Journal of Sensor Technology
Vol. 2 No. 2 (2012) , Article ID: 19676 , 7 pages DOI:10.4236/jst.2012.22010
Optical Chemical Sensor for Screening Cadmium(II) in Natural Waters
Department of Environmental Sciences, Sri Venkateswara University, Tirupati, India
Email: *naidugrk@yahoo.com
Received December 23, 2011; revised January 19, 2012; accepted January 29, 2012
Keywords: Optode; Cd(II) Ions; 1-(2-Pyridylazo)-2-Napthol (PAN); FAAS
ABSTRACT
Membrane based optical chemical sensor (optode) for Cd(II) was developed by the immobilization of a dye 1-(2-Pyridylazo)-2-Napthol (PAN) in the Tri-(2-Ethylhexyl) Phosphate (TEHP) plasticized Cellulose Triacetate (CTA) matrix. Various combinations of PAN immobilized in the cellulose triacetate CTA and Polystyrene (PS) matrices plasticized with Tri-(2-Ethylhexyl) Phosphate TEHP, 2-Nitrophenyl Octyl Ether (NPOE) and Dioctyl Phthalate (DOP) were studied to arrive a suitable composition and found that the optode does not require any extractant to produce a distinct colour change on complexation with Cd(II). On sorption of Cd(II) in the optode matrix, PAN changes color of the optode from golden yellow to violet red having a maximum absorbance (lmax = 553 nm) within 150 min of total equilibration time at pH = 7.5. The optode developed in the present work was studied for its analytical application for Cd(II) in the aqueous samples by spectrophotometry and as well as Flame Atomic Absorption Spectrophotometry (FAAS). This preconcentrated optode showed a linear response by UV-visible spectrophotometry at λmax = 553 nm over a concentration range of 10 ng·mL–1 to 500 ng·mL–1 of Cd(II) ions. Where as the aqueous solutions was also subjected to FAAS before and after equilibration of the optode and found to be linear in the concentration range of 250 ng·mL–1 to 5000 ng·mL–1 of Cd(II) ions. The optode found to be reversible and can be desorbed by equilibrating it with 0.01 mol·L–1 HNO3. The applicability of the developed optode in real samples was studied by determining cadmium in the natural waters spiked with a known amount of Cd(II) ions.
1. Introduction
The large-scale production of a variety of chemical compounds has led to increased discharge of chemicals that causes the global deterioration of the environmental quality. Among the released chemicals, heavy metals are highly toxic and cause serious threat to human and the environment [1,2]. In various toxic metal ions, cadmium (II) is one of the common contaminants which gains due to its high toxic nature even at very low concentrations and it has been recognized as one of the most toxic environmental and industrial contaminants due to its ability to induce severe alterations in various organs and tissues following either acute or chronic exposure [3-6]. According to EPA the permissible limit of cadmium in drinking water is 0.05 mg/L [7]. The tolerance limits for cadmium in potable water are usually between 5 and 10 mg/L and a threshold limit value of 0.05 mg·m−3 for cadmium dust and cadmium salts in workplace atmospheres has been suggested [8]. There are many analytical methods that can be used for the quantification of Cd(II) in different environmental matrices, in which various preconcentration procedures including conventional liquid-liquid extraction, solid phase extraction based on adsorption or ion exchange, cloud point extraction, electroanalytical techniques etc., have been used [9,10]. Optical chemical sensors (optodes) for metal ions detection have found increasing applications over the past several years. These are generally associated with such advantages as simplicity and low cost as well as suitability for remote and continuous monitoring. Optodes for metal ion determination can be fabricated by employing different types of reagents such as chromogenic, fluorescent and ionophoric compounds and enzymes [11]. To improve the analytical signal, preconcentration by several orders of magnitude enhances the accuracy of the results, offer a high degree of selectivity and facilitates calibration. Optodes offer a possibility of simultaneous preconcentration and quantification of a target analyte with a minimum sample manipulation, reasonable selectivity and improved sensitivity without using any reference devices [12].
Optical sensors for Cd(II) ions in the aqueous samples have been developed using various strategies [13-17]. Ensafi et al. have developed optical chemical sensor using 2-Amino-Cyclopentene-1-Dithiocarboxylic Acid (ACDA) on a cellulose triacetate membrane for sensing Cd(II) ions in the ground water samples. Sanchez-Pedren et al., three kinetic methods based on flow injection, flow, and stopped-flow injection was applied for the determination of Cd(II) using an optode membrane that incorporates 1-(2-Pyridylazo)-2-Naphtol (PAN) in a plasticized poly (vinyl) chloride. Czolk et al. have developed optic-chemical sensor (optode) for the detection of heavy metal ions using 5,10,15,20-tetra(p-sulfonatophenyl) porphyrin covalently immobilized onto a polymeric matrix, complexation of ions, such as Cd(II), Pb(II) or Hg(II) [18]. An optical-fiber chemical sensor (optode) have developed for determination of Zn(II) and Cd(II) mixtures using 2-(5-bromo-2-pyridylazo)-5-(diethylamino)phenol immobilized onto XAD-4 (Br-PADAP/XAD-4) by Kuswandi B et al. [19]. Rezaei, B. et al. have developed optode using 1,2-bis(quinoline-2-carboxamido)-4-chloro-benzene (H2Clbqb) as an excellent ionophore in the construction of a cadmium (II)-selective PVC-based membrane sensor [20]. In all the above proposed methods optode membranes required either extractants of ionophores but at the same time membrane shows slow response.
In the present work, a simple colour changeable optode was developed for the determination of ultra-trace levels of cadmium. This was prepared by the immo-bilization of a chromophore 1-(2-Pyridylazo)-2-Naphtol (PAN) in the Tri-(2-Ethylhexyl) Phosphate (TEHP) plasticized Cellulose Triacetate (CTA) matrix. The optode developed in the present work was studied for its analytical application for Cd(II) in the aqueous samples by spectrophotometry and as well as Flame Atomic Absorption Spectrophotometry (FAAS).
2. Experimental
2.1. Materials and Apparatus
Analytical reagent grade cadmium chloride, sodium hydroxide, dichloromethane, chloroform and 1-(2-Pyridylazo)-2-Naphtol (PAN) were obtained from Merck (Mumbai, India). Double distilled water was used throughout the present work. Cellulose triacetate molecular weight 72,000 - 74,000 acetyl value + 43.2% from sigma Aldrich (Steinheim, Switzerland) from Fisher scientific (Hong Kong), Polystyrene (PS) from the Acros Organics (Geel, Belgium), Tri-(2-Ethylhexyl) Phosphate (TEHP) from koch-light laboratories (Coinbrook Bucks, England), 2-Nitrophenyl Octyl Ether (NPOE) from Sigma-Aldrich (Steinheim, Germany) and Dioctyl Phthalate (DOP) from the British Drug Houses Ltd. (Poole, England) were used. The pH of the solution was carried out with Elico LI120 pH meter from Elico Lab, Hyderabad. UV-visible spectrophotometer model UV-1800 from Shimadzu (Japan), was used for recording the absorbance spectra. Flame Atomic Absorption Spectrometry (Perkin-Elmer, Model 2380, USA) with single and multi-elemental hallow cathode lamps of cadmium element was employed in the studies. The pH of the sample was prepared with using 0.01 M of HCl and 0.01 M of NaOH solution. The natural water samples were collected from the surrounding of rural and industrial areas of Tirupati, Chittoor District, A.P., India.
2.2. Optode Preparation
Cellulose Triacetate (CTA) of 0.2 grams was dissolved in 10 mL of dichloromethane. An amount of 0.001 grams of 1-(2-Pyridylazo)-2-Naphtol (PAN) and 0.1 grams of Tri- (2-Ethylhexyl) Phosphate (TEHP) was dissolved in 10 mL of chloroform separately. Both the solutions were mixed and ultrasonicated for 2 min to form a homogeneous casting solution. The casting solutions were spread on a Petri dish (internal diameter of 9 cm) kept on a levelled surface. After two days, the membrane was casted with controlled evaporation of the dichloromethane, the transparent optode was peeled off from Petri dish using water. The optode thus prepared was thoroughly washed with distilled water to remove soluble components. The optode samples were cut in the size of 2 cm × 1 cm and the same size optode was used through the experiment. The chemical structure of Cellulose Triacetate (CTA), Tri-(2-Ethylhexyl) Phosphate (TEHP) and 1-(2-Pyridylazo)-2-Naphtol (PAN) are shown in Scheme 1.
2.3. Measurement Procedure
The optode with the immobilized PAN was cut into 2 cm
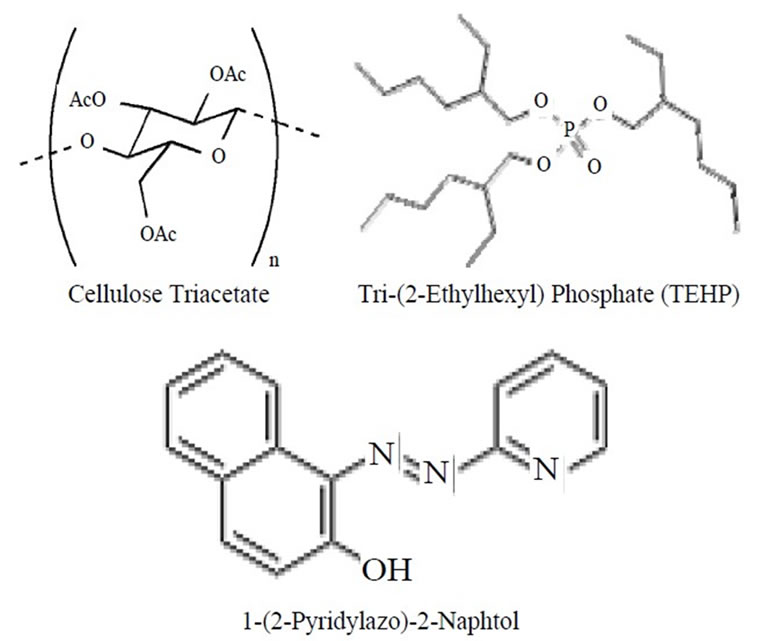
Scheme 1. Structures of the components used in the preparation of the optode.
× 1 cm dimension and placed vertically inside the cuvette and the intensity was measured spectrophotometrically at λmax = 466 and this was treated as blank. The same optode was sorbed with Cd(II) solution at pH = 7.5 for about 150 min and the intensity of Cd(II)-PAN complex formed in the optode sample as a function of cadmium concentration in the equilibrating solution was studied spectrophotometrically. The optode was scanned for the optimum pH for the sorption of Cd(II) at various pH ranging from 2 to 9. The equilibrating solutions of 25 mL were maintained at pH = 7.5 using 0.01 M NaOH solution and known amounts of Cd(II) ions ranging from 10 ng/mL to 5000 ng/mL were added. The sorption of Cd2+ ions in the optode samples was recorded at λmax = 553 corresponding to PAN-Cd2+ complex formed in the optode matrix. The Cd(II) contents were measured according to the calibration graph, produced by the same procedure for different concentrations of Cd(II) ions.
The kinetic studies of optode (2 cm × 1 cm) was equilibrated in a solution of known concentrations of Cd(II) (500 ng/mL, 2000 ng/mL and 5000 ng/mL) ions at pH = 7.5. The optode sample was taken out of the equilibration solution for every 5 min time interval, and the absorbance was measured at 553 nm. This was continued till a constant absorbance value was obtained.
The desorption studies of Cd(II) was studied by equilibrating the optode sample in well stirred 25 mL of 0.01 mol·L–1 HNO3 aqueous solution. The absorbance reading was taken for every 5 min till the optode is completely reversible.
2.4. Cadmium Uptake Studies Optode by FAAS
The uptake studies of cadmium ion in the optode was also studied by using the flame atomic absorption technique. For uptake studies of Cd(II), known amount of cadmium ion was spiked in 25 mL of aqueous solution at pH = 7.5 using 0.01 mol·L–1 NaOH. The optode sample (2 cm × 1 cm) was completely equilibrated for a period of 150 min. The uptake percentage of Cd(II) by the optode was measured by FAAS making use of before and after equilibrated sample solutions. The percentage of uptake of Cd(II) ions in the optode was calculated by the following equation:
Uptake% = 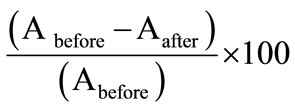
where Abefore and Aafter are the absorbance of Cd(II) ions in the sample taken from before and after equilibrated sample solutions respectively.
2.5. Application to Real Samples
The developed procedure was used for the quantification and determination of Cd(II) ions in the natural water samples by comparison of the absorbance of the optode at 553 nm with the calibration curve. The optode was equilibrated in natural water sample (25 mL) spiked with known amount of Cd(II) for 150 min at pH = 7.5. The optode sample was washed thoroughly with the deionized water and its absorbance was measured at 553 nm against air or blank optode sample.
2.6. Interference Studies
The interference of foreign ions like ,
,  , Na+, Ca2+, Fe3+, Cr3+, EDTA, Mn2+ and Ni2+ was studied by preparing 0.01 mol·L–1 of respective solutions. The optode 2 cm × 1 cm area optode samples was equilibrated with each interfering ion for 150 min at pH = 7.5 and spectra was recorded.
, Na+, Ca2+, Fe3+, Cr3+, EDTA, Mn2+ and Ni2+ was studied by preparing 0.01 mol·L–1 of respective solutions. The optode 2 cm × 1 cm area optode samples was equilibrated with each interfering ion for 150 min at pH = 7.5 and spectra was recorded.
3. Results and Discussion
PAN is known to be terdentate ligand for metal ions and various divalent cations like Zn(II), Cd(II), Hg(II) and Cu(II) forms a coloured complex with PAN in the pH range of 7 - 10.5 [1,21,22]. The 1-(2-Pyridylazo)-2- Naphtol (PAN) immobilized on the surface of Cellulose Triacetate (CTA), acts as a selective optical sensor for Cd(II) ion at pH 7.5. The metal complexation with PAN optode is due to its phenolic rings leaving the hydrophilic moieties (oxygen and azo-nitrogens) available for metal complexation through the formation of one or even two coordination spheres leading to the respective stable five-member rings [21,22]. It may be assumed that 1:2 Cd(II)-PAN complex is formed in the optode membrane, since this was the stoichiometry found in a non-aqueous solution for the Cd(II)-PAN complex. The reaction between the Cd(II) present in the aqueous phase and the optode membrane and the subsequent regeneration of the membrane is shown in Scheme 2.
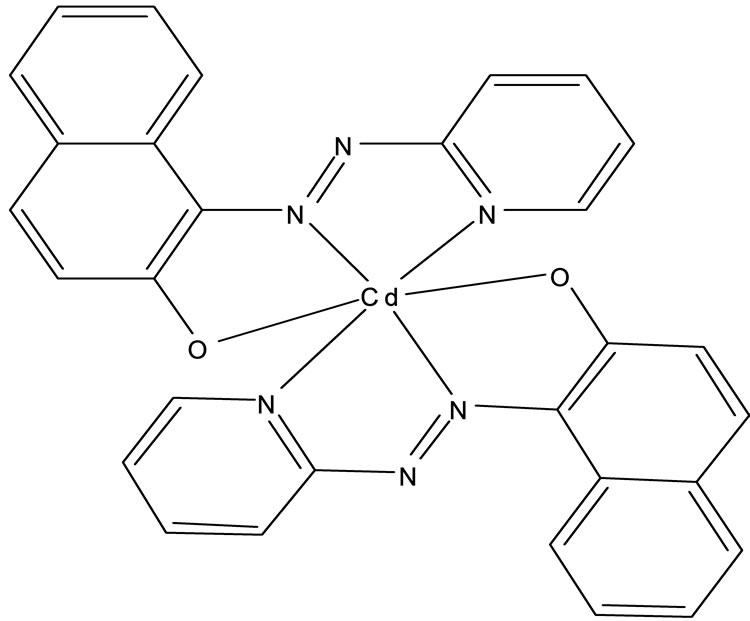
Scheme 2. Chemical structure of Cd(II)-PAN complex.
3.1. Optimization Studies
In the present work, PAN immobilized in a cellulose triacetate plasticized with tri-(2-ethylhexyl) phosphate, was found to produce colour change on equilibration in aqueous solution containing Cd (II) ions in pH 7.5. It was observed that there was no colour change of optode, if PAN is immobilized along with some extractants like Bis (2-ethylhexyl) phosphoric acid (HDEHP), dibromo-pxylene (DBPX) and trioctylmethylammonium chloride (Aliquat-336). It indicates that the developed optode doest not require any extractant which is contrary to our previous work [12]. The proportions of CTA and TEHP in the optode were optimized in terms of better uptake, kinetics, mechanical strength and uniformity. The optimized composition of the optode was found to be: CTA = 0.2 g, TEHP = 0.1 g, PAN = 0.001 g.
3.2. Effect of pH
The pH of the test solution plays a vital role in deriving sensor performance. The influence of pH on the Cd-PAN optode response has been studied by varying pH ranges from 2 to 9. The sorption of Cd(II) ion was found to be maximum at the pH = 7.5. The optimization of pH studies was shown in Figure 1. The decrease in percentage sorption at pH < 7.0 is due to the insufficient formation of the Cd-PAN complex. Hence, in subsequent studies, the pH was adjusted to 7.5 using dil. NaOH.
3.3. Dynamic Response Time
Dynamic response time is an another important analytical factor that measures the sensing ability of the membrane optode. The response time was recorded by equilibrating the optode membrane with a well-stirred solution containing known concentration of Cd(II) ions in 25 mL by adjusting the pH = 7.5. The absorbance of optode was measured at λmax = 553, absorbance was measured for every 5 minutes time interval and it was continued up to 150 minutes till a constant absorbance was obtained. The response time for optodes with concentration 500 ng/mL, 2000 ng/mL & 5000 ng/mL is shown in the Figure 2.
3.4. Linearity Range
The techniques like spectrophotometer and FAAS were used for linearity studies for the Cd(II) uptake in the developed optode membrane. The optode developed in the present work can be used for the preconcentration of Cd(II) from aqueous medium, the concentration range and the detection limit for the which optode can be used. The minimum amount of Cd(II) required to produce detectable response by spectrophotometer is 10 ng/mL. The R2 value for spectrophotometric studies is 0.9642 and the linearity range was shown in the Figure 3. The absorbance
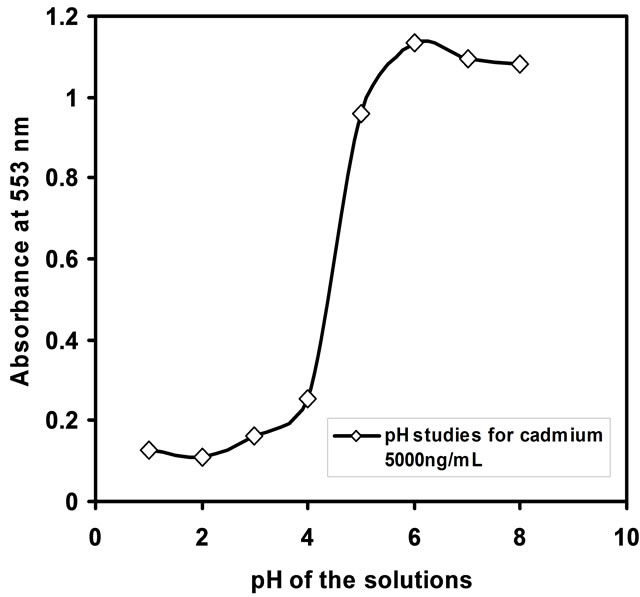
Figure 1. Uptake of Cd(II) (5000 ng/mL) in the optode as a function of pH.
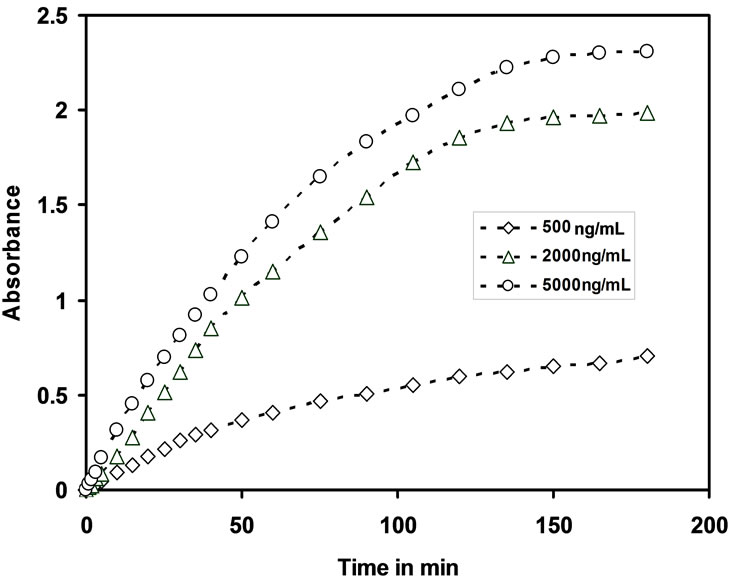
Figure 2. Variation of absorbance of optodes at 553 nm as function of its equilibration time with different concentations of Cd(II).
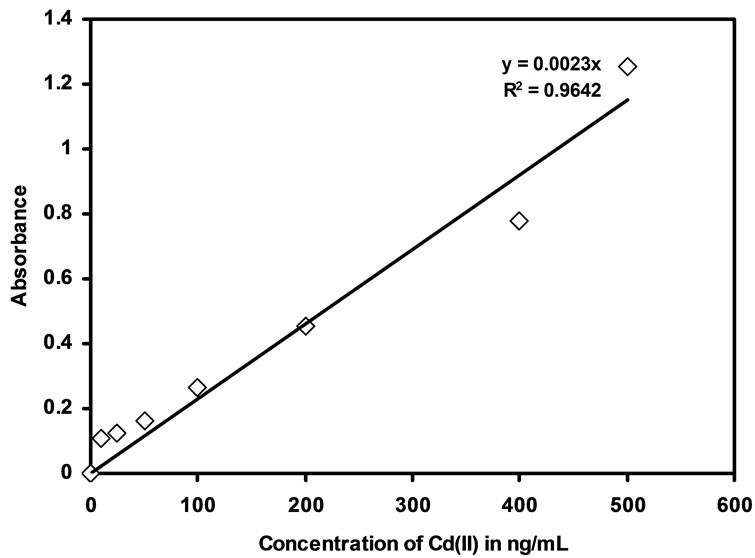
Figure 3. Calibration curve obtained from measurement of the absorbance of optode samples (2 cm× 1 cm) at 553 nm by spectrophotometry.
of the equilibrated optode samples at 553 nm showed a linear response with a concentration range of 10 ng/mL to 500 ng/mL of Cd(II) by UV-visible spectrophotometry.
The uptake percentage of Cd(II) by the optode was measured by FAAS making use of before and after equilibrated sample solutions. The quantitative determination of Cd(II) is dependent upon the volume of the equilibrating solution, the amount of Cd(II) required in the optode matrix to produce detective response and Cd(II) sorption capacity of the optode sample by FAAS with R2 = 0.9857. Detection limit of Cd(II) by FAAS is 250 ng/mL. In order to study the linear response, water samples of 25 mL were spiked with known amount of Cd(II) ions and equilibrated with optode sample for 150 min. The absorbance of the before and after equilibrated sample solutions showed a linear response with a range from 250 ng/mL to 5000 ng/mL. This linear response range by FAAS was shown in the Figure 4.
3.5. Optode Response
The UV-Vis absorption spectra of blank and Cd(II) uploaded with various concentrations are given in Figure 5. It was observed that the uncomplexed PAN optode has the maximum absorbance at λmax = 466 nm. It also observed from the same figure with the increase of Cd(II) concentration the absorption was increased gradually. On complexation with Cd(II), there was a large banthochromic shift in the absorbance of the optode sample from 466 nm to 553 nm. On sorption with Cd(II) ions, the optode changes its colour from golden yellow to violet red. The response in the form of increase in absorption at 553 nm, proportional to the amount of cadmium ions preconcentrated in the optode matrix, is generated by the PAN. The change of colour of the optode samples from golden yellow to violet red with the gradual increase in concentration of cadmium ion was shown in the Figure 6.
3.6. Reproducibility Study
The reproducibility of the optode was studied by repeating three cycles of Cd(II) sorption and desorption from the optode sample (2 cm × 1 cm). For Cd(II) sorption optode was equilibrated in 25 mL of 1000 ng/mL of Cd(II) ions for 150 min and the absorbance was measured at 553 nm. The desorption studies were done by equilibrating the cadmium uploaded optode in 0.01 N HNO3 aqueous solution with well stirring and the readings were taken for every 2 min till the optode is fully reversible. It was clearly observed that Cd(II) ions can be quantitatively desorbed within 15 min. The sorption and desorption of Cd(II) from the optode were studied by monitoring the absorbance at 553 nm. The sorption and desorption
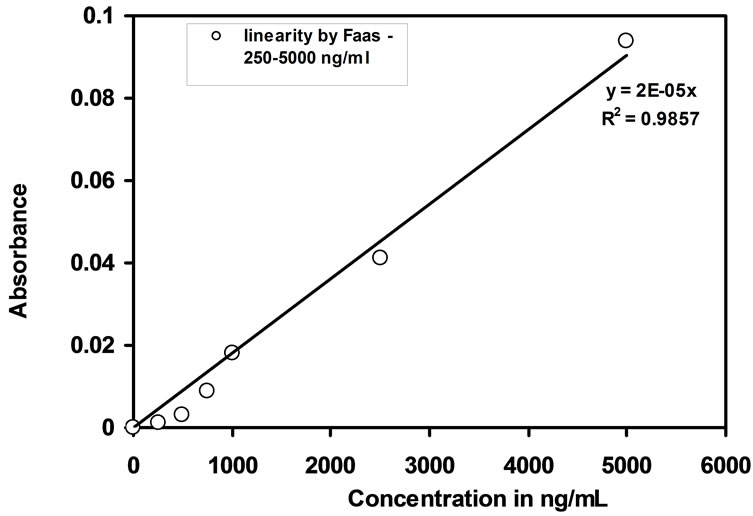
Figure 4. Calibration curve obtained from measurement of the absorbance of equilibrated sample solutions by FAAS.
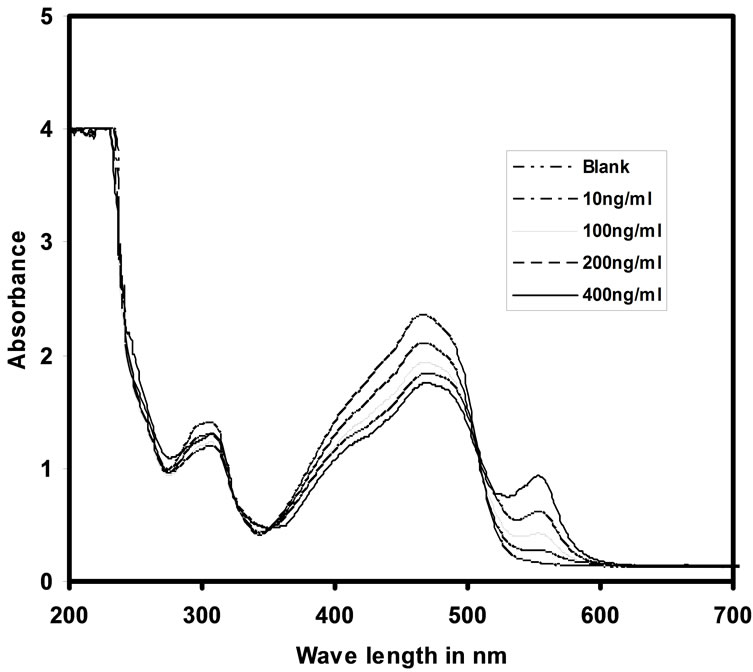
Figure 5. UV-Vis spectra of optode samples spiked with different concentrations of Cd(II) ions.









Figure 6. Change in colour of the optodes with different concentrations of Cd(II) (10 - 500 ng/mL).
of Cd(II) ions profile with three repeated cycles was shown in the Figure 7. It is seen from the figure that the developed membrane optode in the present work is reusable.
3.7. Interference Studies
The selectivity for the developed optode was tested for the determination of Cd(II) in the presence of other foreign anions and cations namely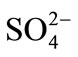 ,
, 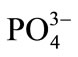 , Na+, Ca2+, Fe3+, Cr3+, EDTA, Mn2+ and Ni2+. The various foreign
, Na+, Ca2+, Fe3+, Cr3+, EDTA, Mn2+ and Ni2+. The various foreign
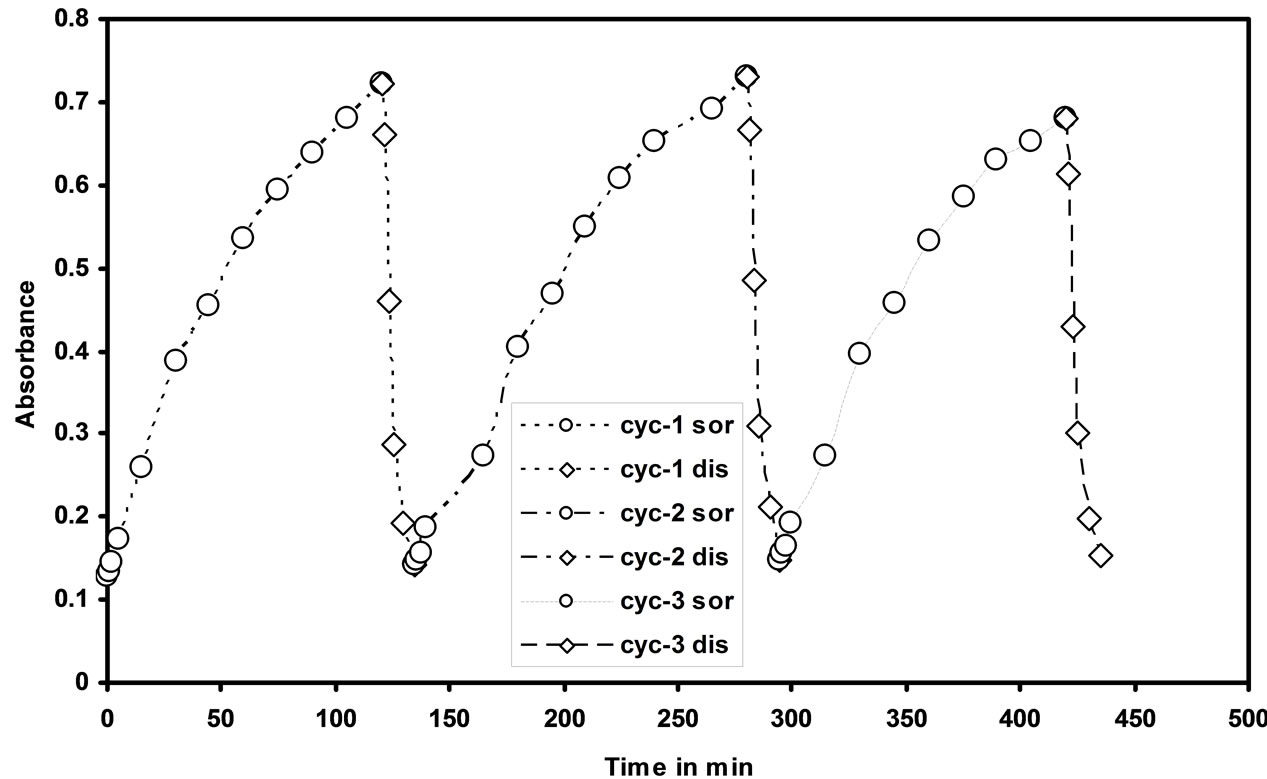
Figure 7. Sorption and desorption cycles of the optode in 25 mL aqueous solution containing 1000 ng/mL of Cd(II) and 0.01 mol·L–1 HNO3 respectively.
ions were prepared in 0.01 mol·L–1 proportion and 2 cm × 1 cm area optode samples was equilibrated with known amount of Cd(II) in presence of each interfering ion for 150 min at pH = 7.5. The obtained tolerance limits of the optode in presence of the foreign ions are summarized in Table 1.
3.8. Analysis of Real Sample
To evaluate the capability of the membrane optode in the analysis of real water samples, the known amount of metal ion solution is spiked in natural waters (25 mL). Optode samples was equilibrated with well-stirring for 150 min by adjusting the pH = 7.5 and the absorbance was measured at 553 nm. The results of the determination are given in Table 2. From this study it is clearly indicated that the optode prepared in this work can be successfully applied for the determination of Cd(II) in natural water samples.
4. Conclusion
The colour changeable optode for the qualitative and quantitative determination of Cd(II) ions in aqueous samples was developed by the immobilization of chromopore 1-(2-Pyridylazo)-2-Naphtol (PAN) in the Tri-(2-Ethylhexyl) Phosphate (TEHP) plasticized cellulose triacetate matrix. The developed optode was used to determine Cd(II) ions in the water samples by using the analytical techniques like spectrophotometer and flame atomic absorption spectroscopy with response with in 150 min. The optode reported in the present work was found to be
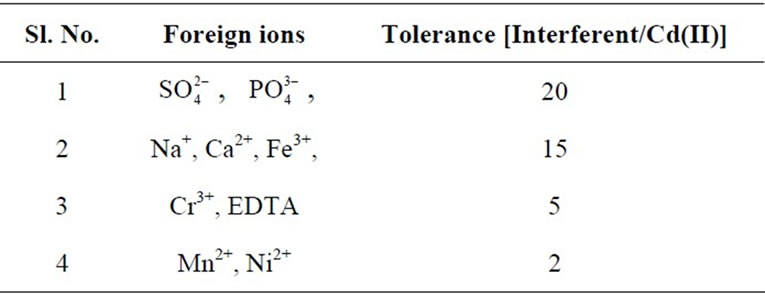
Table 1. Tolerance of foreign ions in the determination of Cd(II) (1 mg/L) by spectrophotometry.
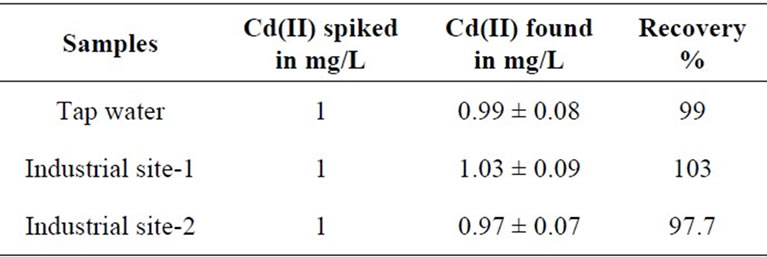
Table 2. Uptake % of Cd(II) in the optode from the natural water samples.
reusable and can be successfully applied for the determination of cadmium in natural water samples.
REFERENCES
- M. Soylak, U. Divrikli, S. Saracoglu and L. Elci, “Membrane Filtration—Atomic Absorption Spectrometry Combination for Copper, Cobalt, Cadmium, Lead and Chromium in Environmental Samples,” Environmental Monitoring and Assessment, Vol. 127, No. 1-3, 2007, pp. 169- 176. doi:10.1007/s10661-006-9271-0
- A. Kaur and U. Gupta, “A Preconcentration Procedure Using 1-(2-Pyridylazo)-2-Napthol Anchored to Silica Nanoparticle for the Analysis of Cadmium in Different Samples,” E-Journal of Chemistry, Vol. 5, No. 4, 2008, pp. 930-939. doi:10.1155/2008/431916
- M. Khosravan and A. S. Saljooghi, “Determination of Cadmium by FAAS after Adsorption and Preconcentration on 2-Aminothiophenol Modified Silica Gel,” European Journal of Scientific Research, Vol. 48, No. 4, 2011, pp. 606-613.
- F. Depault, M. Cojocaru, F. Fortin, S. Chakrabarti and N. Lemieux, “Genotoxic Effects of Chromium(VI) and Cadmium(II) in Human Blood Lymphocytes Using the Electron Microscopy in Situ End-Labeling (EM-ISEL) Assay,” Toxicology in Vitro, Vol. 20, No. 4, 2006, pp. 513- 518. doi:10.1016/j.tiv.2005.09.003
- A. C. Davis, P. Wu, X. Zhang, X. Hou and B. T. Jones, “Determination of Cadmium in Biological Samples,” Applied Spectroscopy Reviews, Vol. 41, No. 1, 2006, pp. 35- 75. doi:10.1080/05704920500385486
- M. R. Ullah and M. E. Haque, “Spectrophotometric Determination of Toxic Elements (Cadmium) in Aqueous Media,” Journal of Chemical Engineering, Vol. 25, No. 1, 2010, pp. 1-12.
- M. Jamaluddin Ahmed, M. Salim and J. Bangladesh Acad, “Second Annual Report on Carcinogens,” Environmental Protection Agency, NTP 81-43, December 1981, pp. 73-80.
- S. J. Haswell, “Atomic Absorption Spectrometry: Theory, Design and Applications,” Elsevier, Amsterdam, 1991.
- A. Erdem and A. E. Eroglu, “Speciation and Preconcentration of Inorganic Antimony in WATERS by Duolite GT-73 Microcolumn and Determination by Segmented Flow Injection-Hydride Generation Atomic Absorption Spectrometry (SFI-HGAAS),” Talanta, Vol. 68, No. 1, 2005, pp. 86-92. doi:10.1016/j.talanta.2005.04.041
- S. L. C. Ferreira, W. N. L. dos Santos, M. A. Bezerra, V. A. Lemos and J. M. Bosque-Sendra, “Use of Factorial Design and Doehlert Matrix for Multivariate Optimisation of an On-Line Preconcentration System for Lead Determination by Flame Atomic Absorption Spectrometry,” Analytical and Bioanalytical Chemistry, Vol. 375, No. 3, 2003, pp. 443-449.
- A. A. Ensafi and Z. N. Isfahani, “A Simple Optical Sensor for Cadmium Ion Assay in Water Samples Using Spectrophotometry,” Journal of Analytical Chemistry, Vol. 66, No. 2, 2011, pp. 151-157. doi:10.1134/S106193481102002X
- Y. Kalyan, A. K. Pandey, G. R. K. Naidu and A. V. R. Reddy, “Membrane Optode for Uranium(VI) Ions Preconcentration and Quantification Based on a Synergistic Combination of 4-(2-Thiazolylazo)-Resorcinol with 8- Hydroxyquinaldine,” Spectrochimica Acta Part A: Molecular and Biomolecular, Vol. 74, No. 5, 2009, pp. 1235- 1241. doi:10.1016/j.saa.2009.09.045
- A. A. Ensafi, S. Meghdadi and E. Fooladgar, “Development of a New Selective Optical Sensor for Cd(II) Ions Based on 4-Hydroxy Salophen,” IEEE Sensor Journal, Vol. 8, No. 11, 2008, pp. 1794-1800. doi:10.1109/JSEN.2008.2005227
- C. Sanchez-Pedreno, M. S. Garcia, J. A. Ortuno, M. I. Albero and R. Exposito, “Kinetic Methods for the Determination of Cadmium(II) Based on a Flow-Through Bulk Optode,” Talanta, Vol. 56, No. 3, 2002, pp. 481-489. doi:10.1016/S0039-9140(01)00571-9
- Y. Kurauchi, R. Hayashi, N. Egashira and K. Ohga, “Fluorometric Determination of Zinc, Cadmium and Gallium Ions with a Fiber-Optic Sensor Having a Pyridoxal Isomer-Modified Chitosan/Agarose Gel as a Sensing Probe,” Analytical Sciences, Vol. 8, No. 6, 1992, pp. 837-840. doi:10.2116/analsci.8.837
- I. Oehme and O. S. Wolfbeis, “Optical Sensors for Determination of Heavy Metal Ions,” Microchimica Acta, Vol. 126, No. 3-4, 1997, pp. 177-192. doi:10.1007/BF01242319
- M. Valcarcel and M. D. L. de Castro, “Flow-Through (Bio)- chemical Sensors,” Elsevier Science, Amsterdam, 1994.
- R. Czolk, J. Reichert and H. J. Ache, “An Optical Sensor for the Detection of Heavy Metal Ions,” Sensors and Actuators B: Chemical, Vol. 7, No. 1-3, 1992, pp. 540-543. doi:10.1016/0925-4005(92)80360-A
- B. Kuswandi, A. A. Vaughan and R. Narayanaswamy, “Simple Regression Model Using an Optode for the Simultaneous Determination of Zinc and Cadmium Mixtures in Aqueous Samples,” Analytical Sciences, Vol. 17, No. 1, 2001, pp. 181-186. doi:10.2116/analsci.17.181
- B. Rezaei, S. Meghdadi and S. Bagherpour, “Cadmium Selective PVC-Membranes Sensor Based on 1,2-Bis (Quinoline-2-Carboxamido)-4-Chlorobenzene as a Neutral Carrier,” Sensors Journal, Vol. 8, No. 8, 2008, pp. 1469-1477. doi:10.1109/JSEN.2008.920719
- D. L. Giokas, E. K. Paleologos, M. I. Prodromidis and M. I. Karayannis, “Development of 1-(2-Pyridylazo)-2-Naphthol-Modified Polymeric Membranes for the Effective Batch Pre-Concentration and Determination of Zinc Traces with Flame Atomic Absorption Spectrometry,” Talanta, Vol. 56, No. 3, 2002, pp. 491-498. doi:10.1016/S0039-9140(01)00572-0
- C. Sanchez-Pedreno, J. A. Ortuno, M. I. Albero, M. S. Garcia and M. V. Valero, “Development of a New Bulk Optode Membrane for the Determination of Mercury(II),” Analytical Chimica Acta, Vol. 414, No. 1-2, 2000, pp. 195-203. doi:10.1016/S0003-2670(00)00809-6
NOTES
*Corresponding author.

