World Journal of Condensed Matter Physics
Vol.3 No.1(2013), Article ID:28283,7 pages DOI:10.4236/wjcmp.2013.31003
Experimental and Theoretical Study on the Ethane and Acetylene Formation from Electron Irradiation of Methane Ices
![]()
1Physics Department of Calabria Univeristy, Rende, Italy; 2National Research Council-CNR, Magna Graecia University, Catanzaro, Italy; 3Astronomy and Physics Department, University of Catania, Catania, Italy.
Email: *mmarianna.barberio@fis.unical.it
Received October 3rd, 2012; revised November 9th, 2012; accepted November 21st, 2012
Keywords: Solid Ices; Ethane Formation; Acetylene Formation; Electron Irradiation
ABSTRACT
In this work we present an experimental and theoretical study on the formation of ethane and acetylene from solid methane condensed at 20 K and irradiated with a 500 - 3000 eV electron beam. The experiments were monitored with Thermal Desorption Spectroscopy. We observe that the electron irradiation induced a dehydrogenation of methane and a consequent formation of CHx (x = 1, 2, 3) fragments. Furthermore, in the solid during irradiation, a simple recombination reaction in the solid between two adjacent CHx molecules may form HC≡CH, H2C=CH2, and H3C-CH3 with a triple, double, and single carbon-carbon bond, respectively. The formed amount of ethane and acetylene increases with irradiation time and reaches a saturation value.
1. Introduction
In the past decades, self assembled monolayers and multilayers of different materials have attracted growing attention in many areas of practical applications such as protein microsensors, electrochemical interfaces, microelectronics development and surface science investigations [1].
In particular the study of interaction between these thin films and charged and energetic particles has been developed for its implications in astrophysics and in spatial device applications [1-3]. In this context it is very important to obtain information about the formation of macromolecules due to the interaction between the interstellar medium and the charged and energetic particles of cosmic radiation.
The interstellar medium (ISM) is composed of gas for 99% and constitutes about 10% of the mass of our Galaxy. Hydrogen and helium represent respectively 93.38% and 6.49% of ISM chemical composition, while oxygen, carbon and nitrogen contribute only with 0.11% (O:C:N = 6:3:1, [4]).
The ISM is not distributed uniformly in the space between stars but settles in the galactic plane, where it “condenses” in large-scale structures as for example the dense interstellar clouds. In these environments a rich chemistry takes place, actually around 140 molecular species have been identified.
Atoms, molecules, and radicals in the gas phase can condense on the surface of dust grains forming an icy mantle with a thickness of about 0.1 μm. The presence of ices is confirmed by IR spectroscopy of obscured stellar sources. Icy mantles are constituted by a solid mixture containing H2O, CO, CH4 and NH3 [5,6]. Ices in the molecular clouds and solar system bodies are subjected to the action of cosmic rays, UV photons and energetic particles. New complex organic species may form during interactions with UV radiations or energetic ions [7], while ionization may occur if the ices are irradiated with cosmic rays. Though the icy mantles are rarely under energetic electron bombardment, the electrons emitted in ionization processes can be energetic enough to further ionize or excite surrounding molecules creating photons, electrons, ions, radicals, and roto-vibronically excited species [8]. In some cases, a simple local warming up may also facilitate some reactions [9].
Therefore it is essential to understand the effects of electron bombardment on the chemical composition of the ices [10]. Several systematic studies have been reported in the literature concerning the interactions of electrons with various astronomically relevant molecular ices as H2O, NH3, CH4 etc. and their mixtures by employing infrared spectroscopy and thermal desorption (TD) techniques [2,10-12].
In particular, studies of chemical composition of comets [11,12] suggest that the formation of hydrocarbons, ethane and acetylene mostly, is originated from the irradiation of methane within interstellar ices. In this framework the study of production of new hydrocarbons formed from the irradiation of methane assumes particular importance. Recently Bennet et al. [3] performed the irradiation of methane ices at 10 K with energetic electrons (5 keV) to mimic the energy transfer process that occur in the track of the trajectories of MeV cosmic ray particles. Their experiment, monitored via FTIR spectroscopy, comes to conclusions that the primary effects of irradiation is the formations of methyl radical CH3 and that subsequently in the matrix two neighboring methyl radicals can recombine to form ethane molecule (C2H6), finally the irradiations of these new molecules generated ethylene, ethyl radical, vinyl radical and acetylene as degradation products. However, in this study the irradiation time is limited to 50 minutes and the ethane production is linearly increasing without showing any sign of turn up or stabilization on a saturation value.
In this work we present an experimental and theoretical investigation on 500 - 3000 eV electron induced chemical reaction in solid methane at 20 K. Our study confirms and improves that one of Bennet et al. Indeed we obtain as Bennet [3] that the primary effects of irradiations is the methyl radical formations, followed by the formations of ethane and of other hydrocarbons. Moreover we demonstrated, for first time, that this process is possible also for low energy electrons (in the range 500 - 3000 eV) and that for very large irradiation time (about 250 minutes) the ethane production leads a saturation value.
2. Experiments
The experiments were conducted in an ultra high vacuum chamber (base pressure in the low 10−10 mbar range) evacuated with a dry turbo pump. A computer controlled quadruple mass spectrometer was used to monitor various gas species released during the thermal desorption processes. For each of them, a mass scan of about 40 data points was taken and the integrated area was used as the signal intensity. To better detect all desorbed gases, an automatically adjusted and differently set sensibility scale was used for each mass.
Methane ice was formed by condensing CH4 onto the stainless steel cold head surface (7 cm2) of a close cycled He refrigerator by introducing CH4 gas (purity of 99.999%) in the chamber through a precision leak valve. Background dosing allowed formation of a uniform film while use of a 2 mm diameter Cu tube with its end reproducibly positioned at <1 mm away from the cold surface enabled the growth of a column-like bulk film. In the latter case, the gas pressure in the tube and the dosing time were kept constant. The sample temperature was monitored with a calibrated Au/0.7% Fe-chromel thermocouple and the adsorption temperature was 20 ± 2 K. Sample heating was achieved by simply turning off the refrigerator compressor. In the temperature range of our interest (40 - 120 K) the heating rate was nearly constant (3 K/min). Quadrupole mass spectra and the sample temperature recording were synchronized.
We observe that, for background dosing, two distinct desorption peaks were observed for as deposited CH4 films with a temperature difference of 10 K and their intensity ratio remained constant as the amount of the adsorbed CH4 varied. This was due to adsorption on two different portions of the cold head, as checked with a set of thermocouples positioned on various parts of the second stage of the refrigerator. The desorption features reported in this paper are relative to those coming from the coldest area which were subjected to electron irradiation.
In studying the effects of interactions with the CH4 ice, the electron beam with energy of 200 - 3000 eV was totally defocalized with a spot size similar to the sample surface area to avoid the local heating. The beam current was in the order of µA and the bombardment time ranged from a few minutes up to 250 minutes.
Tiny traces of H2O, N2 and O2 were detected during desorption but they were absent if the chamber was directly filled with gases suggesting that they were due to condensation of the chamber residual contaminant species.
3. Results and Discussions
3.1. Experimental Results
In Figure 1 we present four typical mass spectra taken at temperatures of T = 47 K and T = 62 K during thermal desorption of a thin solid methane film deposited with 350 L background dosing 1 L = 1 × 10−6 Torr∙sec. Spectra (a) and (b) are for the sample irradiated with 2000 eV electrons while spectra (c) and (d) are relative to an as deposited film. For the purpose of comparison we also show the mass spectra taken by filling the chamber at 1 × 10−6 mbar with room temperature CH4, C2H6, and H2 gases (curves (e)-(g), respectively).
We first note that the quadrupole filament (operating at 60 V [13,14]) produced a great amount and variety of fragments for methane and ethane. This certainly overshadowed the effects of electron irradiation on solid CH4. Indeed, the intensity ratios of CHx/CH4 and C2Hx/C2H6 are very similar for curves (a), (c) and (e) and for curves
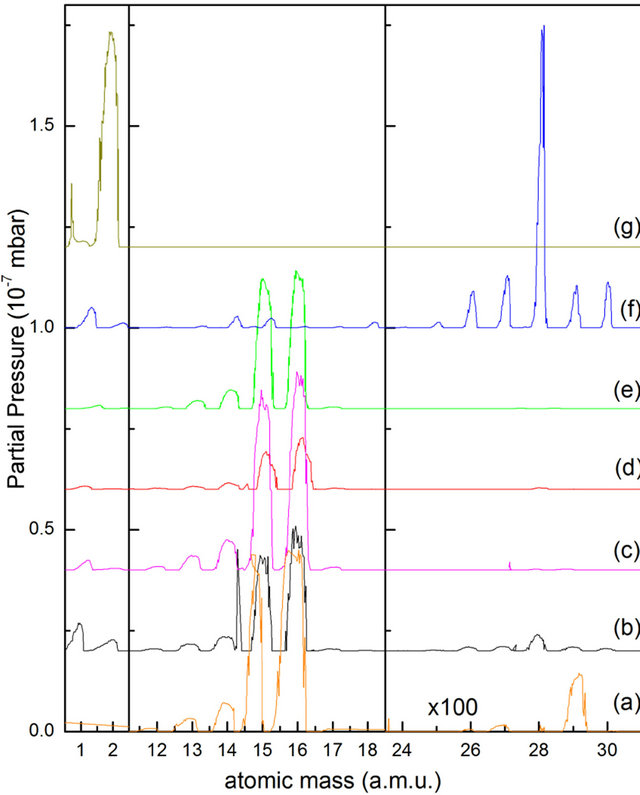
Figure 1. Quadruple mass spectra taken during thermal desorption of a 2 keV electron bombarded thin CH4 film at 47 K (curve (a)) and 62 K (curve (b)) and for an as-deposited film (curves (c) and (d)). Also shown are the scans for background filled methane, ethane and hydrogen gases at 1 × 10−6 mbar (curves (e)-(g), respectively). For the sake of clarity, all curves have been shifted.
(b), (d) and (f), respectively, clearly indicating that cracking due to the quadrupole filament is by far the most dominant mechanism for the observed dissociation of methane and ethane.
Thermal desorption curves of the relevant species for an electron bombarded sample are illustrated in Figure 2. The film was obtained by background dosing and its thickness was approximately 1350 Å (or 350 layers) allowing the primary electrons of 2000 eV to penetrate through. The electron flux was Φ = 2 × 1016 electrons/cm2. The spectra can be gathered into three groups which appear separately in different temperature ranges. Methane and its derivates CHx are released at about 47 K, atomic and molecular hydrogen signals are seen at 29 K and together with CHx at 47 K, whereas the C2Hx species desorb at higher temperatures with two wider features centered at 62 and 75 K. We notice that for T > 50 K, the TPD curve for CH4 is structureless though its intensity is much higher than those of C2Hx species. For as deposited films no dicarbonic molecules were detected.
To further study the irradiation effects, we condensed a column-like bulk CH4 with relatively small quantity of methane gas avoiding the over pressure during desorp-
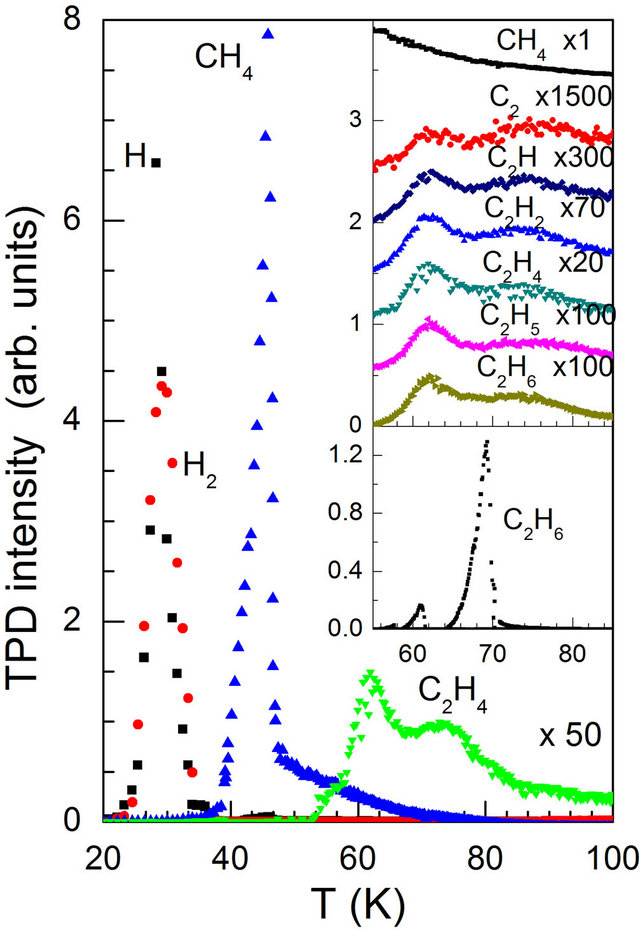
Figure 2. Thermal desorption traces of some relevant species for a background dosed methane film of 450 L after 60 min of 2 keV electron bombardment at a current density of 880 μA/cm2. In the upper panel of the inset are shown the traces of C2Hx while that illustrated in the lower panel is for an as deposited ethane film.
tion. The thickness was estimated to be a few tens of mm and quite reproducible as judged by the total desorbed CH4 signal. The monotonous increase of the amount of desorbed C2H6 plotted in Figure 3 as a function of the electron irradiation time clearly demonstrates the effects of electron stimulated reactions.
In discussing the electron induced dehydrogenation occurring in the solid methane matrix, we mention that the energy required for CH4 dissociation is only a few eV so that the impinging primary electrons and also seconddary electrons generated in the cascade could produce CHx fragments with CH3 being the most important one [Shirai et al. [15], Table 1]. Because of the dominant quadrupole cracking effects on desorbing methane molecules from a large portion of cold surfaces which were not subjected to electron bombardment, a direct detection of these CHx reaction products through thermal desorption spectra was difficult. However, these reactions could still be inferred from the observation of molecular hydrogen during electron irradiation. Indeed, turning on and off the electron beam with use of a mechanical shuttle resulted in a quite reproducible on-off behavior of the H2 signals.
In the solid, to the molecular dissociation may follow a recombination process, which is negligible in gas phase because of the large intermolecular distance. The most favored recombination reactions are those with very low binding enthalpies, as the formation of hydrogen molecules, acetylene, ethylene, and ethane. A simple recombination reaction between two adjacent CHx (x = 1, 2, 3) molecules may form HC≡CH, H2C=CH2, and H3C-CH3 with a triple, double, and single carbon-carbon bond, respectively.

Figure 3. Total C2H6 (a) and C2H2 (b) intensity produced by 2 keV electron bombardment on a thick solid CH4 film as a function of irradiation time. The continuous curve is a best fit by using a simple rate-equation based recombination model (see text).
The fact that all C2Hx TPD curves have nearly the same line shape (upper panel of the inset of Figure 2) would strongly suggest an essentially common origin of these species. A comparison of the intensity ratios seen in the TPD curves between C2Hx and C2H6 with those recorded for ethane gas (curve (g) of Figure 1) reveals that virtually all observed dicarbonic species could be attributed to the quadrupole filament cracking of desorbed ethane molecules except for acetylene. Indeed, in this later case, the detected intensity ratio is much higher than the cracking faction. This observation leads us to conclude that a substantial portion of C2H2 must have formed in the CH4 matrix upon electron irradiation.
A comparison with the TPD curve of a solid C2H6 film (lower panel of the inset in Figure 2) show that the binding environments of electron irradiation produced ethane are much more complex with two broad structures centered at about 62 and 73 K, in contrast to the sharp desorption peak of bulk ethane at 69 K.
3.2. Theoretical Model
We point out that the difference in the desorption temperatures for CH3 (CH) and C2H6 (C2H2) species unambiguously indicates that ethane (acetylene) molecules are formed in the solid during electron irradiation or during thermal annealing, but certainly not in gas phase during desorption.
To get some estimates on the process of CH4 dehydrogenation into CH3 and the subsequent CH3 recombination into C2H6, we first note that the probability that a molecule within the reaction depth l gets hit by a 2 keV electron in a second is 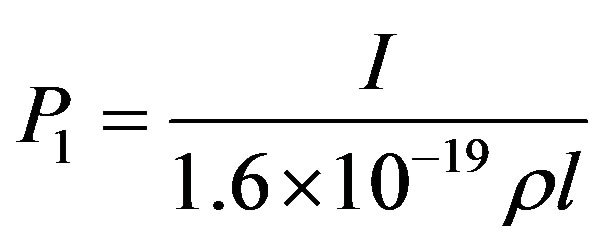 where I = 8.8 × 10−3 A∙m−2 is the electron beam density and ρ = 1.694 × 1028 m−3 the site number density. The probability a methane molecule undergoes a collision with a primary electron is
where I = 8.8 × 10−3 A∙m−2 is the electron beam density and ρ = 1.694 × 1028 m−3 the site number density. The probability a methane molecule undergoes a collision with a primary electron is 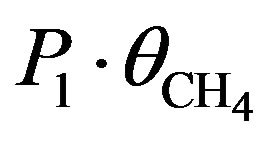 with
with 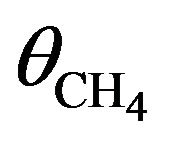 denoting the CH4 concentration:
denoting the CH4 concentration: 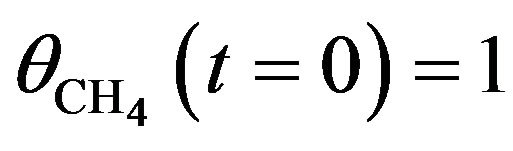 and
and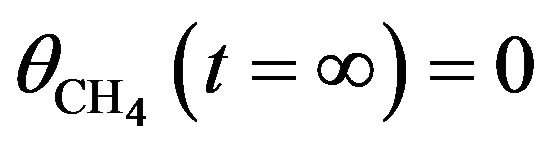 . In the case of background dosing as that shown in Figure 2, the film thickness d = 135 nm was smaller than the primary electron penetration length λ [16-18] so that chemical reaction took place through the whole sample thickness:
. In the case of background dosing as that shown in Figure 2, the film thickness d = 135 nm was smaller than the primary electron penetration length λ [16-18] so that chemical reaction took place through the whole sample thickness:
Table 1. cross section (cm2) vs electron energy for emission of product from CH4 electron irradiation.
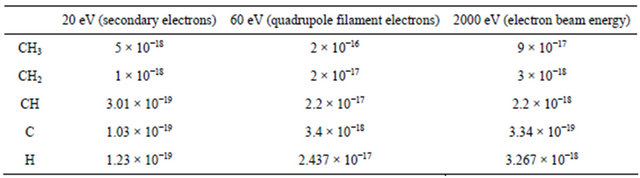
l = d, P1 = 2.45 × 10−5. The dissociation probability for CH4 is 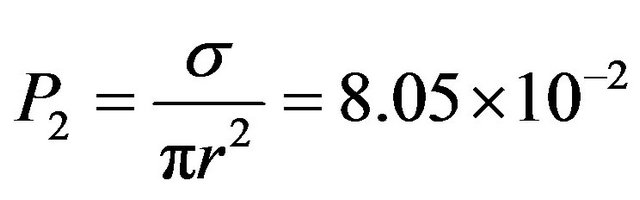 with σ = 9.5 × 10−17 cm2
with σ = 9.5 × 10−17 cm2
being the cross section at 2 keV (Table 1) and r = 1.95 Å the molecular mean radius. Therefore, the probability that a CH4 molecule could be dissociated in a second is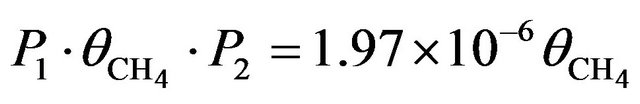 . Considering that a 2 keV electron produces many secondary electrons, we may reasonable assume the total number of hits to be at least 50 with a similar s, then the real probability of dissociating a CH4 molecule in a second can be as high as 1 × 10−4
. Considering that a 2 keV electron produces many secondary electrons, we may reasonable assume the total number of hits to be at least 50 with a similar s, then the real probability of dissociating a CH4 molecule in a second can be as high as 1 × 10−4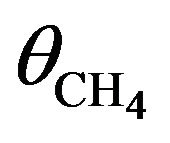 . For
. For 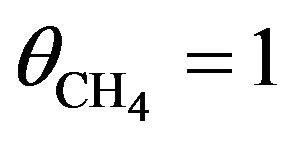 the total probability of dehydrogenation in an hour is P = 36%, thus as a first approximation we may take a simple average value of
the total probability of dehydrogenation in an hour is P = 36%, thus as a first approximation we may take a simple average value of
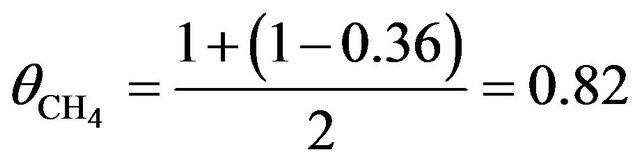 so that P = 30%.
so that P = 30%.
Experimentally, the intensity ratio between C2H6 (including its cracking fragments) and CH4 originating from electron bombarded region is about 1.8 × 10−2, thus that between CH3 involved in the recombination and CH4 is 3.6 × 10−2. This later value indicates that only about 10% of produced CH3 recombine to form C2H6.
To somehow quantitatively describe the kinetics of electron induced reactions occurring in the solid matrix (case of column-like thick film), we assume that the film thickness to be much larger than the electron penetration length and denote with 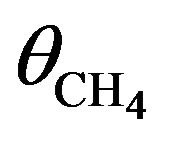 the concentration of CH4 molecules. The rate equation for methane [2] can be written as
the concentration of CH4 molecules. The rate equation for methane [2] can be written as
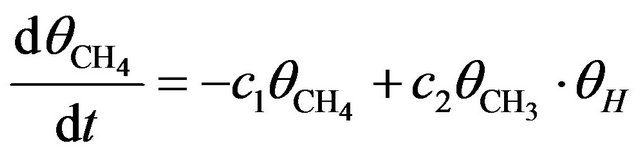 (1)
(1)
where c1 is the rate constant for reduction of CH4 into CHx (x = 0, 1, 2, 3) and c2 that for reformation from CH3 and H. Because of the preferential formation of H2 and its instant release from the solid, we can reasonably assume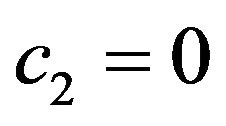 . Equation (1) then has a simple solution
. Equation (1) then has a simple solution
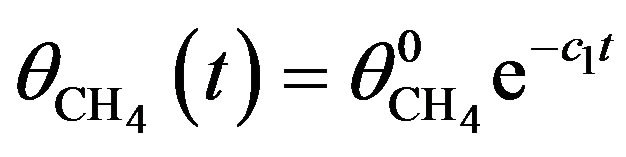 (2)
(2)
where  denotes the initial density of CH4.
denotes the initial density of CH4.
The rate equation for CH3 molecules [2] is given by
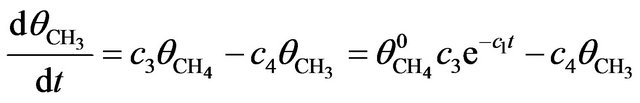 (3)
(3)
where c3 is the rate constant for dissociation of CH4 into CH3 and c4 that for fragmentation of CH3 into smaller ones. The solution of (3) satisfying the initial condition  is:
is:
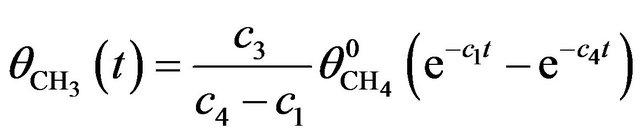 (4)
(4)
As the CH3 + CH3 àC2H6 recombination is concerned, if it occurs at low temperature during electron irradiation, then its rate equation is given as
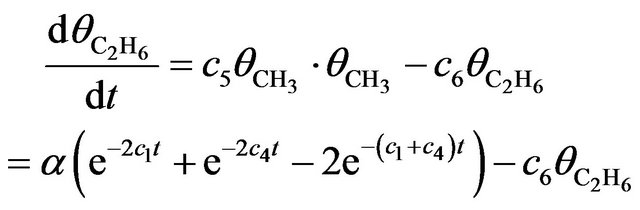 (5)
(5)
with c5 denoting the recombination rate and c6 the dissociation rate. The solution for (5) is
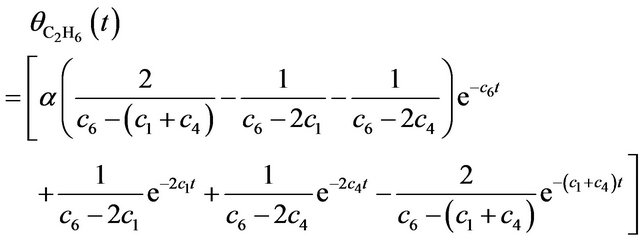 (6)
(6)
However, if the recombination process occurs during thermal heating (in the solid and before desorption) then the probability of formation of C2H6, P, is simply proportional to the square of 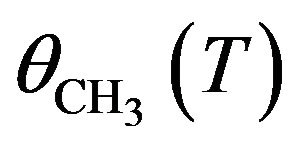 with T indicating the irradiation time T
with T indicating the irradiation time T
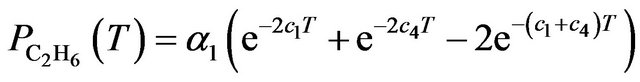 (7)
(7)
As shown in Figure 3, fitting to the total intensity of C2H6 produced in the solid, sum of all its detected fragments, as a function of electron irradiation time T yields 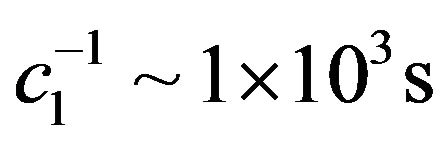 and
and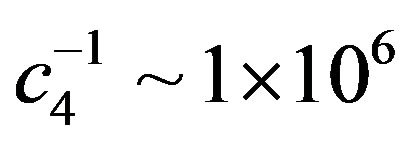 .
.
An independent evaluation of the rate of electron induced dehydrogenation of a methane molecule in a solid matrix A can be made as follows: the reaction depth d =λ = 300 nA; P1 = 1.13 × 10−5, the probability that a CH4 molecule to be dissociated in a second is 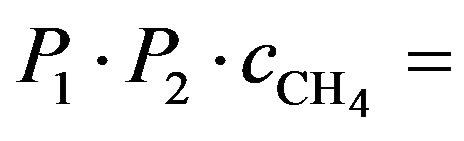
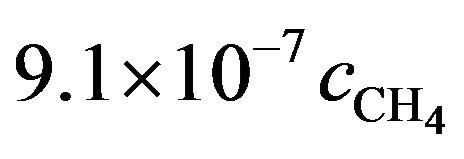 . We may reasonable assume the total number of hits to be at least 100 with a similar s,
. We may reasonable assume the total number of hits to be at least 100 with a similar s, 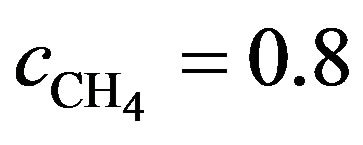 then the real probability of dissociating a CH4 molecule can be 7.3 × 10−5 or more or a constant rate of 1.4 × 104 s.
then the real probability of dissociating a CH4 molecule can be 7.3 × 10−5 or more or a constant rate of 1.4 × 104 s.
This estimate is just about a factor of 14 off the experimental value of A = 1 × 103 s. Considering the roughness of this simple model with large uncertainties in the parameters used such as λ and the average hits per incident electron, and of the assumption of a uniform distribution of electron-CH4 collision probability over the depth, we may certainly regard the experimentally determined value of A reasonable. We also point out that in 100 hits only 8% will produce dissociation with a total energy loss of only 40 eV or something more.
Further, by assuming that all CH is produced in the solid via dissociation of CH4 just as in the case of CH3, we fitted the intensity of C2H2 after subtracting the cracking fraction of C2H6 with use of Equation (5) to (lower panel of Figure 3). The so determined time constant A = 2 × 103 s is quite similar to the value obtained for C2H6, though the assumed hypothesis is quite rough.
3.3. Monte Carlo Simulation
For a better knowledge of our experimental results we also developed a simple Monte Carlo code which simulates the irradiation of a solid methane sample with electrons. The code follows the basic ideas of the program CASINO.
CASINO (monte CArlo SImulation of electroNs in sOlids) has been programmed by the research team of Raynald Gauvin and it is well described in Drouin et al. [19]. Essentially the main part of the Monte Carlo program is the simulation of a complete electron trajectory. The electron lands randomly on the sample with an initial penetration angle fixed by the user. The distance between two successive collisions is evaluated using the equations
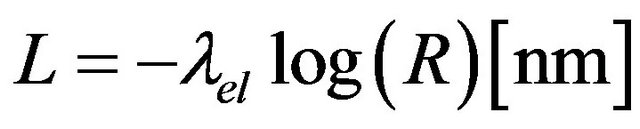 (8)
(8)
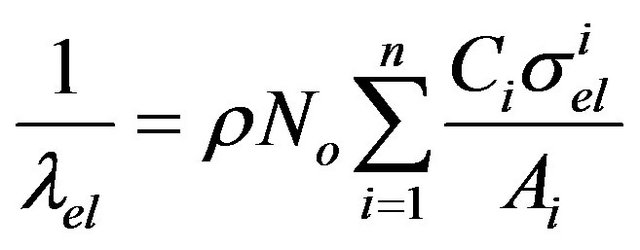 (9)
(9)
where Ci and Ai are the weight fraction and atomic weight of element i, r is the density of the region (g/cm3), No is the Avogrado’s constant and siel is the electron total cross section.
The energy between collisions can be calculated as
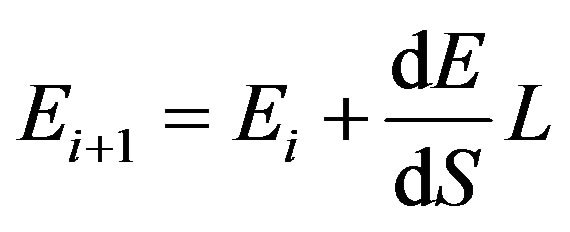 (10)
(10)
with 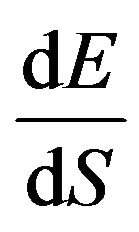 given in Drouin et al. [19]. The scattering angle is determined as described in Hovington et al. [20]. The steps given above are repeated until the electron energy is less than a predetermined value or the electron escapes from the sample.
given in Drouin et al. [19]. The scattering angle is determined as described in Hovington et al. [20]. The steps given above are repeated until the electron energy is less than a predetermined value or the electron escapes from the sample.
Our code, MethDiss (Methane Dissociation), will be described in depth in a separate paper. Here we briefly report that we consider a solid CH4 sample with dimensions of (300 ´ 300 ´ 300) nm3. A layer of CH4 molecules has a depth of 3 nm, the outer layer is on the xy plane with z = 0, the inner layers have z < 0. Electrons with a given energy come from z > 0 in direction perpendicular to the sample with a flux rescaled by the experimental situation. Electrons path in the solid is followed by using the steps given above. At each collision we considered the possibility that the molecule hit by the electron is dissociated or ionized. Cross sections (total, dissociation, ionization) for electron collisions with hydrocarbons are taken from Shirai et al. [15]. Upon electron irradiation the sample, initially formed by CH4 molecules only, becomes populated by CHx and C2Hx hydrocarbons. We assumed that formation of more complex hydrocarbons can take place when two nearest neighbour molecules react together. With these assumptions we obtained, in our simulations, the formation of all the molecules detected during the experiments. Simulations and data analysis of the most comprehensive model are not completed yet. We took into account dissociation of CHx and C2Hx hydrocarbons (which brings to the production of neutral and ionized species) and chemical reactions between them, H2 production and diffusion, H recombination, neutralization of ionized species due to low energy electrons. Our goal is to be able to fit the concentrations of all the detected species as a function of the irradiation time. Here we give the results of a preliminary, very simple, simulation. We considered only dissociation of CH4 molecules in CH3 and H. In Figure 4 we show the comparison between normalized experimental data (black squares) and simulation (black line) for C2H6 formation. So, using the simulated irradiation time as the only free parameter our simulation is able to reproduce experimental data.
4. Conclusion
In this work we presented an experimental and theoretical investigation on 500 - 3000 eV electron induced chemical reactions in solid methane at 20 K. Our study confirms and improves that one of Bennet et al. [3]. We
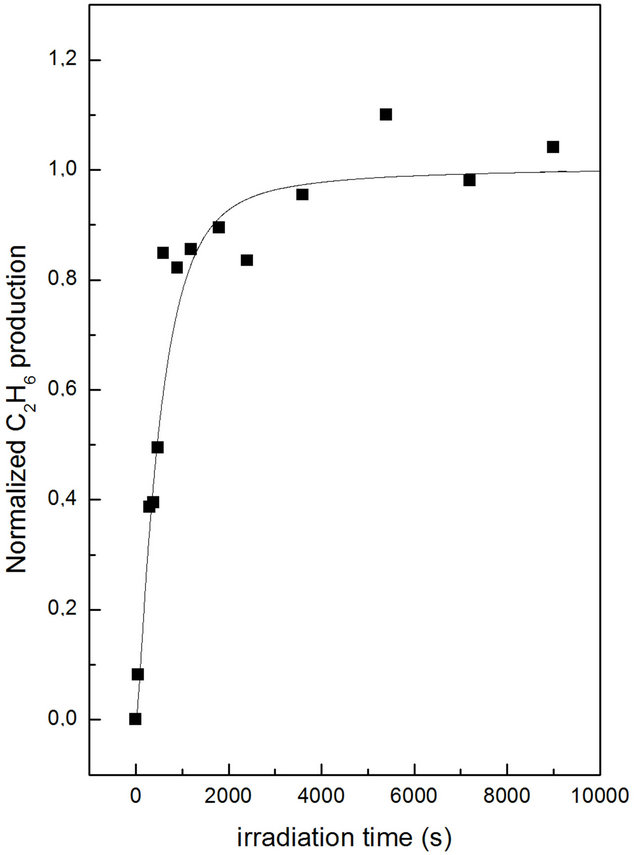
Figure 4. Total C2H6 (a) intensity produced by 2 keV electron bombardment on a thick solid CH4 film as a function of irradiation time. The continuous curve is obtained using the CASINO results (see text).
obtain that the primary effects of irradiations is the formations of CH3, CH2 and CH fragments from the dehydrogenation of methane. The formations of ethane and acetylene is obtained, during solid ice irradiation, from a simple recombination reaction between two adjacent CH3 or CH radicals. Our experimental results are confirmed with the implementation of a theoretical model and a Monte Carlo simulation that discussed the ethane (acetylene) formation in terms of association of two adjacent methyl radicals CH3 (CH2) during electron irradiation. At this stage we assume that the effect of thermal desorption is negligible, but more efforts are necessary to introduce the effects of thermal desorption on dicarbonic species formation.
REFERENCES
- E. Garand and P. A. Rowntree, “The Mechanism of Hydrogen Formation Induced by Low-Energy Electron Irradiation of Hexadecanethiol Self-Assembled Monolayers,” The Journal of Physical Chemistry B, Vol. 109, No. 26, 2005, pp. 12927-12934. doi:10.1021/jp050817k
- H. F. Winters, “Dissociation of Ethane by Electron Impact,” Chemical Physics, Vol. 36, No. 3, 1979, pp. 353- 364. doi:10.1016/0301-0104(79)85019-3
- C. J. Bennet, C. S. Jamieson, Y. Osamura and R. I. Kaiser, “Laboratory Studies on the Irradiation of Methane in Interstellar, Cometary, and Solar System Ices,” The Astrophysical Journal, Vol. 653, No. 1, 2006, p. 792. doi:10.1086/508561
- Scheffler and Elsasser, “Physics of the Galaxy and Interstellar Matter,” Springer, Berlin, 1988.
- D. C. B. Whittet, “Observations of Molecular Ices,” In: T. J. Millar and D. A. Williams, Eds., Dust and Chemistry in Astronomy, Cambridge University Press, Cambridge, 1993, p. 201.
- Hollenbach and Thronson, “Interstellar Processes,” Proceedings of the Symposium on Interstellar Processes, Grand Teton National Park, 1-7 July 1986, Vol. 134.
- W. A. Schutte, “Production of Organic Molecules in Interstellar Ices,” Advances in Space Research, Vol. 30, No. 6, 2002, pp. 1409-1417. doi:10.1016/S0273-1177(02)00500-8
- R. I. Kaiser and K. Roessler, “Theoretical and Laboratory Studies on the Interaction of Cosmic-Ray Particles with Interstellar Ices. III. Suprathermal Chemistry-Induced Formation of Hydrocarbon Molecules in Solid Methane (CH4), Ethylene (C2H4), and Acetylene (C2H2),” The Astrophysical Journal, Vol. 503, No. 2, 1998, p. 959. doi:10.1086/306001
- A. Wada, N. Mochizuki and K. Hiraoka, “Methanol Formation from Electron-Irradiated Mixed H2O/CH4 Ice at 10 K,” The Astrophysical Journal, Vol. 644, No. 1, 2006, p. 300. doi:10.1086/503380
- Zheng, Jewitt, Osamura and Kaiser, “Formation of Nitrogen and Hydrogen-bearing Molecules in Solid Ammonia and Implications for Solar System and Interstellar Ices,” The Astrophysical Journal, Vol. 674, No. 2, 2008, p. 1242. doi:10.1086/523783
- P. Ehrenfreund, S. B. Charneley and D. Wooden, “Comets II,” University of Arizona Press, Tucson, 2004.
- T. Y. Brooke, A. T. Tokunaga, H. A. Weaver, J. Crovisier, D. Bockelée-Morvan and D. Crisp, “Detection of Acetylene in the Infrared Spectrum of Comet Hyakutak,” Nature, Vol. 383, 1996, pp. 606-608. doi:10.1038/383606a0
- http://www.sisweb.com/referenc/source/exactmas.htm
- M. Barberio, P. Barone, R. Vasta, G. Manicò and F. Xu, “Formation of Molecular Nitrogen and Diazene by Electron Irradiation of Solid Ammonia,” Thin Solid Film, Vol. 520, No. 16, 2012, pp. 5479-5481. doi:10.1016/j.tsf.2012.03.112
- T. Shirai, et al., “Analytic Cross Sections for Electron Collisions with Hydrocarbons: CH4, C2H6, C2H4, C2H2, C3H8, and C3H6,” Atomic Data and Nuclear Data Tables, Vol. 80, No. 2, 2002, pp. 147-204. doi:10.1006/adnd.2001.0878
- D. J. Alberas-Sloan and J. M. White, “Low-Energy Electron Irradiation of Methane on Pt(111),” Surface Science, Vol. 365 No. 2, 1996, pp. 212-228. doi:10.1016/0039-6028(96)00720-0
- http://xdb.lbl.gov/Section3/Sec_3-2.html
- M. A. Huels, P. C. Dugal and L. Sanche, “Degradation of Functionalized Alkanethiolate Monolayers by 0 - 18 eV Electrons,” Journal of Chemical Physics, Vol. 118, No. 24, 2003, pp. 11168-11179. doi:10.1063/1.1574791
- D. Drouin, A. R. Couture, D. Joly, X. Tastet, V. Aimez and R. Gauvin, “CASINO V2.42—A Fast and Easy-toUse Modeling Tool for Scanning Electron Microscopy and Microanalysis Users,” Scanning, Vol. 29, No. 3, 2007, p. 92.
- P. Hovington, D. Drouin and R. Gauvin, “CASINO: A New Monte Carlo Code in C Language for Electron Beam intEraction—Part I: Description of the Program,” Scanning, Vol. 19, No. 1, 1997, pp. 1-14. doi:10.1002/sca.4950190101
NOTES
*Corresponding author.

