Journal of Diabetes Mellitus
Vol.07 No.01(2017), Article ID:74242,9 pages
10.4236/jdm.2017.71003
Clinical Parameters of Metabolic Control (HbA1c) and Deterioration of Peripheral Arterial Perfusion in Type 2 Diabetes
Ma. de Lourdes Zúñiga-Martínez1, Yolanda Terán-Figueroa2*, Laura Escarlet Guerrero-Cruz1, Ángel Antonio Vértiz-Hernández1
1Academic Coordination Altiplano Region, Autonomous University of San Luis Potosi, San Luis Potosi, Mexico
2Faculty of Nursing and Nutrition, Autonomous University of San Luis Potosi, San Luis Potosi, Mexico

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: December 7, 2016; Accepted: February 18, 2017; Published: February 21, 2017
ABSTRACT
Objective: To determine the relationship between clinical parameters (HbA1c) whit metabolic control and deterioration of peripheral arterial perfusion in diabetic patients. Methodology: 108 medical records of patients with type 2 diabetes mellitus were evaluated. We obtained averages of: blood glucose (162.3 ± 73.10 mg/dl), glycated hemoglobin (HbA1c = 7.64% ± 1.77%), cholesterol (189.28 ± 35.25 mg/dl), triglycerides (189.11 ± 87.76 mg/dl), Systolic Blood Pressure (SBP = 119.69 ± 14.95 mmHg), Diastolic Blood Pressure (DBP = 77.15 ± 9.55 mmHg) and Media Blood Pressure (MBP = 91.36 ± 9.89 mmHg). We correlated variable HbA1c with vascular injury symptomatology. Results: Cor- relation was found between sensitivity dysfunction and HbA1c with a statistical significance of p = 0.01, and a correlation Kendal coefficient w = 0.01, any other parameter of metabolic control was not correlated with symptoms of vascular injury. Conclusion: It is remarkable that the sensitivity dysfunction is a symptom of poorly vascularized lower extremities caused for both functional impairment and structural changes in diabetic patients’ peripheral nerves, even in the preclinical stage of vascular disease. The HbA1c could also be investigated as a likely sensitivity dysfunction biomarker in DM due to the correlation presented in this study but more studies must be realized.
Keywords:
Protein Glycation, Vascular Disease, HbA1c, Type 2 Diabetes Mellitus

1. Introduction
Diabetes mellitus (DM) remains a major health care problem worldwide due to associated complications and prevalence both in developing and developed countries [1] . The people with it face an array of health issues such as: 1) It is the leading cause of lower-limb amputation non-traumatic, new cases of blindness, and kidney failure complications that affect the daily activity; 2) It is also a major contributor to cardiovascular disease, the number one cause of death in world. It is predicted that within 15 years (2030), there will be 500 million people worldwide with diabetes if we do not take the necessary measures to prevent the spread of this serious and costly disease.
The medical costs for treating patients with DM represent an important spending, the projections for 2020 close to 200 billion USD, or even higher. It also causes loss of productivity resulting in spend for the patient and his family [2] . In Mexico, the National Institute of Public Health (2010), ranked the DM as third cause of disability in men and the fifth in the women [3] , 80% of the disabilities in diabetics are caused for vascular complications could be averted through adequate prevention and early intervention. The strategies are focused on prevention and intensive treatment and are cost-effective when the first focus on people at high risk of developing the disease and second in hypertension control, cholesterol and glucose levels among diagnosed people [4] [5] .
In the past two decades, they have accumulated considerable evidence that supporting the potentially pathogenic role of some number of mechanisms that lead to the development of vascular complications mainly the diabetic foot among which are: nerve ischemia, oxidative stress, glycosylation and products and other molecular disorders. These changes cause damage to nerve fibers and lead to the development of peripheral vascular disease where endothelial dysfunction is the most serious result as it affects limb microcirculation [6] .
The chronic hyperglycemia in diabetic patients leads to changes at biomolecular, anatomical and physiologic level. These changes induce the development of vascular complications such as the peripheral arterial disease (PAD) and thus to poorly vascularized extremities whit considerable risk for amputation contribute to diminished quality of life and even death of diabetics [7] .
Advanced glycation end products (AGEs) contribute to a variety of microvascular and macrovascular injuries mostly leads to Peripheral Vascular Disease (PVD) cause of blindness, chronic renal failure and nontraumatic lower-limb amputation in diabetic patient in Mexico [8] [9] . The National Nutrition Health Survey in 2012 [5] founded 38% (2.4 millions) of patients with a previous DM diagnosis reported the presence of burning, pain or loss of sensation in the feet. Besides, also reported a prevalence of amputations or 2% (182 thousand people) and the latter were associated with an evolution of the disease of approximately 12 years [6] [10] . Nontraumatic lower-limb amputation is usually preceded by a foot ulcer in 85% of patients, and cases so that the association between ulcers and lower limb amputations is obvious, it is also known that patients require subsequent amputation and that the greater part of the amputated, there are higher risk of death within 5 years [11] .
In general terms, Treatment of Peripheral Vascular Disease (PVD), like other complications of DM, should focus first on metabolic control and then the specific actions, that is, emphasize early detection risk factors such as: hyperglycemia, dyslipidemia, hypertension, etc. [8] . In some contexts of health, as in some Mexican provinces, is difficult to carry out good metabolic control in patients with DM as it has not reactive required for this, it is why it is necessary to have risk markers with high specificity and sensitivity in identifying PVD.
This study addresses the issue on the identification of risk markers to determine the peripheral vascular damage in patients with Type 2 DM, specifically HbA1c, so as to enable health personnel identify potential damage service users and intervene in an effective and timely manner. 97% of hemoglobin corresponding to a molecular form represented as HbA1, 2.5% or less to HbA2 form and less than 1% to the HbF form the union of different sugars favors the formation of HbA1a, HbA1b y HbA1c; the latter is the most abundant (approximately 80%) in the bloodstream. HbA1c it has been used for over 10 years, to establish the diagnosis of DM in different populations, to determine the degree of metabolic control that the patient has had in the last three months and as a marker of coronary risk tissue [12] . The purpose of this study was to establish whether there a relationship between clinical parameters of metabolic control (blood pressure, plasma glucose, triglycerides and HbA1c) with the deterioration of peripheral arterial perfusion in patients with a clinical diagnosis of type 2 DM current or later production of electronic products.
2. Materials and Methods
2.1. Study Design and Sample Size
This study was documentary quantitative and the study subjects were clinical records of patients with type 2 Dibetes Mellitus and deterioration of peripheral arterial perfusion symptoms (n = 108 records). The inclusion criteria were patients with type 2 DM records with follow-up consultation record (of one year); patients who have test HbA1c results, blood glucose, cholesterol, triglycerides and that the clinical records mentions the presence of symptomatology of vascular dysfunction. The exclusion criteria were records of diabetic patients with any type of anemia, with renal failure and iron pharmacological therapy. These three conditions alter the results of glycosylated hemoglobin.
2.2. Data Collection
The parameters obtained were: HbA1c, blood glucose, blood pressure (BP), Mean Arterial Pressure (MAP), cholesterol and triglycerides these values were related with skin disorders or signs and symptoms to deterioration of peripheral arterial perfusion.
2.3. Data Assessment
The clinical parameters were related with the percentages of symptoms related to vascular damage and overall error and standard deviation of each variable was calculated. The Pearson correlation coefficient (r=) Kendall coefficient, (w=) between HbA1c and decreased peripheral pulses, paleness in lower limbs, delayed capillary refill, changes in temperature, sensitivity dysfunction, alteration nailbeds, onychomycosis, venous insufficiency ulcers, hyperkeratosis were obtained.
2.4. Ethical Concerns of the Study
The development of this research was based on Article 17 of the General Health Law in research without risk [13] . The protocol included the authorization of general manager of Mexican Social Security Institute (Clinic 14, in March 7, 2016; number of trade Of. Dir. 084/16).
3. Results
The records were constituted for 82 women files and 26 men files (n = 108) in this study. In the clinical records the patients were 59.19 ± 13.86 years old and the predominant marital status was married with 76.9%, the single status was 0.9%. The year’s evolution for the disease had an average of 9.12 ± 6.42 years (see Table 1).
The average of clinical disease evolution was 9.12 ± 6.42 years and the metabolic control parameters with 12 months of follow-up are on the Table 1, nevertheless, the greater data deviation was plasma glucose (>140 mg/dL), followed cholesterol (<160 mg/dl), see Table 2.
No results found of decreased peripheral pulses, delay in capillary refill, and changes in temperature or pain nevertheless, our results showed that paleness of the limbs had an average of 4.26% ± 0.79%, sensitivity dysfunction was 2.93% ±
Table 1. Socio-demographic data of patients with type 2 diabetes mellitus (n = 108).
1.67%, presence of recorded edema was 4.5% ± 2.01%, alteration in nail beds found an average of 2.70% ± 1.25% among others parameters (see Table 3).
In relation with the correlation of variables, we found no statistical significance between most of them except as regards HbA1c and the presence of altered sensitivity. Pearson correlation coefficient was r = −0.20, with a value p < 0.50. Regarding the correlation coefficient Kendall, it was w = 0.01, with a value p < 0.01, which is statistically significant (Figure 1).
Table 2. Average clinical parameters of metabolic control in patients with type 2 diabetes mellitus (n = 108).
Data represent 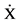 ± DE a monthly readings of a study records corresponding to patients with Type 2 DM. 1Glycated hemoglobin fraction 1c, 2Systolic Blood Pressure, 3Diastolic Blood Pressure, 4Media Blood Pressure.
± DE a monthly readings of a study records corresponding to patients with Type 2 DM. 1Glycated hemoglobin fraction 1c, 2Systolic Blood Pressure, 3Diastolic Blood Pressure, 4Media Blood Pressure.
Table 3. Average values of the different symptoms of vascular damage (n = 108).
Data represent  ± DE a monthly readings of a study records corresponding to patients with Type 2 DM.
± DE a monthly readings of a study records corresponding to patients with Type 2 DM.
Figure 1. Correlation between HbA1c variable with the sensitivity dysfunction. (Data represent % of HbA1c and % of sensitivity dysfunction an average of 12 months; n = 108; p < 0.01).
4. Discussion
Diabetes and its complications put a great economic burden on patients, their families, healthcare systems and countries [1] . A correlation was found between the awareness about the disease complications and mean self-care agency scores of the diabetic subjects. Those who were aware of disease complications had higher self-care agency scores than those who were unaware of them [14] . However, a study showed that there was no significant correlation between the presence of complications and self-care [15] .
Our results show a clear metabolic breakdown in all patients throughout the study period since each parameter was found to be well above normal values [16] . In spite of the above, we did not find statistical relation of the registered parameters and symptomatology of vascular damage except between HbA1c and sensitivity dysfunction, which is consistent with studies that have associated HbA1c as a marker of risk with cardiovascular disease [17] [18] [19] [20] [21] , mainly in coronary arteries, not in damage to other organs such as the kidney [22] or liver [23] . Ramesh and col (2015), evaluated the role of hepato-biliary function as a marker of predictor Coronary Artery Disease (CAD) in patient with Type 2 DM. 100 subjects included 50 T2DM patients with CAD and 50 T2DM without CAD were evaluated and their finding implies that decreased serum bilirubin increases the risk of CAD in patients with Type 2 DM and it shows inverse correlation between HbA1c and bilirubin [24] .
On the other hand, results found in our study regarding the symptomatology of sensitivity dysfunction differ from those reported by different authors [25] [26] [27] , studies in which, claudication and pain are the most prevalent symptoms in the PVD during stadiums I and II. In the present study, the most prevalent symptom was the presence of onychomycosis followed by hyperkeratosis, probably due to changes in endothelial cell proliferation which compromise the activity of the immune system-inhibition of fibroblasts and damage to the basement membrane of keratinocytes [27] . Hyperkeratosis has been associated with collagen glycosylation in keratinocytes which results in thick skin, dry and rough in the lower limbs and its presence has been associated with ulcerations in the diabetic foot [28] .
Presence of sensitivity dysfunction is the main symptom of diabetic polyneuropathy―loss of peripheral nerve fiber function―considered the most common predictor of diabetic foot ulceration and symptom associated with a lack of metabolic control of the patient [29] . This result indicates that HbA1c could also be investigated as a likely marker of risk of diabetic neuropathy―Necessary condition for the appearance of ulcers in Type 2 DM patients. In other studies [30] [31] HbA1c has shown its effectiveness both in the diagnosis of Type 2 DM, as well as cardiovascular risk, damage to the retina and diagnosis of DM. There are no other studies related to HbA1c and sensitivity dysfunction in lower limbs.
5. Conclusions and Suggestions
- In this study, a correlation was established between HbA1c and sensitivity dysfunction in lower limbs.
- The correlation found in this study is an important finding of clinical impact which would allow emphasizing the importance of metabolic control through the measurement of HbA1c in patients with Type 2 DM.
- The authors suggest that this positive correlation can be used as a likely marker of loss of sensitivity.
Conflict of Interests
The authors declare that they have no conflict of interests.
Authors’ Contribution
This study was designed by Ma. de Lourdes Zúñiga-Martínez and Ángel Antonio Vértiz-Hernández. Patient selection and data collecting were organized and made by Yolanda Terán-Figueroa and Laura Escarlet Guerrero-Cruz Statistical analyses were done by Ángel Antonio Vértiz-Hernández, statistical expert. The paper was written by Ma. de Lourdes Zúñiga-Martínez, Yolanda Terán-Figueroa and Ángel Antonio Vértiz-Hernández.
Cite this paper
de Lourdes Zúñi- ga-Martínez, Ma., Terán-Figueroa, Y., Gu- errero-Cruz, L.E. and Vértiz-Hernández, Á.A. (2017) Clinical Parameters of Metabolic Con- trol (HbA1c) and Deterioration of Periphe- ral Arterial Perfusion in Type 2 Diabetes. Journal of Diabetes Mellitus, 7, 31-39. https://doi.org/10.4236/jdm.2017.71003
References
- 1. T. R. Ministry of Health Public Health Agency of Turkey (2014) Turkey Diabetes Program 2015-2020. The Kuban Printing Publishing, Ankara.
- 2. International Diabetes Federation (2008) International Curriculum for Diabetes Health Professional Education. B-1000 Brussels, 30-45.
- 3. Lozano, R., Gómez-Dantés, H., Garrido-Latorre, F., Jiménez-Corona, A., Campuzano-Rincón, J., Franco-marina, F., Medina-Mora, M., et al. (2013) Burden of Disease, Injuries, Risk Factors and Challenges for the Health System in Mexico. Salud Pública de México, 55, 580-594.
https://doi.org/10.21149/spm.v55i6.7304 - 4. Escobar, C., Blanes, I., Ruíz, A., Vinuesa, D., Montero, M., Rodríguez, M., Barbera, G. and Manzano, L. (2011) Prevalence and Clinical Profile and Management of Peripheral Arterial Disease in Elderly Patients with Diabetes. Europan Journal of Internal Medicine, 22, 275-281.
https://doi.org/10.1016/j.ejim.2011.02.001 - 5. Hernández-ávila, M., Gutiérrez, J. and Reynoso-Noverón, N. (2013) Diabetes Mellitus in Mexico. Status of the Epidemic. Salud Pública de México, 55, SI 29-SI 36.
- 6. Okosun, I., Seale, P., Lyn, R. and Davis-Smith, M. (2015) Improving Detection of Prediabetes in Children and Adults: Using Combinations of Blood Glucose Tests. Frontiers in Public Health, 3, 260.
https://doi.org/10.3389/fpubh.2015.00260 - 7. Guzmán, J.R., Lyra, R., Aguilar-Salinas, C., Cavalcanti, S., Escano, F., Tambasia, M. and Duarte, E. (2010) Treatment of Type 2 Diabetes in Latin America: A Consensus Statement by the Medical as Associations of 17 Latin American Countries. Pan American Journal of Public Health, 28, 463-471.
https://doi.org/10.1590/S1020-49892010001200008 - 8. Health Secretary (2013) Diagnosis, Outpatient Control Goals and Timely Reference to Pre-Diabetes and Diabetes Mellitus Type 2 in Adults at the First Level of Care. Health Secretary, Mexico.
- 9. Aguilar, F.R., Terán, J. and Escobedo, J.D. (2011) The Pathogenesis of the Diabetic Foot Ulcer: Prevention and Management. In: Dinh, T., Ed., Global Perspective on Diabetic Ulcerations, In Tech, 155-182.
https://doi.org/10.5772/30325 - 10. Healh Secretary, National Commission for Social Protection in Health, Human Development Program Opportunities, Institute of Security and Social Services for State Workers, National Institute of Public Health. National Health and Nutrition Survey 2012 (ENSANUT). United States of Mexico, 2012.
- 11. Bowker, J. and Pfeifer, M. (2011) Major and Minor Amputations and Dislocations of the Lower Limb in Patients with Diabetes Mellitus. In: Levin and O’Neal, Eds., The Diabetic Foot, Elsevier Mosby, Espana, 409-412.
- 12. Hernández, G.A., Jiménez, A.A. and Bacardí, M.G. (2015) Effect of Carbohydrate Diets on Weight Loss and Glycosylated Hemoglobin in People with Type 2 Diabetes: Systematic Review. Hospital Nutrition, 32, 1960-1966.
- 13. General Secretariat, Office of Parliamentary Services, Chamber of Deputies of the H. Congress of the Union (2016) General Law of Health. United Mexican States.
- 14. Istek, N. and Karakur, P. (2016) Effect of Activities of Daily Living on Self-Care Agency in Individuals with Type 2 Diabetes. Journal of Diabetes Mellitus, 6, 247-262.
ttp://www.scirp.org/journal/jdmh
https://doi.org/10.4236/jdm.2016.64026 - 15. Gao, J., Wang, J., Zheng, P., Haardorfe, R., Kegler, M.C., Zhu, Y. and Fu, H. (2013) Effects of Self-Care, Self-Efficacy, Social Support on Glycemic Control in Adults with Type 2 Diabetes. BMC Family Practice, 14, 66.
https://doi.org/10.1186/1471-2296-14-66 - 16. Health Secretary (2003) Official Mexican Standard NOM 037-SSA2-2002, for Prevention, Treatment and Control of Dyslipidemias.
http://www.salud.gob.mx/unidades/cdi/nom/037ssa202.html - 17. Arnold, L.W., Hoy, W.E., Sharma, S.K. and Wand, Z. (2016) The Association between HbA1c and Cardiovascular Disease Markers in a Remote Indigenous Australian Community with and without Diagnosed Diabetes. Journal of Diabetes Research, 2016, Article ID: 5342304.
https://doi.org/10.1155/2016/5342304 - 18. Lu, J., Bi, Y., Wang, T., Wang, W., Mu, Y., Zhao, J., Liu, Ch., Chen, L., Shig, L., Li, Q., Wan, Q., Wu, S., Qin, G., Yang, T., Yan, L., Liu, Y., Wang, G., Luo, Z., Tang, X., Chen, G., Huo, Y., Gao, Z., Su, Q., Ye, Z., Wang, Y., Deng, H., Yu, X., Shen, F., Chen, L., Zhao, L., Dai, M., Xu, M., Xu, Y., Chen, Y., Lai, S. and Ning, G. (2014) The Relationship between Insulin-Sensitive Obesity and Cardiovascular Diseases in a Chinese Population: Results of the REACTION Study. International Journal of Cardiology, 172, 388-394.
https://doi.org/10.1016/j.ijcard.2014.01.073 - 19. Sahu, A., Gupta, T., Kavishwar, A. and Singh, R.K. (2015) Cardiovascular Diseases Risk Prediction by Homocysteine in Comparison to Other Markers: A Study from Madhya Pradesh. Journal of the Association of Physicians of India, 63, 37-40.
- 20. Mostafa, S.A., Khunti, K., Srinivasan, B.T., Webb, D., Gray, L.J. and Davies, M.J. (2010) The Potential Impact and Optimal Cut-Points of Using Glycated Haemoglobin, HbA1c, to Detect People with Impaired Glucose Regulation in a UK Multi-Ethnic Cohort. Diabetes Research and Clinical Practice, 90, 100-108.
https://doi.org/10.1016/j.diabres.2010.06.008 - 21. Scicali, R., Giral, P., Gallo, A., Di Pino, A., Rabuazzo, A.M., Purrello, F., Cluzel, P., Redheuil, A., Bruckert, E. and Rosenbaum, D. (2016) HbA1c Increase Is Associated with Higher Coronary and Peripheral Atherosclerotic Burden in Non Diabetic Patients. Atherosclerosis, 255, 102-108.
https://doi.org/10.1016/j.atherosclerosis.2016.11.003 - 22. Yoon, H.-J., Lee, Y.-H., Ra, S.K., Hyungtaek, T.R., Young, E.L., Seok, E.K., Cha, B.-S., Chul, H.L. and Lee, B.-W. (2015) Glycated Albumin and the Risk of Micro- and Macrovascular Complications in Subjects with Type 1 Diabetes. Cardiovascular Diabetology, 14, 53.
https://doi.org/10.1186/s12933-015-0219-y - 23. Fiorentino, T.V., Andreozzi, F., Mannino, G.C., Pedace, E., Perticone, M., Sciacqua, A., Perticone, F. and Sesti, G. (2016) One-Hour Postload Hyperglycemia Confers Higher Risk of Hepatic Steatosis to HbA1c-Defined Prediabetic Subjects. The Journal of Clinical Endocrinology & Metabolism, 101, 4030-4038.
https://doi.org/10.1210/jc.2016-1856 - 24. Ramesh, R., Adhishwar Kumaran, N., KuzhandaiVelu, V., Reeta, R., SathishBabu, M. and Niranjan, G. (2015) Association between Hepato-Biliary Status and HbA1C in Type 2 Diabetes Mellitus with Coronary Artery Disease (CAD). Journal of Diabetes Mellitus, 5, 67-71.
https://doi.org/10.4236/jdm.2015.52008 - 25. Dyck, P.J., Albers, J.W., Andersen, H., Arezzo, J.C., Biessels, G.J., Bril, V., Feldman, E.L., Litchy, W.J., O’Brien, P.C., Russell, J.W. and Toronto Expert Panel on Diabetic Neuropathy (2011) Diabetic Polyneuropathies: Update on Research Definition, Diagnostic Criteria and Estimation of Severity. Diabetes/Metabolism Research and Reviews, 27, 620-628.
- 26. Centro Nacional de Excelencia Tecnológica en Salud (2009) Diagnóstico y tratamiento de la enfermedad arterial periférica. Secretaría de Salud, México.
- 27. Schaper, N.C., Andros, G., Apelqvist, J., Bakker, K., Lammer, J., Lepantalo, M., Mills, J.L., Reekers, J., Shearman, C.P., Zierler, R.E. and Hinchliffe, R.J. (2012) Diagnosis and Treatment of Peripheral Arterial Disease in Diabetic Patients with a Foot Ulcer. A Progress Report of the International Working Group on the Diabetic Foot. Diabetes Metabolism Research and Reviews, 28, 218-224.
https://doi.org/10.1002/dmrr.2255 - 28. Montero-Monterroso, J.L., Gascón-Jiménez, J.A., Vargas-Rubio, M.D., Quero-Salado, C., Villalba-Marín, P. and Pérula-de Torres, L.A. (2015) Prevalencia y factores asociados a la enfermedad arterial periférica en pacientes con diabetes mellitus tipo 2 en atención primaria. SEMERGEN—Medicina de Familia, 41, 183-190.
https://doi.org/10.1016/j.semerg.2014.05.004 - 29. Pataky, Z., de LeonRodriguez, D., Allet, L., Golay, A., Assal, M., Assal, J.P. and Hauert, C.A. (2010) Biofeedback for Foot Offloading in Diabetic Patients with Peripheral Neuropathy. Diabetic Medicine, 27, 61-64.
- 30. Clayton, W.J. and Elasy, T.A. (2009) A Review of the Pathophysiology, Classification, and Treatment of Foot Ulcers in Diabetic Patients. Clinical Diabetes, 27, 52-58.
https://doi.org/10.2337/diaclin.27.2.52 - 31. Huijberts M.S., Schaper, N.C. and Schalkwijk, C.G. (2008) Advanced Glycation End Products and Diabetic Foot Disease. Diabetes Metabolism Research and Reviews, 24, S19-S24.
https://doi.org/10.1002/dmrr.861



