Journal of Diabetes Mellitus
Vol.3 No.3(2013), Article ID:35007,6 pages DOI:10.4236/jdm.2013.33017
The effect of demethylasterriquinone B-1 on insulin secretion in rat pancreas
![]()
1Department of Veterinary Medicine, College of Veterinary Medicine, National Chung Hsing University, Taichung City, Chinese Taipei; *Corresponding Author: cyang@dragon.nchu.edu.tw
2Bureau of Animal and Plant Health Inspection and Quarantine, Council of Agriculture, Taipei City, Chinese Taipei
Copyright © 2013 Min-Chuan Lai et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 18 April 2013; revised 20 May 2013; accepted 28 May 2013
Keywords: DMAQ-B1; Insulin Secretion; PI3 Kinase; Tyrosine Kinase; Wortmannin
ABSTRACT
A small nonpeptidyl compoud extracted from Pseudomassaria sp. was found to induce the activity of human insulin receptor tyrosine kinase in vitro. The compound was identified as demethylasterriquinone B-1 (DMAQ-B1). DMAQB1 also induced an increase in [Ca2+]i and insulin secretion in mice pancreatic beta-cells at low glucose (3 mM) concentration via insulin receptor substrate-1/phosphatidylinositol-3- kinase (PI3 kinase) pathway. By using rat pancreatic perfusion technique, we found that 10 μM DMAQ-B1 directly stimulated insulin secretion up to 240% in normal rat pancreas. In the dosage from 1 to 20 μM, DMAQ-B1 stimulated insulin secretion in a dose dependent manner. Furthermore, DMAQ-B1 enhanced glucose-induced insulin secretion by 17.6% (first stage) and 19.0% (second stage), respectively. The PI3 kinase inhibitors, LY 294002 (3.9 μM) or wortmannin (100 nM), inhibited DMAQ-B1-induced insulin secretion by 46.3% and 57.4%, respectively. LY 294002 or wortmannin also inhibited DMAQ-B1 with 10 mM glucose-induced insulin secretion by 70.3% and 79.0%, respectively. All the results suggested that DMAQ-B1 directly stimulated insulin secretion and enhanced glucose-induced insulin secretion. The effect of DMAQ-B1 may mediate through the activation of PI3 kinase pathway to stimulate insulin secretion in normal rat pancreas.
1. INTRODUCTION
Oral therapies for type 2 diabetes mellitus are widely used until today. Thus, the development of an orally insulin mimetic drug was the way for the treatment of diabetes mellitus. In order to discover a new compound that could activate the human insulin receptor tyrosine kinase, Zhang et al. established a cell-based assay to screen a collection of synthetic chemicals and natural products extracted from fungus [1]. Following the analysis with high-performance liquid chromatography and nuclear magnetic resonance, a small nonpeptidyl compound isolated from Pseudomassaria sp. was characterized and identified as demethylasterriquinone B-1 (DMAQ-B1), and also named as L-783, 281 [1]. It was found that DMAQ-B1 had hormone-like action in vitro. DMAQ-B1 increased the activity of human insulin receptor tyrosine kinase and increased the tyrosine phosphorylation of IR subunit. DMAQ-B1 also activated insulin receptor tyrosine kinase downstream signaling proteins, including insulin receptor substrate 1 (IRS-1), ERK1/2, SHC, Phosphatidylinositol 3-kinase (PI3 kinase), p70 S6 kinase, Akt and glycogen synthase kinase-3 (GSK-3) [1- 3]. DMAQ-B1 also induced an increase in [Ca2+]i involved release of Ca2+ from intracellular stores and stimulated insulin secretion in mice pancreatic beta-cells at nonstimulatory glucose (3 mM) concentrations via insulin receptor substrate-1/phosphatidylinositol-3-kinase (PI3 kinase) pathway [4]. In addition, DMAQ-B1 could improve glucose intolerance and inhibit hyperinsulinemia in db/db and ob/ob mice [1]. Strowski et al. found that administration of DMAQ-B1 could decrease hyperglycemia and obesity in a nongenetic mouse model of type 2 diabetes mellitus [5]. Velliquette et al. found that the oral treatment of DMAQ-B1 didn’t increase body weight and food intake in the spontaneously hypertensive obese rat model of metabolic syndrome X [6]. Chronic treatment with DMAQ-B1 had no significant effect on the maximal insulin-induced insulin receptor tyrosine phosphorylation or IRS-1- associated PI3 kinase activity in skeletal muscle in vivo [6]. According to previous studies, we explored the direct effect of DMAQ-B1 on insulin secretion and the potential signaling mechanism of stimulatory effect of DMAQ-B1 by using an isolated rat pancreas perfusion system.
2. MATERIALS AND METHODS
2.1. Animals and Chemicals
Sprague-Dawley rats (200 - 300 g) originated from BioLasco Taiwan Co., Ltd were kept at room temperature (~25˚C) in plastic cages under a 12-h cycle of light. The rats were given free access to tap water and were fed ad libitum with commercial diet (Fwusow; Sha-Lu, Taichung, Taiwan). After an overnight fast (>12 hr), rats were anesthetized with a 125 mg/kg intraperitoneal injection of Urethane (Fluka Chemie Gmbh, China). Access to the pancreas was gained through a ventral midline incision, and the animals’ celiac arteries and portal veins were cannulated with polyvinyl tubing’s of 0.625 and 1.2 mm (internal diameter), respectively. The rats were maintained at 37˚C throughout the experiments. KrebsRinger bicarbonate buffer supplemented with 10 mM N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid
(HEPES), 5.5 mM glucose, 0.1% dextran, and 0.2% bovine serum albumin, was used as the perfusate (basal medium). This solution was continuously aerated with 95% O2/5% CO2, and the pH value was maintained at 7.4. All chemicals were purchased from Sigma Chemical (St. Louis, MO), except for DMAQ-B1, dimethylsulfoxide (DMSO) and LY294002, which were provided by Merck (U.S. and Canada), Merck (Darmstadt, Germany) and Eli Lilly (Indianapolis, IN), respectively. DMAQ-B1 was dissolved in 100 μM DMSO. The animal use protocol was reviewed and approved by the Institutional Animal Care and Use Committee of National Chung Hsing University.
2.2. Pancreatic Perfusion
By using a method modified from Grodsky and Fanska [7], the in situ in living rat pancreatic perfusion with an open system was performed at 37˚C. The pancreatic perfusion was maintained at a flow rate of 1 ml/min, which did not cause noticeable edema or impair insulin release. After the cannulation of celiac artery and portal vein, the rat pancreatic perfusion preparations were perfused with KRB basal medium for 20 min as an equilibration period. Subsequently, the effluent fluid was collected every minute from the cannula of the portal vein for 50 min. In experiment 1, after a baseline period of 10 min, the perfusate containing DMAQ-B1 (10 μM) or DMSO (100 μM) was administered for 30 min, followed by the basal medium for 10 min. In experiment 2, after a baseline period of 10 min, the perfusate containing DMAQ-B1 (1, 5, 10, 15 or 20 μM) was administered for 30 min, followed by the basal medium for 10 min. In experiment 3, the perfusate containing 10 mM glucose with or without DMAQ-B1 (10 μM) was administered for 30 min, followed by a basal medium washout for the last 10 min. In experiment 4, the medium containing PI3K inhibitors (3.9 μM LY294002 or 100 nM wortmannin) was administered for 5 min and was followed by LY294002 (3.9 μM) with DMAQ-B1 (10 μM) or wortmannin(100 nM) with DMAQ-B1(10 μM) for another 25 min, respectively. In experiment 5, the medium containing LY294002 (3.9 μM) was administered for 5 min as pretreatment and was followed by LY294002 (3.9 μM) with DMAQ-B1 (10 μM) or LY294002 (3.9 μM) with DMAQ-B1 (10 μM) and 10 mM glucose for another 25 min. In experiment 6, the medium containing wortmannin (100 nM) was administered for 5 min as pretreatment and was followed by wortmannin (100 nM) with DMAQ-B1 (10 μM) or wortmannin (100 nM) with 10 mM glucose and DMAQ-B1 (10 μM) for another 25 min. The collected effluent fluid was kept at 4˚C and subsequently assayed within 12 h for insulin by using radioimmunoassay (RIA), as previously described by Hale and Randle [8]. Rat insulin was used as standard for the RIA.
2.3. Data Expression and Statistical Analysis
Data of effluent insulin concentrations were expressed as percentage of the baseline level (mean of 10 baseline values) in means ± SE. Data were analyzed by using analysis of variance (ANOVA) to determine the significance of treatment and time. The treatment multiplied by time interaction was used as an error term to determine the effect of treatment. The significance of treatment was determined from the conservative F value. Tukey’s highly significant difference test was used to determine the differences between treatments for which the ANOVA indicated a significant (P < 0.05) F ratio. For analyzing the first and second phases of insulin secretion during glucose perfusion, the areas under the curve (AUCs) of the percentage increase over baseline were calculated and compared by using Student’s t tests. P < 0.05 was considered statistically significant.
3. RESULTS
3.1. Effect of DMAQ-B1 on Insulin Secretion
The direct effect of DMAQ-B1 (10 μM) on insulin secretion in normal living rat in situ pancreas was shown in Figure 1. The increase of insulin secretion was observed
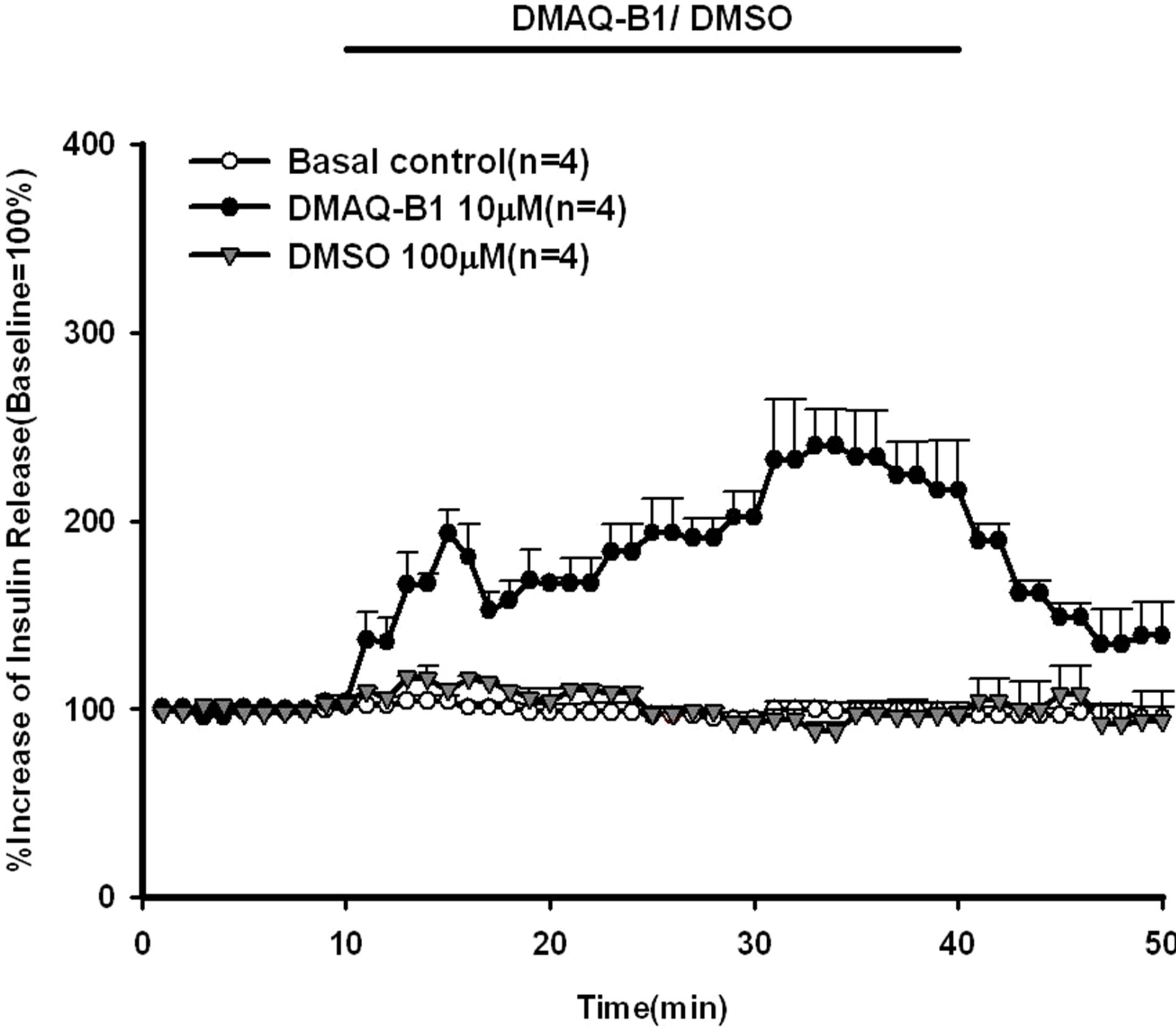
Figure 1. Effect of demethylasterriquinone B-1 on insulin secretion in the perfused rat pancreas. In pancreatic perfusion experiments, an equilibration period of 20 min preceded time 0. After a baseline period of 10 min, 10 μM DMAQ-B1 and 100 μM DMSO were administered for 30 min followed by the basal medium (KRB), respectively. Values are shown as means ± SE (n = 4). The baseline effluent concentrations of insulin were 4172 ± 277 pg/ml, 1385 ± 265 pg/ml, and 2462 ± 565 pg/ml for the control, 10 μM DMAQ-B1, and 100 μM DMSO groups, respectively.
within 4 minutes and reached a statistical significant level at 21 minutes. DMAQ-B1 (10 μM) stimulated a 240% increase in the peak effluent concentration of insulin at 21 minutes. In order to determine the dose-effect of DMAQ-B1 on insulin secretion, various concentrations of DMAQ-B1 (1, 5, 10, 15, 20 μM) were infused into rat pancreas. As shown in Figure 2, insulin secretion was increased by DMAQ-B1 in a dose-dependent manner. DMAQ-B1 (1, 5, 15 and 20 μM) induced increases in the peak effluent insulin concentrations were 126%, 172%, 256% and 291%, respectively, above the basal control group. The insulin secretion response was declined after discontinuation of DMAQ-B1 perfusion.
3.2. Effect of DMAQ-B1 on Glucose-Induced Insulin Secretion
As shown in Figure 3, glucose (10 mM) with or without DMAQ-B1 (10 μM) were infused via in situ pancreatic perfusion. Glucose (10 mM) alone induced a biphasic insulin secretory response. After the administration of DMAQ-B1 in perfusate containing 10 mM glucose, the glucose-induced second phase of insulin secretion were enhanced compared with those with glucose alone (P < 0.01). By comparing the AUCs of the percentage increase over baseline, DMAQ-B1 (10 μM) enhanced 17.6% and 19.0% insulin secretion in the first and second phases of insulin secretion, respectively (Table 1).

Figure 2. Dose-dependent effect of DMAQ-B1 on insulin secretion in the perfused rat pancreas. In pancreatic perfusion experiments, an equilibration period of 20 min preceded time 0. After a baseline period of 10 min, different dosage of DMAQB1 was administered for 30 min followed by the basal medium (KRB). Values are shown as means ± SE (n = 4). The baseline effluent concentrations of insulin were 4172 ± 277 pg/ml, 2556 ± 296 pg/ml, 1733 ± 259 pg/ml, 1385 ± 265 pg/ml, 1381 ± 74 pg/ml and 842 ± 175 pg/ml for the control, 1, 5, 10, 15 and 20 mM DMAQ-B1 groups, respectively.
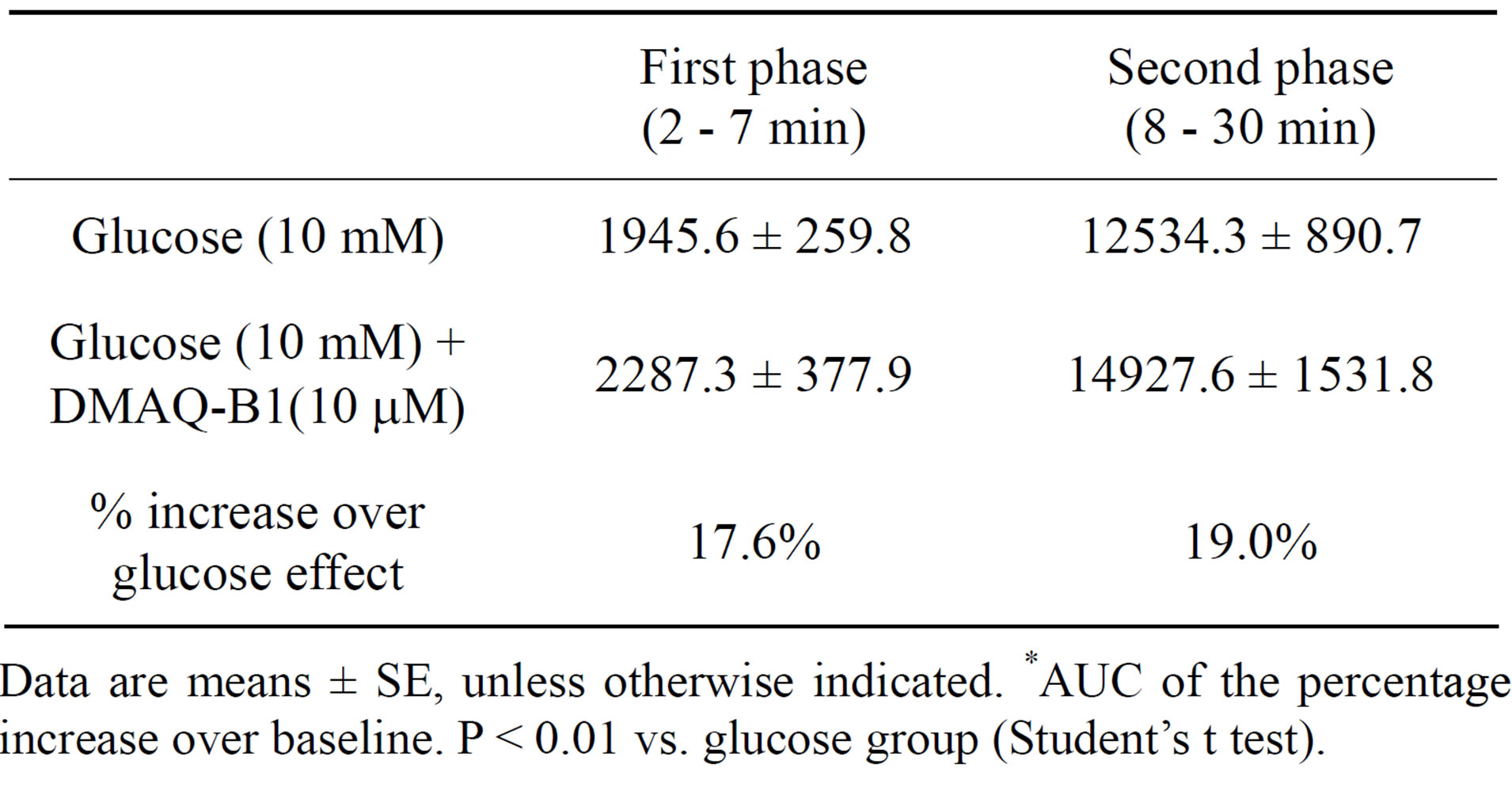
Table 1. Effect of DMAQ-B1 on glucose-induced first and second phases of insulin secretion.
Data are means ± SE, unless otherwise indicated. *AUC of the percentage increase over baseline. P < 0.01 vs. glucose group (Student’s t test).
3.3. Effect of PI3 Kinase Inhibitors on DMAQ-B1-Induced Insulin Release
Two PI3 kinase inhibitors, LY294002 and wortmannin, were included in the perfusate to explore the possible signaling pathways leading to the effect of DMAQ-B1 on insulin secretion. As shown in Figure 4, LY294002 (3.9 μM) and wortmannin (100 nM) dramatically decreased DMAQ-B1-induced insulin secretion by 46.3% and 57.4%, respectively. The results indicated a possible role of the PI3 kinase pathway involving the effect of DMAQ-B1 on insulin secretion. In Figures 5 and 6,
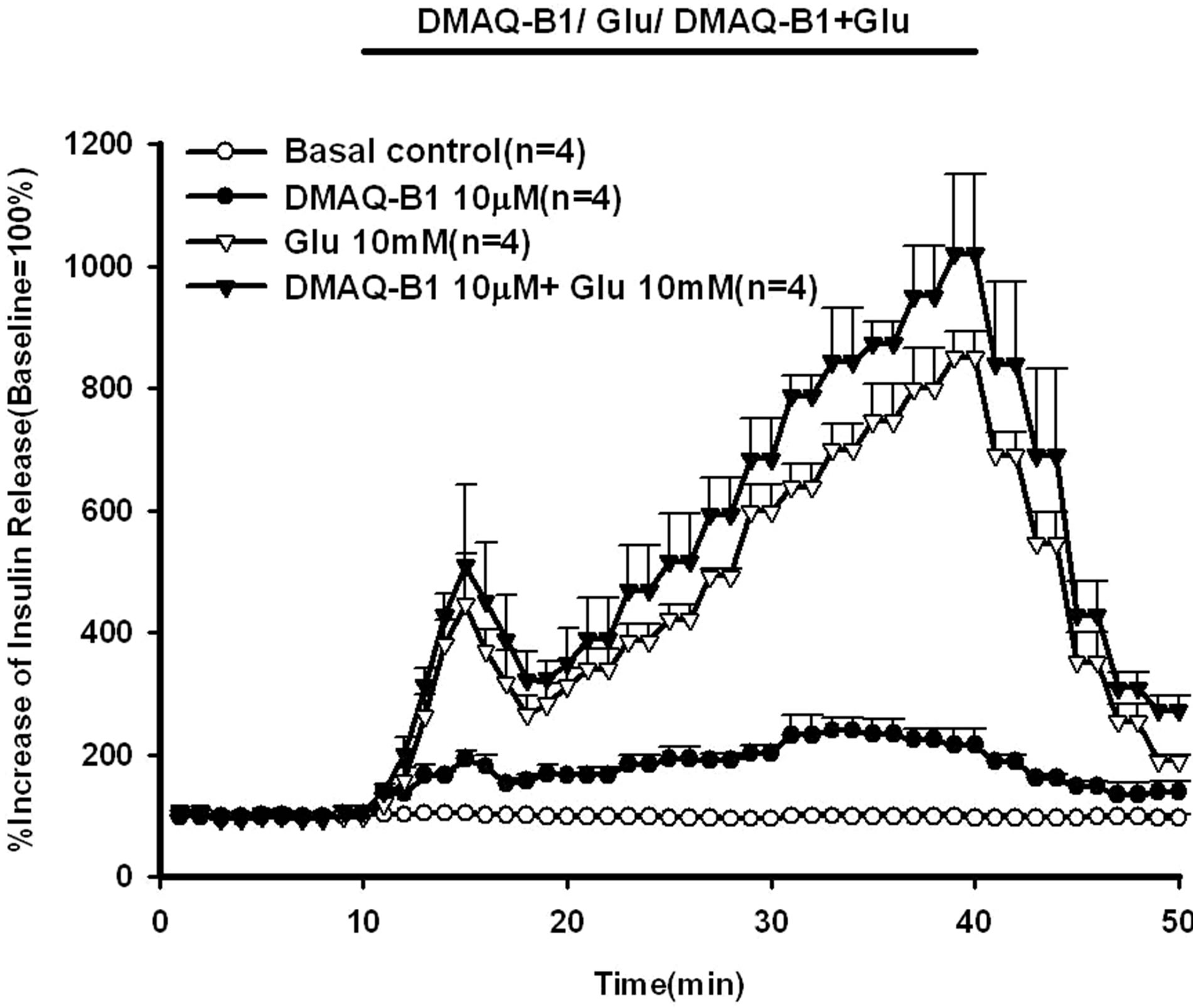
Figure 3. Effect of 10 μM DMAQ-B1 in the medium containing 10 mM glucose on insulin secretion in the perfused rat pancreas. In pancreatic perfusion experiments, an equilibration period of 20 min preceded time 0. After a baseline period of 10 min, 10 μM DMAQ-B1 with or without 10 mM glucose was administered for 30 min followed by basal medium perfusion. Values are means ± SE (n = 4). The baseline effluent concentrations of insulin were 4172 ± 277 pg/ml, 1385 ± 265 pg/ml, 634 ± 156 pg/ml and 836 ± 194 pg/ml for the control, 10 μM DMAQ-B1, 10 mM glucose and 10 μM DMAQ-B1 + 10 mM glucose groups, respectively.
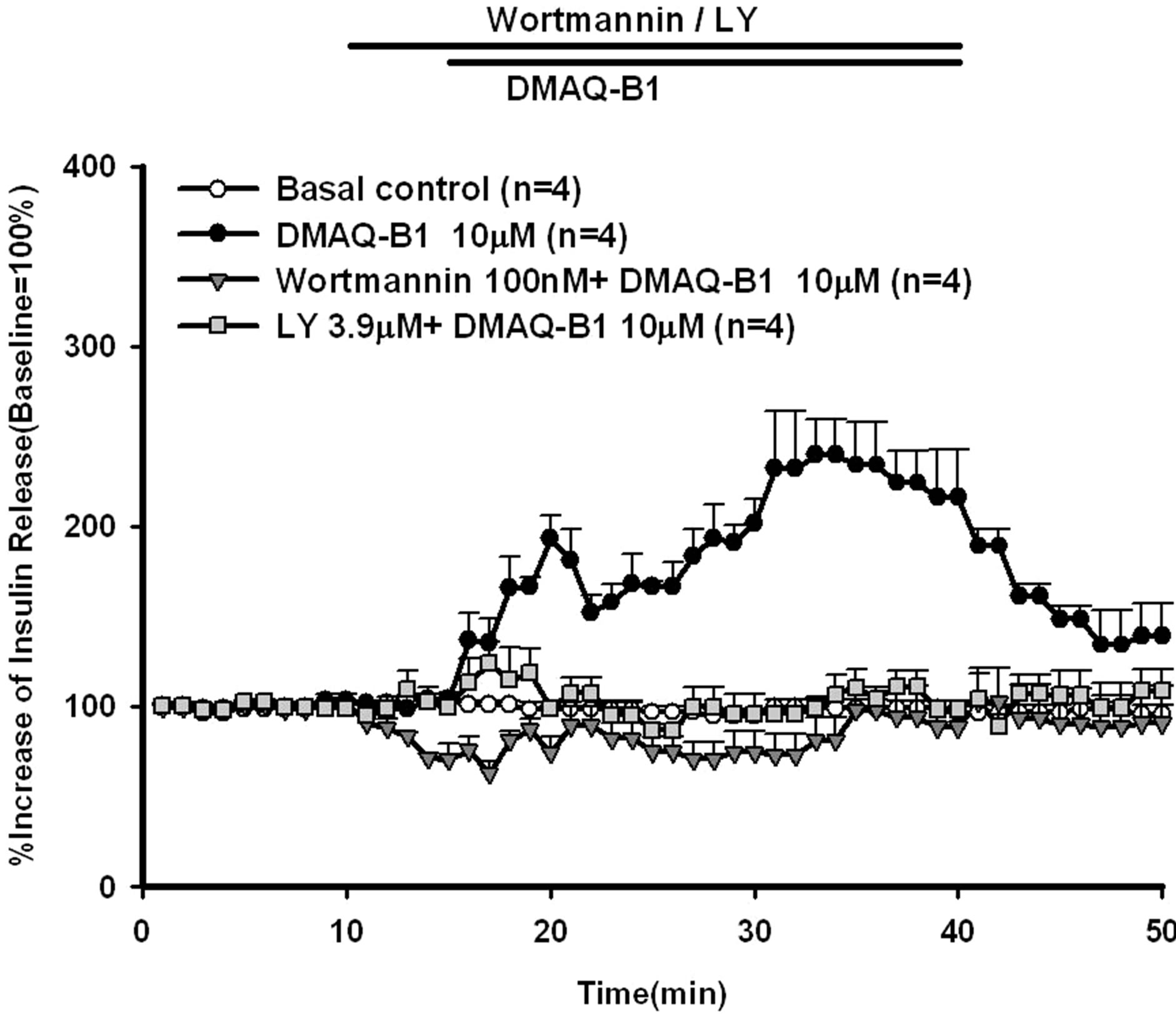
Figure 4. Effect of LY294002 or wortmannin on DMAQ-B1- induced insulin release in the perfused rat pancreas. In pancreatic perfusion experiments, an equilibration period of 20 min preceded time 0. After a baseline period of 10 min, 3.9 μM LY294002 or 100 nM wortmannin was administered for 5 min, and then 10 mM DMAQ-B1 was added in the medium for 30 min followed by basal medium perfusion. Values are means ± SE (n = 4). The baseline effluent concentrations of insulin were 4172 ± 277 pg/ml, 1385 ± 265 pg/ml, 2871 ± 253 pg/ml and 3179 ± 642 pg/ml for the control, 10 μM DMAQ-B1, 10 μM DMAQ-B1 + 3.9 μM LY294002 and 10 μM DMAQ-B1 + 100 nM wortmannin groups, respectively.
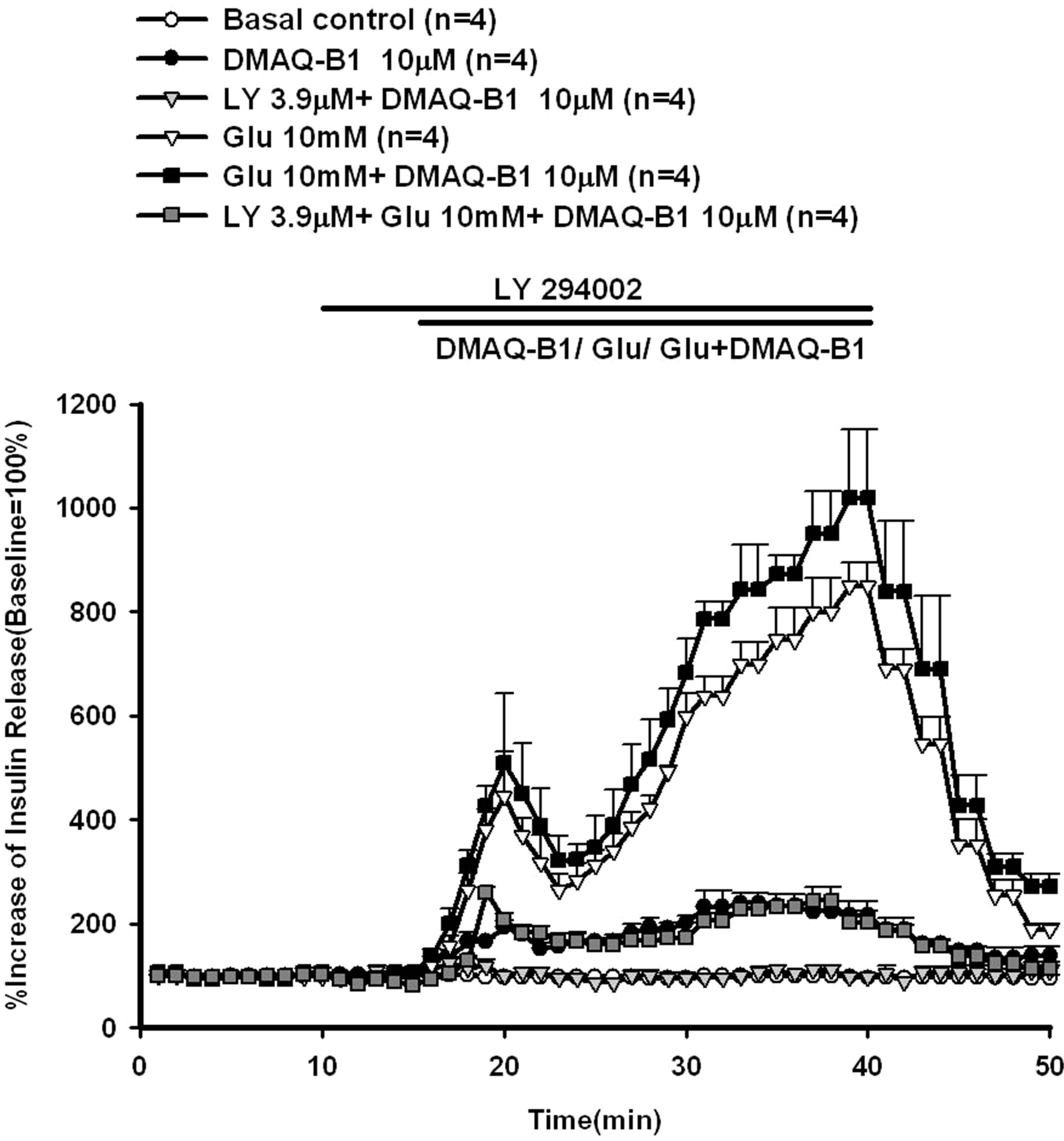
Figure 5. Effect of LY294002 on DMAQ-B1- or DMAQ-B1 mixed with 10 mM glucose-induced insulin release in the perfused rat pancreas. In pancreatic perfusion experiments, an equilibration period of 20 min preceded time 0. After a baseline period of 10 min, 3.9 μM LY294002 was administered for 5 min, and then 10 μM DMAQ-B1 or 10 μM DMAQ-B1 with 10 mM glucose was added in the medium for 30 min followed by basal medium perfusion. Values are means ± SE (n = 4). The baseline effluent concentrations of insulin were 4172 ± 277 pg/ml, 1385 ± 265 pg/ml, 2871 ± 253 pg/ml, 634 ± 156 pg/ml, 836 ± 194 pg/ml and 2670 ± 376 pg/ml for the control, 10 μM DMAQ-B1, 3.9 μM LY294002 + 10 μM DMAQ-B1, 10 mM glucose, 10 mM glucose + 10 μM DMAQ-B1 and 3.9 μM LY294002 + 10 mM glucose + 10 μM DMAQ-B1 groups, respectively.
LY294002 (3.9 μM) or wortmannin (100 nM) were perfused before DMAQ-B1 with 10 mM glucose, LY294002 or wortmannin inhibited glucose (10 mM) mixed with DMAQ-B1-induced insulin secretion by 70.3% and 79.0%, respectively.
4. DISCUSSION
In 1999, through extensive screening of over 50,000 mixtures of synthetic compounds and natural products, Zhang et al. identified and isolated a small molecular IR activator, demethylasterriquinone B-l (DMAQ-B1) from a fungal extract (Pseudomassaria sp.) by using Chinese hamster ovary cells expressed human IR [1]. DMAQ-Bl has greater activity l0-fold selectivity for insulin receptor tyrosine kinase than insulin-like growth factor receptor (IGHF-IR), epidermal growth factor receptor (EGFR) and platelet-derived growth factor receptor (PDGFR) [1- 3]. Zhang et al. also found that the interaction of DMAQ-B1 with IR kinase domain appears to alter the
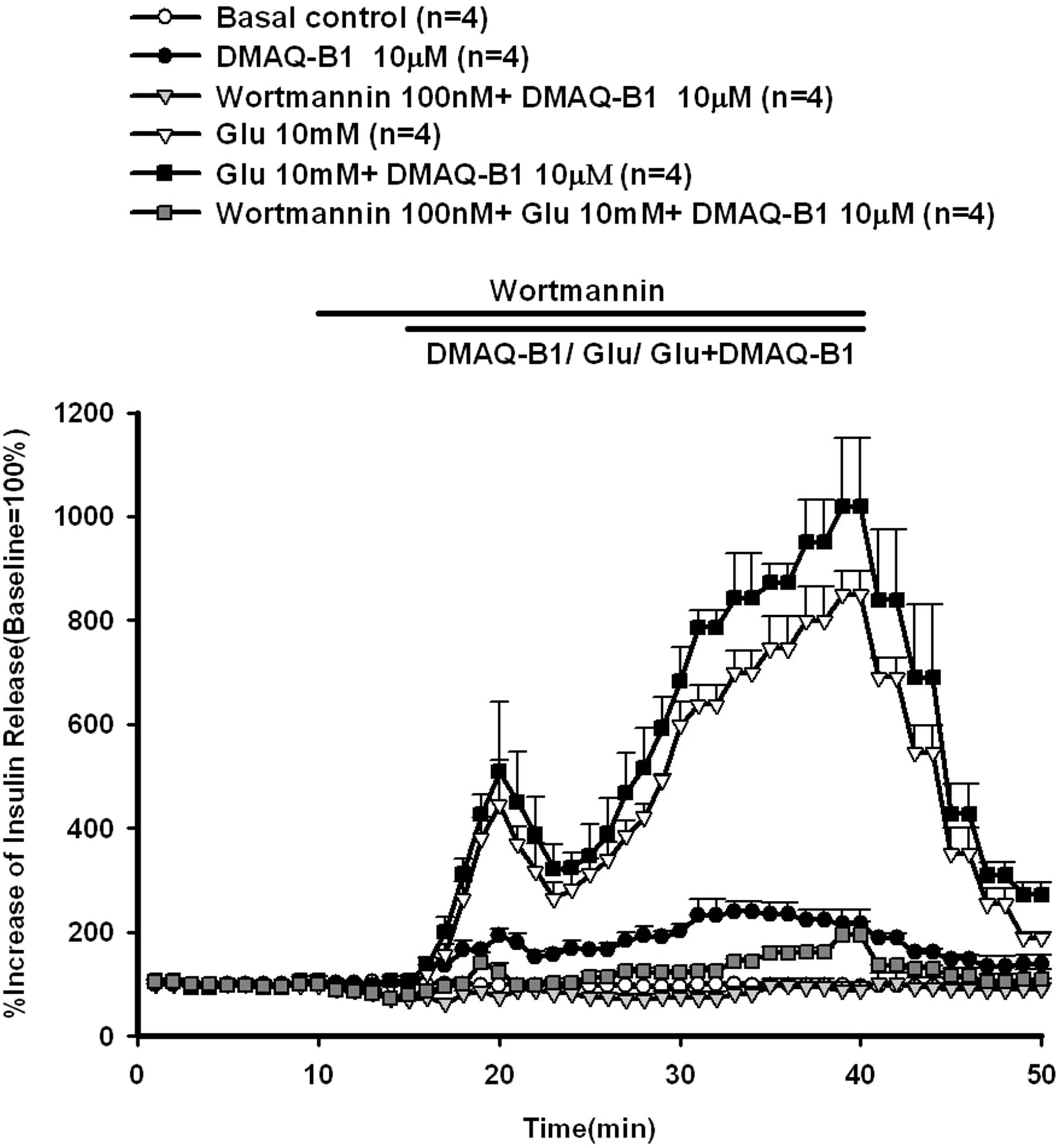
Figure 6. Effect of wortmannin on DMAQ-B1- or DMAQ B1 mixed with 10 mM glucose-induced insulin release in the perfused rat pancreas. In pancreatic perfusion experiments, an equilibration period of 20 min preceded time 0. After a baseline period of 10 min, 100 nM wortmannin was administered for 5 min, and then 10 μM DMAQ-B1 or 10 μM DMAQ-B1 with 10 mM glucose was added in the medium for 30 min followed by basal medium perfusion. Values are means ± SE (n = 4). The baseline effluent concentrations of insulin were 4172 ± 277 pg/ml, 1385 ± 265 pg/ml, 3179 ± 742 pg/ml, 634 ± 156 pg/ml, 836 ± 194 pg/ml and 5439 ± 1912 pg/ml for the control, 10 μM DMAQ-B1, 100 nM wortmannin + 10 μM DMAQ-B1, 10 mM glucose, 10 mM glucose + 10 μM DMAQ-B1 and 100 nM wortmannin + 10 mM glucose + 10 μM DMAQ-B1 groups, respectively.
conformation of the protein in the region encompassing the ATP binding site [1].
DMAQ-B1 also stimulated glucose uptake in rat primary adipocytes and in isolated soleus muscle from lean mice [1]. In addition, DMAQ-B1 dose-dependently increased glucose transport in 3T3L1 adipocytes [2]. Therefore, DMAQ-B1 can increase glucose uptake in peripheral tissues. In 2002, Roper et al. found that DMAQ-B1 dose-dependently induced intracellular calcium release in CD1 (ICR) mice and C57Bl/6 mice pancreas [4]. The pretreatment of thapsigargin reduced the DMAQ-B1- induced intracellular calcium release [4]. They also found that nifidipine can decrease DMAQ-B1-induced calcium increase by 33% ± 6%. It revealed that DMAQB1 induced Ca2+ entry through voltage-dependent Ca2+ channel [4]. In addition, 10 µM DMAQ-B1 can stimulate 119% ± 25% insulin release in IRS-1+/+ mice, whereas, 10 µM DMAQ-B1 can only stimulate 1.3% ± 0.1% insulin release in IRS-1−/− mice [4].
Zhang et al. found that oral administration of DMAQBl dose-dependently reduced blood glucose concentration in ob/ob mice. DMAQ-Bl also improved the glucose tolerance and reduced insulin concentration in ob/ob mice [1]. In 2004, Strowski et al. found that oral DMAQB1 (11.5 mg/kg) for 3 weeks could reduced postprandial blood glucose levels by approximately 50% in STZ-induced type 2 diabetic mellitus C57BL/6 mice fed with high fat diet [5].
Our results showed that DMAQ-B1 directly stimulated insulin release in normal rat pancreas in a dose-dependent manner. The administration of DMAQ-B1 in 10 mM glucose perfusate enhanced glucose-induced insulin secretion to 1019% compared with those in the perfusion experiments with DMAQ-B1 alone by 240% and glucose alone by 850%.
Zhang et al. found that DMAQ-B1 phosphorylated insulin receptor beta subunit and insulin receptor substrate-1 (IRS-1) in CHO.IR cells and activated PI 3- kinase and AKT [1]. Ding et al. reported that DMAQ-B1 could phosphorylate p70S6 kinase in CHO.IR cells [3]. P70 S6 kinase is another key enzyme downstream of the PI3-kinase pathway, and wortmannin can inhibit DMAQB1 induced phosphorylation of p70S6 kinase [3].
There are two specific PI3 kinase inhibitors, wortmannin and LY294002. Wortmannin was produced from penicillium wortmannin and LY294002 was designed from flavonoid quercetin as a synthetic PI3 kinase inhibitor [9]. Although the IC50 of LY294002 is about 500-fold higher than that of wortmannin, LY294002 is widely used in cell biology as a specific PI3K inhibitor because it is much more stable in solution than wortmannin [10]. The results showed that LY294002 (3.9 µM) or wortmannin (100 nM) inhibited DMAQ-B1- induced insulin secretion by 46.3% and 57.4%. LY294002 (3.9 µM) or wortmannin (100 nM) also inhibited glucose mixed with DMAQ-B1-induced insulin secretion by 70.3% and 79.0%, respectively. Pharmaceutical intervention aimed at augmenting insulin receptor function ultimately may prove beneficial as a novel therapeutic option in patients with diabetes [11].
All the results suggested that DMAQ-B1 directly stimulated insulin secretion in a slow release pattern in normal rat pancreas. In addition, glucose-induced insulin secretion was enhanced by DMAQ-B1. The results also showed that LY294002 or wortmannin inhibited DMAQB1-induced insulin secretion and glucose mixed with DMAQ-B1-induced insulin secretion. Therefore, the effect of DMAQ-B1 may mediate through the activation of PI3 kinase pathway to stimulate insulin secretion.
REFERENCES
- Zhang, B.B., Salituro, G.M., Szalkowski, D., Li, Z., Zhang, Y., Royo, I., Vilella, D., Diez, M.T., Pelaez, F., Ruby, C., Kendall, R.L., Mao, X., Griffin, P., Calaycay, J., Zierath, J.R., Heck, J.V., Smith, R.G. and Moller, D.E. (1999) Discovery of a small molecule insulin mimetic with antidiabetic activity in mice. Science, 284, 974-977. doi:10.1126/science.284.5416.974
- Webster, N.J., Park, K. and Pirrung, M.C. (2003) Signaling effects of demethylasterriquinone B1, a selective insulin receptor modulator. Chembiochem, 4, 379-385. doi:10.1002/cbic.200200468
- Ding, V.D.H., Qureshi, S.A., Szalkowski, D., Zhihua, L. I., Biazzo-ashnault, D.E., XIE, D., Liu, K., Jones, A.B., Moller, D.E. and Zhang, B.B. (2002) Regulation of insulin signal transduction pathway by a small-molecule insulin receptor activator. Biochemical Journal, 367, 301-306. doi:10.1042/BJ20020708
- Roper, M.G., Qian, W.J., Zhang, B.B., Kulkarni, R.N., Kahn, C.R. and Kennedy, R.T. (2002) Effect of the insulin mimetic L-783, 281 on intracellular Ca2+ and insulin secretion from pancreatic beta-cells. Diabetes, 51, S43- S49. doi:10.2337/diabetes.51.2007.S43
- Strowski, M.Z., Li, Z., Szalkowski, D., Shen, X., Guan, X.M., Juttner, S., Moller, D.E. and Zhang, B.B. (2004) Small molecule insulin mimetic reduces hyperglycemia and obesity in a non-genetic mouse model of type 2 diabetes. Endocrinology, 145, 5259-5268. doi:10.1210/en.2004-0610
- Velliquette, R.A., Friedman, J.E., Shao, J., Zhang, B.B. and Ernsberger, P. (2005) Therapeutic actions of an insulin receptor activator and a novel peroxisome proliferator-activated receptor γ agonist in the spontaneously hypertensive obese rat model of metabolic syndrome X. J. Journal of Pharmacology and Experimental Therapeutics, 314, 422-430. doi:10.1124/jpet.104.080606
- Grodsky, G.M. and Fanska, R.E. (1975) The in vitro perfused pancreas. Methods in Enzymology, 39, 364-372. doi:10.1016/S0076-6879(75)39033-2
- Hale, C.N. and Randle, P.J. (1963) Immunoassay of insulin with insulin antibody precipitate. Biochemical Journal, 88, 137-146.
- Vlahos, C.J., Matter, W.F., Hui, K.Y. and Brown, R.F. (1994) A specific inhibitor of phosphatidylinositol 3- kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). The Journal of Biological Chemistry, 269, 5241-5248.
- Walker, E.H., Pacold, M.E., Perisic, O., Stephens, L., Hawkins, P.T., Wymann, M.P. and Williams, R.L. (2000) Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Molecular Cell, 6, 909-919. doi:10.1016/S1097-2765(05)00089-4
- Salituro, G.M., Pelaez, F. and Zhang, B.B. (2001) Discovery of a small molecule insulin receptor activator. Recent Progress in Hormone Research, 56, 107-126. doi:10.1210/rp.56.1.107

