Molecular Biology, 38, 526-532.
- Lowry, O.H., Rosebrough, N.J., Farr, A.R. and Randall, R.J. (1951) Protein measurements with folin-phenol reagent. The Journal of Biological Chemistry, 193, 265- 275.
- Spiro, R.G. (1966) Analysis of sugars found in glycoproteins. Methods in Enzymology, 8, 3-26.
- Reisfeld, R.A., Lewis, O.J. and Williams, D.E. (1962) Disc electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature, 145, 281-283.
- Davis, B.J. (1964) Disc electrophoresis: Methods and applications to human serum proteins. Annals of the New York Academy of Sciences, 121, 404-427.
- Bryan, J.K. (1977) Molecular weights of proteins multimers from polyacrylamide gel electrophoresis. Analytical Biochemistry, 78, 513-519.
- Laemmli, U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 277, 680-685.
- Robertson, E.F., Dannelly, H.K., Malloy, P.J. and Reeve, H.C. (1987) Rapid isoelectric focusing in a vertical polyacrylamide minigel system. Analytical Biochemistry, 167, 290-294.
- Andrews, P. (1964) Estimation of the molecular weights of proteins by sephadex gel-filtration. Biochemical Journal, 91, 222-233.
- Paulova, M., Entlicher, G., Ticha, M., Kostir, J.V. and Kocourek, J. (1971) Studies of phytohemagglutinins. Vii. Effect of Mn2+ and Ca2+ on hemagglutinin of phytohemagglutinin of Pisum sativum l. Biochimica et Biophysica Acta, 237, 513-518.
- Spande, T.F. and Witkop, B. (1967) Determination of tryptophan content of protein with n-bromosuccinimide. Methods in Enzymology, 11, 498-532.
- Horton, H.R. and Koshland, Jr., D.E. (1972) Modification of proteins with active benzyl halides. Methods in Enzymology, 25, 468-477.
- Riordan, J.F., Wacker, W.E.C. and Vallee, B.L. (1965) N-acteyl imidazole: A reagent for determination of free tyrosyl residues of proteins. Biochemistry, 4, 1758-1765.
- Wang, H., Ye, X.Y. and Ng, T.B. (2001) Purification of chrysancorin, a novel antifungal protein with mitogenic activity from garland chrysanthemum seeds. Biological Chemistry, 382, 947-951.
- Mosmann, T. (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods, 65, 55-63.
- Boyum, A. (1968) Separation of leukocytes from blood and bone marrow. Scandinavian Journal of Clinical and Laboratory Investigation, 21, 77-89.
- Monks, A., Scudiero, D., Skehan, P., Shoemaker, R., Paul, K. and Vistica, D. (1991) Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. Journal of the National Cancer Institute, 83, 757-766.
- van Damme, E.J., Goossens, K., Smeets, K., van Leuven, F., Verhaert, P. and Peumans, W.J. (1995) The major tuber storage protein of araceae species is a lectin. Characterization and molecular cloning of the lectin from Arum maculatum. Plant Physiology, 107, 1147- 1158.
- Bains, J.S., Dhuna, V., Singh, J., Kamboj, S.S., Nijjar, K. K. and Agrewala, J.N. (2005) Novel lectins from rhizomes of two acorus species with mitogenic activity and inhibitory potential towards murine cancer cell lines. International Immunopharmacology, 5, 1470-1478.
- van Damme, E.J.M., Goldstein, I.J. and Peumans, W.J. (1991) A comparative study of mannose-binding lectins from amaryllidaceae and alliaceae. Photochemistry, 30, 509-514.
- Ito, N., Imai, S., Haga, S., Nagaike, C., Morimura, Y. and Hatake, K. (1996) Localization of binding sites of Ulex europaeus, Helix pomatia and Griffonia simplicifolia i-b4 lectins and analysis of their backbone structures by several glycosidases and poly-n-acetyllactosamine-specific lectins in human breast carcinoma. Histochemistry and Cell Biology, 106, 331-339.
- Green, E.D., Adelt, G., Baenziger, J.U., Wilson, S. and van Halbeek, H. (1988) The asparagine-linked oligosaccharides on bovine fetuin. Structural analysis of n-glycanase-released oligosaccharides by 500-megahertz 1h nmr spectroscopy. The Journal of Biological Chemistry, 263, 18253-18268.
- van Damme, E.J.M., Allen, A.K. and Peumans, W.J. (1988) Related mannose-specific lectins from different species of the family amaryllidaceae. Plant Physiology, 73, 52-57.
- Chandra, N.R., Prabu, M.M., Suguna, K. and Vijayan, M. (2001) Structural similarity and functional diversity in proteins containing the legume lectin fold. Protein Engineering, 11, 857-866.
- Pang, Y., Shen, G.A., Liao, Z.H., Yao, J.H., Fei, J., Sun, X.F. and Tang, X. (2003) Molecular cloning and characterization of a novel lectin gene from Zephyranthes candida. DNA Sequence, 14, 163-167.
- Hayes, C.E. and Goldstein, I.J. (1974) An alphad-galactosyl-binding lectin from bandeiraea simplicifolia seeds. Isolation by affinity chromatography and characterization. The Journal of Biological Chemistry, 249, 1904-1914.
- van Damme, E.J., Smeets, K., Torrekens, S., van Leuven, F., Goldstein, I.J. and Peumans, W.J. (1992) The closely related homomeric and heterodimeric mannosebinding lectins from garlic are encoded by one-domain and two-domain lectin genes, respectively. European Jou nal of Biochemistry, 206, 413-420.
- Devyani, N. and Mala, R. (1998) Structural and functional role of tryptophan in xylanase from an extremophilic bacillus: Assessment of the active site. Biochemical and Biophysical Research Communications, 249, 207-212.
- Zand, R., Agrawal, B.B.L. and Goldstein, I.J. (1971) pH-dependent conformational changes of concanavalin A. Proceedings of the National Academy of Sciences, USA, 68, 2173-2376.
- Kaur, M., Singh, K., Rup, P.J., Saxena, A.K., Khan, R.H., Ashraf, M.T., Kamboj, S.S. and Singh, J. (2006) A tuber lectin from Arisaema Helleborifolium schott with antiinsect activity against melon fruit fly Bactrocera Cucurbitae (coquillett) and anti-cancer effect on human cancer cell lines. Archives of Biochemistry and Biophysics, 445, 156-165.
- Desai, N.N., Allen, A.K. and Neuberger, A. (1983) The properties of potato (Solanum tuberosum) lectin after deglycosylation by triflouromethanesulphonic acid. Biochemical Journal, 211, 273-276,
- Konozy, E.H.E., Mulay, R., Faca, V., Aard, R.J., Greene, L.J., Roque-barriera, M.C., Sabharwal, S. and Bhide, S.V. (2002) Purification, some properties of a d-galactosebinding leaf lectin from Erythrina indica and further characterization of seed lectin. Biochimie, 84, 1035-1043.
- Konozy, E.H.E., Bernardes, E.S., Rosa, C., Faca, V. Greene, L.J. and Ward, R.J. (2003) Isolation, purification, and physicochemical characterization of a d-galactosebinding lectin from seeds of Erythrina speciosa. Archives of Biochemistry and Biophysics, 410, 222-229.
- Sultan, N.A.M., Kenoth, R. and Swamy, M.J. (2004) Purification, physicochemical characterization, saccharide specificity, and chemical modification of a gal/galnac specific lectin from the seeds of Trichosanthes dioica,” Archives of Biochemistry and Biophysics, 432, 212-221.
- Yu, L.G., Fernig, D.G., White, M.R., Spiller, D.G., Appleton, P. and Evans, R.C. (1999) Edible mushroom (Agaricus bisporus) lectin, which reversibly inhibits epithelial cell proliferation, blocks nuclear localization sequence-dependent nuclear protein import. The Journal of Biological Chemistry, 274, 4890-4899.
- Wang, H.X., Ng, T.B., Liu, W.K., Ooi, V.E. and Chang, S.T. (1995) Isolation and characterization of two distinct lectins with antiproliferative activity from the cultured mycelium of the edible mushroom Tricholoma mongolicum. International Journal of Peptide and Protein Research, 46, 508-513.
- Ngai, P.H. and Ng, T.B. (2004) A mushroom (Ganoderma capense) lectin with spectacular thermostability, potent mitogenic activity on splenocytes, and antiproliferative activity toward tumor cells. Biochemical and Biophysical Research Communications, 314, 988-993.

